Abstract
OBJECTIVE
This study aims to investigate the association between chronic idiopathic axonal polyneuropathy (CIAP) and the metabolic syndrome or its individual components.
RESEARCH DESIGN AND METHODS
A total of 249 patients with CIAP and 709 controls underwent fasting laboratory studies, and blood pressure and waist circumference were measured. The metabolic syndrome was diagnosed if three or more of the following Adult Treatment Panel III criteria were present: impaired fasting glucose, hypertension, abdominal obesity, reduced HDL cholesterol, and hypertriglyceridemia. Subgroup analysis was performed for patients with a painful predominantly sensory CIAP, because this phenotype is most similar to diabetic polyneuropathy. Statistical analysis was performed with adjustment for age and gender.
RESULTS
Fifty-five percent of all patients fulfilled the metabolic syndrome criteria compared with 34% of controls (odds ratio 2.2 [95% CI 1.7–3.0]). Multivariate analysis shows hypertension (2.9 [1.7–4.9]) and abdominal obesity (3.3 [2.4–4.6]) to be significantly more prevalent in patients than in controls. Of the patients classified as having a painful predominantly sensory CIAP, 62% fulfilled the metabolic syndrome criteria (3.1 [2.0–4.8]). In this subgroup, hypertension and abdominal obesity also were significantly more prevalent compared with controls.
CONCLUSIONS
Abdominal obesity and hypertension seem to be the most consistent contributing components of the metabolic syndrome in patients with CIAP. Evaluation and appropriate treatment of these risk factors in patients with CIAP would be advocated.
Polyneuropathy has an estimated prevalence of 75–125 per 100,000 people (1,2). Despite extensive evaluation, no cause can be established in 5–30% of patients with chronic polyneuropathy (1,3–5). Most of these patients present in the sixth decade of life with a predominantly sensory or sensorimotor axonal polyneuropathy, a so-called chronic idiopathic axonal polyneuropathy (CIAP) (4). CIAP progresses very slowly, >10 years after disease onset, the extent of disability is not severe, and there is no loss of ambulation (6).
Diabetes mellitus is a common established cause of chronic axonal polyneuropathy, but whether prediabetes (impaired fasting glucose [IFG] or impaired glucose tolerance) is an etiological factor remains a matter of debate (7–9). Sural nerve biopsy studies showed similarly increased basal lamina thickness of endoneurial vessels in patients with CIAP or patients with diabetic neuropathy when compared with controls, suggesting a similar pathophysiological mechanism (10). The observed endoneurial vessel changes may result in axonal injury from chronic ischemia and could be etiologically associated with conditions that predispose to microvascular disease, such as (pre)diabetes and cardiovascular risk factors.
Various cardiovascular risk factors and their association with polyneuropathy have been investigated. Only a limited number of studies considered chronic idiopathic polyneuropathy, but diagnostic criteria are quite variable or insufficiently descriptive. Consequently, the associations (or lack thereof) in these studies cannot be readily extrapolated to CIAP. The metabolic syndrome is composed of interrelated cardiovascular risk factors, i.e., hypertension, IFG, hypertriglyceridemia, reduced HDL cholesterol, and abdominal obesity (11). This study aimed to investigate the association between the metabolic syndrome or its individual components and CIAP.
RESEARCH DESIGN AND METHODS
Patients
The current study is part of an ongoing study of the etiology of CIAP initiated in 2008 at the University Medical Center Utrecht and was approved by the Medical Ethics Committee. Patients were referred to the University Medical Center Utrecht neuromuscular outpatient clinic by their general practitioner or by a neurologist in a general hospital. All patients (n = 249) in whom CIAP was diagnosed after evaluation in the University Medical Center Utrecht between October 1, 2008 and December 1, 2011, were included in this study.
Patient evaluation
Patients were evaluated for their polyneuropathy in a standardized fashion by an experienced neuromuscular specialist. The clinical evaluation consisted of thorough history-taking (including questioning about disease course, neurologic symptoms, signs of autonomic dysfunction, comorbidity, intoxications, and medication use), anthropometric measurements, and neurologic examination. The onset of polyneuropathy should have occurred after the age of 45. A detailed kinship history was taken and appropriate genetic testing was performed when necessary. If the possibility of a hereditary polyneuropathy could not be ruled out, then patients were excluded.
The severity of the polyneuropathy was graded by calculation and summation of sensory and motor sum scores (6,12). The sensory sum score ranges from 0 to 28, and considers the anatomical distribution of sensory deficits bilaterally in the lower limbs regarding pinprick and light touch sensation (scored as: normal = 4; up to ankle abnormal = 3; up to distal half lower leg abnormal = 2; up to knee abnormal = 1; above knee abnormal = 0), vibration sense (evaluated with a graduated Rydel-Seiffer 64-Hz tuning fork and scored as: normal = 4; decreased/absent at big toe = 3; decreased/absent at ankle = 2; decreased/absent at knee = 1; decreased/absent at crista iliaca = 0), and propriocepsis of distal interphalangeal joint of the big toe (scored as: normal/immediate perception of movement = 2; reduced/delayed perception of movement = 1; absent perception of movement = 0) (13–15). For the motor sum score, manual muscle strength testing was scored according to the Medical Research Council scale in four distal lower limb muscles (tibialis anterior, gastrocnemius, peroneal, toe extensors), resulting in a score from 0 to 40 for both lower limbs (12,15). Those patients with CIAP who had painful sensory symptoms and signs but no significant weakness (i.e., no weakness or only mild weakness of the toe extensors) were classified as having a “painful predominantly sensory CIAP,” because this phenotype is most similar to diabetic polyneuropathy.
Waist circumference was measured in standing position in a horizontal plane midway between the inferior margin of the rib and the superior border of the iliac crest. Blood pressure was measured sitting in the upright position using an Omron Intellisense 907 Professional Automatic Blood Pressure Monitor.
Ancillary investigations
Extensive laboratory investigations were performed in all patients. Laboratory studies included complete blood count, erythrocyte sedimentation rate, glucose, HbA1c, insulin, renal function, liver enzymes, creatine kinase, C-reactive protein, vitamin B1, vitamin B6, folic acid, vitamin B12, homocystein, cholesterol, triglycerides, HDL cholesterol, LDL cholesterol, thyroid-stimulating hormone, serum and urine M-protein, and serological screening for celiac disease (total IgA level and IgA antibodies against tissue transglutaminase and endomysium). An oral glucose tolerance test (OGTT) was performed in a selected group of patients with a fasting glucose ≥5.6 mmol/L. If indicated, then additional studies (e.g., a lumbar puncture, chest radiograph, magnetic resonance imaging of the spine, or additional laboratory studies such as immunoserology, genetic testing) were performed. Standardized nerve conduction studies were performed for all patients (6,16).
Diagnostic criteria of CIAP
The diagnosis of CIAP was defined according to the following criteria (4,6): presence of symmetrical distal sensory or sensorimotor symptoms such as numbness, pins and needles, tightness, coldness, unsteadiness, muscle cramps, and weakness with onset in the lower limbs, compatible with polyneuropathy; presence of symmetrical distal sensory or sensorimotor signs with evidence of large nerve fiber involvement such as decreased sense of touch, vibration, and propriocepsis, usually in the presence of decreased pin prick/temperature sense, decreased/absent tendon reflexes, or slight muscle weakness on neurologic examination, compatible with polyneuropathy; an insidious onset and slow or no progression of the polyneuropathy over the course of at least 6 months; no identifiable cause for the polyneuropathy after thorough history-taking, clinical examination, and extensive laboratory testing; no suggestion of a hereditary polyneuropathy based on detailed kinship history (i.e., one or more affected family member), neurologic examination, or confirmation by genetic analysis; and nerve conduction studies excluding a demyelinating polyneuropathy and confirming large nerve fiber involvement if the findings on neurologic examination were equivocal considering the patient’s age (16–19). Electrophysiological criteria for demyelination provided that distal compound motor action potential (CMAP) baseline to negative peak amplitude >1 mV were: reduction of motor nerve conduction velocity to <80% of lower limit of normal value (LLN) if distal CMAP >80% of LLN, or to <70% of LLN if CMAP was <80% of LLN; prolongation of distal motor latency to >125% of upper limit of normal value (ULN) if CMAP >80% of LLN, or to >150% of ULN if CMAP <80% of LLN; prolongation of F-waves to >120% of ULN if CMAP >80% of LLN, or to >150% of ULN if CMAP <80% of LLN; conduction block >50% area reduction or, in upper limb nerves, >30% amplitude reduction in proximal CMAP relative to distal; and abnormal temporal dispersion in upper limb nerves >30% duration increase or in lower limb nerves >100% duration increase in proximal CMAP relative to distal (20).
Controls
We used participants in previously conducted population-based studies at the Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, as controls for the current study. Female controls were sampled from the PROSPECT cohort, a Dutch cohort participating in the European Prospective Investigation into Cancer and Nutrition (21). In short, a total of 17,395 healthy women presenting for breast cancer screening, aged 49–70 years and living in Utrecht and the surrounding area, were prospectively enrolled in PROSPECT between 1993 and 1997. In 1999–2000, a subgroup of women (n = 533 from a total of 1,803) who had experienced natural menopause, had an intact uterus and at least one intact ovary, and had not used sex steroids after the reported date of last menstruation agreed to participate in a new study. Women were considered sufficiently healthy if they were able to visit the study center without assistance (n = 402); in these women, fasting laboratory studies were performed, blood pressure and waist circumference were measured, and medication use was recorded. After exclusion of all female controls with (newly) diagnosed type 1 or type 2 diabetes or incomplete data (n = 27), 375 female controls were included in our study.
As male controls, subjects from the HAMLET study were used (22). Briefly, in the HAMLET study, 400 independently living men aged 40–80 years were prospectively enrolled between 2001 and 2002 to investigate the relationship between endogenous testosterone, sex hormone–binding globulin, dehydroepiandrosterone sulfate, estradiol, and the metabolic syndrome. The subjects were recruited by asking female participants of other studies by letter whether they knew a possible interested male volunteer aged 40–80 years; 240 men volunteered to participate. In addition, a randomly selected male population aged 40–80 years was drawn from the municipal register of Utrecht and 1,230 invitation letters were sent. From this group, 390 men volunteered to participate. From the 630 volunteers, those who did not live independently and subjects who were not physically or mentally able to visit the study center independently (n = 16) were excluded. Of the remaining 614 men, eventually 400 men were randomly selected to participate. All male controls had undergone fasting laboratory studies, had blood pressure and waist circumference measured, and medication use was recorded. After exclusion of all male controls with (newly) diagnosed type 1 or type 2 diabetes, or incomplete data (n = 66), 334 male controls were included in our study.
Diagnostic criteria of the metabolic syndrome
The metabolic syndrome was diagnosed if three or more of the following Adult Treatment Panel III criteria were present: fasting glucose ≥5.6 mmol/L (≥100 mg/dL); systolic blood pressure ≥130 mmHg and/or diastolic blood pressure ≥85 mmHg; waist circumference >102 cm (>40 in) in men or >88 cm (>35 in) in women; HDL cholesterol <1.03 mmol/L (<40 mg/dL) in men or <1.29 mmol/L (<59 mg/dL) in women; and triglycerides ≥1.7 mmol/L (150 mg/dL) (11). Additionally, patients or controls using antihypertensive medication and/or lipid-lowering agents (fibrates, nicotinic acid, or statins) were considered to fulfill the criteria for hypertension, reduced HDL cholesterol, and hypertriglyceridemia, respectively.
Statistical analysis
In the Netherlands, the prevalence of the metabolic syndrome in the nondiabetic general population aged 40 years and older is at least ∼25% (23,24). If the true prevalence of the metabolic syndrome in patients with CIAP is twice as high (i.e., 50%), then a power analysis (power 1 – β = 0.85; associated type I error probability α = 0.05) to determine the sample size for a case-control study shows that 66 cases and 66 controls are needed to be able to reject the null hypothesis that the prevalence rates for cases and controls are equal (PS Program version 2.1.31). Statistical analyses were performed using SPSS version 15.0. The observed prevalence rates in patients and controls were used to calculate odds ratios (ORs) with 95% confidence intervals (CIs), and χ2 analysis (univariate) and binary logistic regression analysis (multivariate) were used to investigate the association between CIAP and the metabolic syndrome or its individual components. The correlation between the severity of the polyneuropathy, based on the sum of the sensory and motor sum score, and the severity of the metabolic syndrome, based on the number of metabolic syndrome criteria that were met, was evaluated with the Spearman correlation coefficient. A subgroup analysis was performed for patients classified as having painful predominantly sensory CIAP. All analyses were performed with adjustment for age, gender, and metabolic syndrome criteria if appropriate. Statistical significance was set at P < 0.05.
RESULTS
Characteristics of the 249 included patients (32% women) and 709 controls (53% women) are outlined in Table 1. The mean duration of symptoms was 5.6 years (0.2–26.3 years). All patients had sensory symptoms and/or signs, 48% of patients reported painful sensory symptoms, and 38% of patients were classified as having painful predominantly sensory CIAP.
Table 1.
Characteristics of patients with CIAP and controls
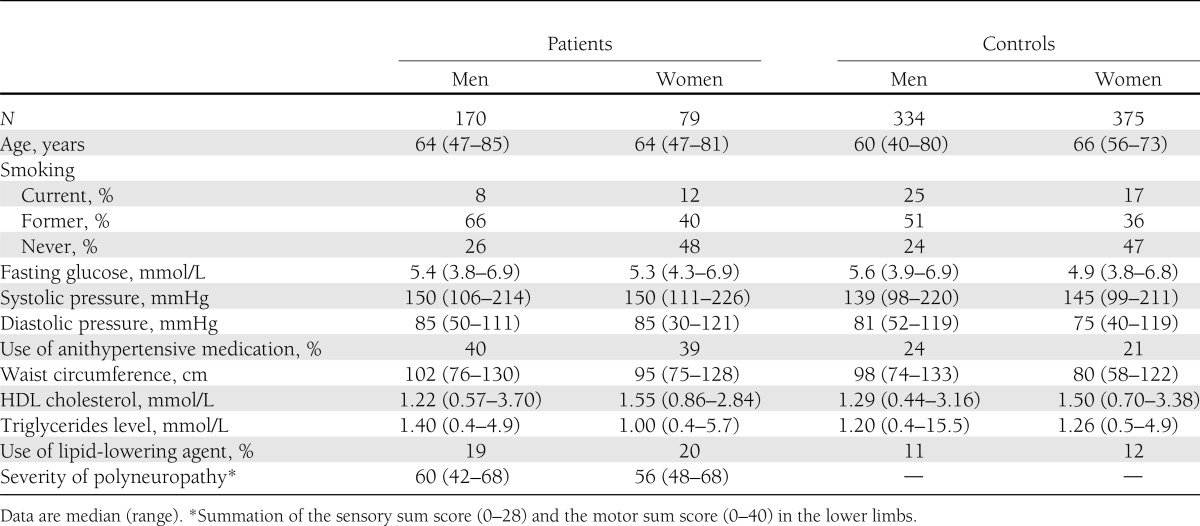
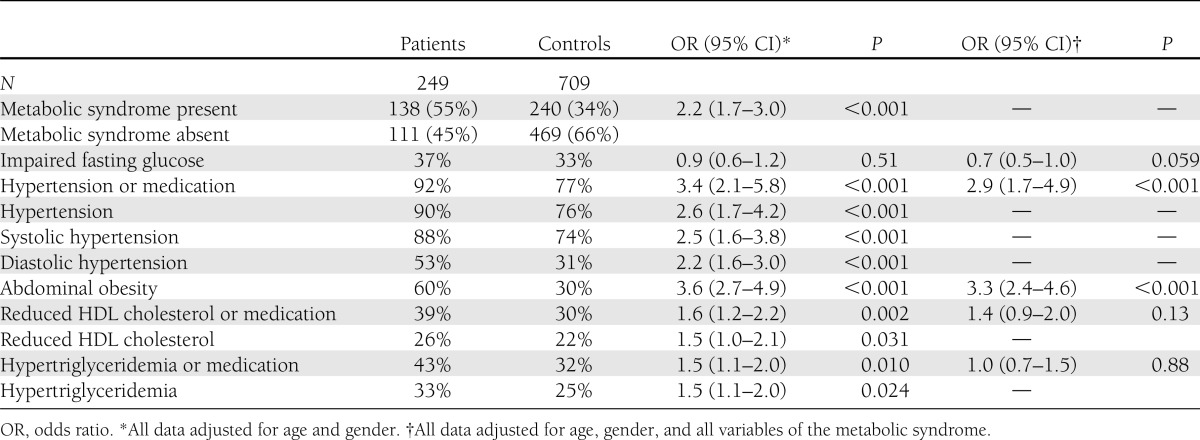
Table 2 details the prevalence of the metabolic syndrome and its individual components in patients and controls. Of the patients, 55% fulfilled the metabolic syndrome criteria compared with 34% of the controls (OR 2.2 [95% CI 1.7–3.0]). Except for impaired fasting glucose, all metabolic syndrome criteria were significantly more prevalent in patients compared with controls when adjusted for age and gender. Multivariate analysis, with adjustment for age, gender, and metabolic syndrome criteria, shows hypertension (2.9 [1.7–4.9]) and abdominal obesity (3.3 [2.4–4.6]) to be significantly more prevalent in patients than in controls. Adjustment for smoking did not affect the results. There was no correlation between the severity of the polyneuropathy and the severity of the metabolic syndrome (Spearman correlation coefficient −0.068; P = 0.35).
Table 2.
Prevalence of the metabolic syndrome and its components in patients with CIAP and controls
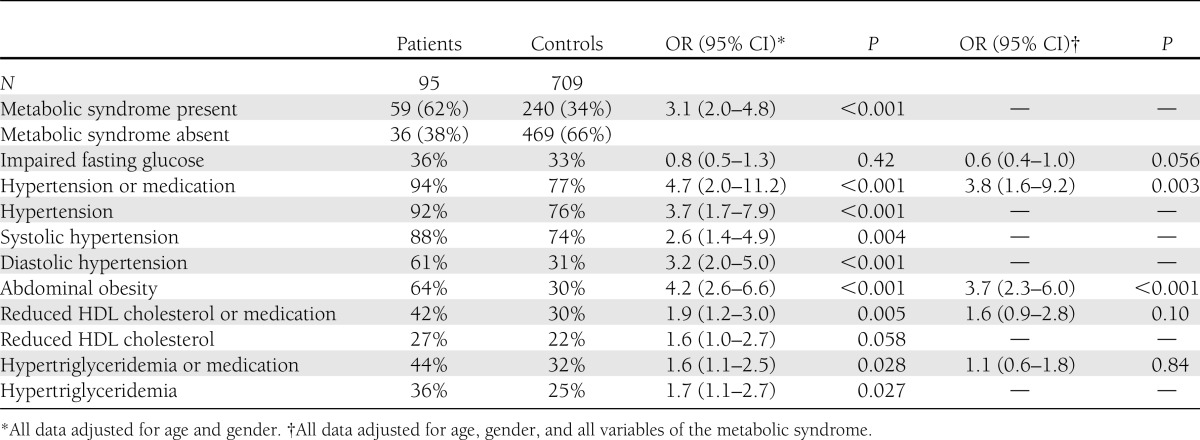
Of the patients classified as having a painful predominantly sensory CIAP, 62% fulfilled the metabolic syndrome criteria (OR 3.1 [95% CI 2.0–4.8]). In this subgroup, hypertension and abdominal obesity also were significantly more prevalent compared with controls (Table 3). Glucose levels in the 95 patients with painful predominantly sensory CIAP (median, 5.4; range, 4.0–6.8) were comparable with glucose levels in the remaining 154 patients (median, 5.4; range, 3.8–6.9), and impaired fasting glucose was not more prevalent in either patient group compared with controls.
Table 3.
Prevalence of the metabolic syndrome and its components in patients with painful predominantly sensory CIAP and controls
CONCLUSIONS
The current study shows that the metabolic syndrome is significantly more prevalent in patients with CIAP compared with controls from the general population, and that abdominal obesity and hypertension seem to be the most consistent contributing components.
The prospective recruitment of patients with CIAP and a control group derived from the general population in the Netherlands distinguishes our study from previous studies. Although controls were obtained from historical Dutch cohorts, we had access to individual data; this enabled appropriate direct comparisons between patients and controls. To retain the use of data from a large control group, all analyses were performed with adjustment for gender and age. The prevalence of the metabolic syndrome in the Netherlands has not changed much in the past decade. A population-based study in 2006 found a prevalence of the metabolic syndrome of 29% in persons 40–70 years old (23). In the most recent population-based study performed in 2009–2010, the prevalence of the metabolic syndrome was 35% in persons 40–70 years old (25). These prevalence rates are comparable with the prevalence of 34% for the metabolic syndrome in the controls (data collection in 1999–2002) used in our study. It is therefore unlikely that the observed association between the metabolic syndrome and CIAP can be largely explained by a change in prevalence of the metabolic syndrome (i.e., increased in patients with CIAP) over time. Controls in general (although not necessarily) may constitute a healthier representation of the population. However, except for being nondiabetic and sufficiently physically and mentally healthy to visit the study center, no other possibly relevant additional health-related eligibility criteria were used for our control groups. Controls were not evaluated for polyneuropathy, but it is not to be expected that more than a few controls did have polyneuropathy after exclusion of patients with diabetes and given the low estimated prevalence of polyneuropathy (1,2). Altogether, our findings were probably not influenced to an important degree by certain selective control characteristics.
A previous case-control study observed a comparable association between hypertension and CIAP (26). Hypertension remained a risk factor for chronic symmetric polyneuropathy after adjustment for a few common causes of polyneuropathy in a population sample of Italian subjects aged 55 years or older and screened for the presence of (sub)clinical signs of polyneuropathy (27). A cross-sectional study found a negative association between current blood pressure or history of hypertension and presence of (sub)clinical signs suggestive of polyneuropathy in subjects older than 65 years without a history of common diseases known to cause polyneuropathy, but this association was not observed when use of antihypertensive medication was taken into account (28).
No studies have reported on the association between abdominal obesity as measured by waist circumference and CIAP. The use of waist circumference instead of BMI is relevant because abdominal obesity appears a more robust risk factor for cardiovascular disease and type 2 diabetes than BMI (29). Studies that used BMI as a measure of obesity vary considerably in mean BMI values and prevalence rates of obesity in the investigated study populations. A controlled study did not find a higher prevalence of an abnormal BMI in patients with CIAP (26). Another cohort study showed no association between BMI and the presence of (sub)clinical polyneuropathy in subjects without diseases commonly known to cause peripheral neuropathy when age and hypertension-related variables were taken into account (28). One small controlled study, however, found that patients with painful CIAP had a higher mean BMI than healthy controls, but this was not observed for patients without pain (30).
The association between dyslipidemia and CIAP has not been studied extensively before. One case-control study reported hypercholesterolemia to be more prevalent among patients with CIAP (26). Another controlled study found no difference in fasting cholesterol levels in patients with CIAP, but increasing fasting triglycerides level was significantly associated with the likelihood of CIAP after adjusting for BMI, age, and gender (30). A recent, small, controlled study did not demonstrate a significantly higher frequency of dyslipidemia or fasting cholesterol level, but fasting triglycerides level was borderline nonsignificantly lower in patients with idiopathic neuropathy (31). The divergent observations could be explained by markedly higher fasting triglycerides levels in patients and lower levels in controls in the former study when compared with patients and controls in the latter study and our study (30,31).
In the Netherlands, treatment of dyslipidemia with fibrates or nicotinic acid is uncommon and only prescribed when statins are not tolerated or are insufficiently effective. Because statins have an effect by lowering triglycerides and increasing HDL cholesterol, we chose to include statin treatment as a defining criterion in addition to the Adult Treatment Panel III metabolic syndrome criteria for dyslipidemia. The frequency of dyslipidemia thus could have been overestimated; however, because this would equally concern patients and controls, we believe it did not skew our results. Furthermore, when only considering hypertriglyceridemia and reduced HDL cholesterol without taking the use of statins into account, data analyses showed similar results (data not shown).
Previous North American studies have suggested an association between chronic polyneuropathy or, more specifically, idiopathic painful sensory neuropathy/small fiber neuropathy and prediabetes (32–35). Our study did not find IFG to be more prevalent in patients with CIAP compared with controls. Even subgroup analysis of patients with painful predominantly sensory CIAP, a CIAP phenotype most similar to diabetic polyneuropathy, demonstrated no association with IFG. Meanwhile, the established associations of CIAP with hypertension and abdominal obesity appear even stronger in this subgroup. The absence of an association with IFG is in accordance with a small controlled study, which did, however, find an elevated 2-h glucose level as determined by an OGTT suggestive of impaired glucose tolerance in patients with painful CIAP, and a recently published prospective population-based study that showed a similar prevalence of polyneuropathy in subjects with normal and impaired glycemia (8,30). Subjects in North American studies tend to have a higher BMI compared with those in European studies; given the intricate association between overweight/obesity and prediabetes, this may distort the association between prediabetes and CIAP. A routinely performed OGTT could have resulted in a higher percentage of patients and controls considered to have prediabetes in our study, but this would not have influenced our findings because impaired glucose tolerance is not a metabolic syndrome criterion. A systematic review showed that the reproducibility of the OGTT in prediabetes is, on average, only 49% compared with 73% in diabetes and 93% in normal glucose tolerance (36). Thus, an OGTT as advocated by some investigators, and especially if only performed once, has limited usefulness and reliability to establish prediabetes and its association with idiopathic polyneuropathy.
We found abdominal obesity and hypertension as risk factors for CIAP. Although our study was not designed to elucidate the precise pathofysiological mechanisms, the results support a microvascular hypothesis (10). For abdominal obesity (which probably plays a pivotal role among all metabolic syndrome factors), the mechanism is mainly related to a proinflammatory state at the level of the microvasculature, including endoneurial microvessels and endothelial cells, that could lead to microvascular changes, chronic ischemia, and consequently axonal injury. For hypertension, the mechanisms could be characterized by structural and functional changes in the microvasculature (37–39).
This study is the largest case-control study of the etiology of CIAP. It shows there is a convincing association between CIAP and the metabolic syndrome, which is determined mostly by metabolic syndrome components hypertension and abdominal obesity. In contrast to most previous studies, no significant association was found with IFG. Considering these results, we advocate a proactive attitude to look for simultaneous occurrence of these conditions in patients and initiate appropriate treatment that could, perhaps, not only prevent but also alleviate progression of the polyneuropathy.
Acknowledgments
This study was supported by a grant (WAR 07-24) from the Prinses Beatrix Fonds. Data collection for female controls was supported by the Netherlands Organization for Health Research and Development (ZonMw no. 2100.0011).
No potential conflicts of interest relevant to this article were reported.
N.A.V., A.F.J.E.V., and N.C.N. included patients. N.A.V. researched data and wrote the manuscript. A.F.J.E.V. contributed to the discussion. A.F.J.E.V., Y.T.v.d.S., L.H.v.d.B., and N.C.N. reviewed and edited the manuscript. Y.T.v.d.S. provided the control data. N.A.V. and A.F.J.E.V. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Jan Veldink, University Medical Center Utrecht, for statistical advice.
References
- 1.Mygland A, Monstad P. Chronic polyneuropathies in Vest-Agder, Norway. Eur J Neurol 2001;8:157–165 [DOI] [PubMed] [Google Scholar]
- 2.Rudolph T, Farbu E. Hospital-referred polyneuropathies—causes, prevalences, clinical- and neurophysiological findings. Eur J Neurol 2007;14:603–608 [DOI] [PubMed] [Google Scholar]
- 3.Jann S, Beretta S, Bramerio M, Defanti CA. Prospective follow-up study of chronic polyneuropathy of undetermined cause. Muscle Nerve 2001;24:1197–1201 [DOI] [PubMed] [Google Scholar]
- 4.Notermans NC, Wokke JH, Franssen H, et al. Chronic idiopathic polyneuropathy presenting in middle or old age: a clinical and electrophysiological study of 75 patients. J Neurol Neurosurg Psychiatry 1993;56:1066–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg NR, Vermeulen M. Should coeliac disease be considered in the work up of patients with chronic peripheral neuropathy? J Neurol Neurosurg Psychiatry 2005;76:1415–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vrancken AF, Franssen H, Wokke JH, Teunissen LL, Notermans NC. Chronic idiopathic axonal polyneuropathy and successful aging of the peripheral nervous system in elderly people. Arch Neurol 2002;59:533–540 [DOI] [PubMed] [Google Scholar]
- 7.Dyck PJ, Dyck PJ, Klein CJ, Weigand SD. Does impaired glucose metabolism cause polyneuropathy? Review of previous studies and design of a prospective controlled population-based study. Muscle Nerve 2007;36:536–541 [DOI] [PubMed] [Google Scholar]
- 8.Dyck PJ, Clark VM, Overland CJ, et al. Impaired glycemia and diabetic polyneuropathy: the OC IG Survey. Diabetes Care 2012;35:584–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajabally YA. Neuropathy and impaired glucose tolerance: an updated review of the evidence. Acta Neurol Scand 2011;124:1–8 [DOI] [PubMed] [Google Scholar]
- 10.Teunissen LL, Notermans NC, Jansen GH, Banga JD, Veldman H, Wokke JH. Thickness of endoneurial vessel basal lamina area in chronic idiopathic axonal polyneuropathy. Acta Neuropathol 2000;100:445–450 [DOI] [PubMed] [Google Scholar]
- 11.Grundy SM, Cleeman JI, Daniels SR, et al. American Heart Association. National Heart, Lung, and Blood Institute Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735–2752 [DOI] [PubMed] [Google Scholar]
- 12.Merkies IS, Lauria G. 131st ENMC international workshop: selection of outcome measures for peripheral neuropathy clinical trials 10-12 December 2004, Naarden, The Netherlands. Neuromuscul Disord 2006;16:149–156 [DOI] [PubMed] [Google Scholar]
- 13.Erdmann PG, Teunissen LL, van Genderen FR, et al. Functioning of patients with chronic idiopathic axonal polyneuropathy (CIAP). J Neurol 2007;254:1204–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merkies IS, Schmitz PI, van der Meché FG, van Doorn PA, The Inflammatory Neuropathy Cause and Treatment (INCAT) Group Reliability and responsiveness of a graduated tuning fork in immune mediated polyneuropathies. J Neurol Neurosurg Psychiatry 2000;68:669–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajabally YA, Narasimhan M. Characteristics and correlates of sensory function in chronic inflammatory demyelinating polyneuropathy. J Neurol Sci 2010;297:11–14 [DOI] [PubMed] [Google Scholar]
- 16.Franssen H, Notermans NC, Wieneke GH. The influence of temperature on nerve conduction in patients with chronic axonal polyneuropathy. Clin Neurophysiol 1999;110:933–940 [DOI] [PubMed] [Google Scholar]
- 17.England JD, Gronseth GS, Franklin G, et al. American Academy of Neurology. American Association of Electrodiagnostic Medicine. American Academy of Physical Medicine and Rehabilitation Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2005;64:199–207 [DOI] [PubMed] [Google Scholar]
- 18.Vrancken AF, Kalmijn S, Brugman F, Rinkel GJ, Notermans NC. The meaning of distal sensory loss and absent ankle reflexes in relation to age: a meta-analysis. J Neurol 2006;253:578–589 [DOI] [PubMed] [Google Scholar]
- 19.Vrancken AF, Notermans NC, Wokke JH, Franssen H. The realistic yield of lower leg SNAP amplitudes and SRAR in the routine evaluation of chronic axonal polyneuropathies. J Neurol 2008;255:1127–1135 [DOI] [PubMed] [Google Scholar]
- 20.Rajabally YA, Nicolas G, Piéret F, Bouche P, Van den Bergh PY. Validity of diagnostic criteria for chronic inflammatory demyelinating polyneuropathy: a multicentre European study. J Neurol Neurosurg Psychiatry 2009;80:1364–1368 [DOI] [PubMed] [Google Scholar]
- 21.Lebrun CE, van der Schouw YT, Bak AA, et al. Arterial stiffness in postmenopausal women: determinants of pulse wave velocity. J Hypertens 2002;20:2165–2172 [DOI] [PubMed] [Google Scholar]
- 22.Muller M, Grobbee DE, den Tonkelaar I, Lamberts SW, van der Schouw YT. Endogenous sex hormones and metabolic syndrome in aging men. J Clin Endocrinol Metab 2005;90:2618–2623 [DOI] [PubMed] [Google Scholar]
- 23.van den Donk M, Bobbink IW, Gorter KJ, Salomé PL, Rutten GE. Identifying people with metabolic syndrome in primary care by screening with a mailed tape measure: a survey of 14,000 people in the Netherlands. Prev Med 2009;48:345–350 [DOI] [PubMed] [Google Scholar]
- 24.Dekker JM, Girman C, Rhodes T, et al. Metabolic syndrome and 10-year cardiovascular disease risk in the Hoorn Study. Circulation 2005;112:666–673 [DOI] [PubMed] [Google Scholar]
- 25.Blokstra A, Vissink P, Venmans LMAJ, et al. Measuring the Netherlands. A monitoring study of risk factors in the general population, 2009-2010. The National Institute for Public Health and the Environment (RIVM) report 260152001/2011:1-27. Available at: www.rivm.nl/en Accessed July 9, 2012
- 26.Teunissen LL, Franssen H, Wokke JH, et al. Is cardiovascular disease a risk factor in the development of axonal polyneuropathy? J Neurol Neurosurg Psychiatry 2002;72:590–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zarrelli MM, Amoruso L, Beghi E, Apollo F, Di Viesti P, Simone P, Italian General Practitioner Study Group Arterial hypertension as a risk factor for chronic symmetric polyneuropathy. J Epidemiol Biostat 2001;6:409–413 [DOI] [PubMed] [Google Scholar]
- 28.Cho DY, Mold JW, Roberts M. Further investigation of the negative association between hypertension and peripheral neuropathy in the elderly: an Oklahoma Physicians Resource/Research Network (OKPRN) Study. J Am Board Fam Med 2006;19:240–250 [DOI] [PubMed] [Google Scholar]
- 29.Lee CM, Huxley RR, Wildman RP, Woodward M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta-analysis. J Clin Epidemiol 2008;61:646–653 [DOI] [PubMed] [Google Scholar]
- 30.Hughes RA, Umapathi T, Gray IA, et al. A controlled investigation of the cause of chronic idiopathic axonal polyneuropathy. Brain 2004;127:1723–1730 [DOI] [PubMed] [Google Scholar]
- 31.Rajabally YA, Shah RS. Dyslipidaemia in chronic acquired distal axonal polyneuropathy. J Neurol 2011;258:1431–1436 [DOI] [PubMed] [Google Scholar]
- 32.Hoffman-Snyder C, Smith BE, Ross MA, Hernandez J, Bosch EP. Value of the oral glucose tolerance test in the evaluation of chronic idiopathic axonal polyneuropathy. Arch Neurol 2006;63:1075–1079 [DOI] [PubMed] [Google Scholar]
- 33.Novella SP, Inzucchi SE, Goldstein JM. The frequency of undiagnosed diabetes and impaired glucose tolerance in patients with idiopathic sensory neuropathy. Muscle Nerve 2001;24:1229–1231 [DOI] [PubMed] [Google Scholar]
- 34.Smith AG, Rose K, Singleton JR. Idiopathic neuropathy patients are at high risk for metabolic syndrome. J Neurol Sci 2008;273:25–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology 2003;60:108–111 [DOI] [PubMed] [Google Scholar]
- 36.Balion CM, Raina PS, Gerstein HC, et al. Reproducibility of impaired glucose tolerance (IGT) and impaired fasting glucose (IFG) classification: a systematic review. Clin Chem Lab Med 2007;45:1180–1185 [DOI] [PubMed] [Google Scholar]
- 37.Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol 2012;11:521–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dharmashankar K, Widlansky ME. Vascular endothelial function and hypertension: insights and directions. Curr Hypertens Rep 2010;12:448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jonk AM, Houben AJ, de Jongh RT, Serné EH, Schaper NC, Stehouwer CD. Microvascular dysfunction in obesity: a potential mechanism in the pathogenesis of obesity-associated insulin resistance and hypertension. Physiology (Bethesda) 2007;22:252–260 [DOI] [PubMed] [Google Scholar]


