Abstract
OBJECTIVE
We investigated the impact of two different injection strategies on the pharmacokinetics and pharmacodynamics of insulin aspart in vivo in an open-label, two-period crossover study and verified changes in the surface-to-volume ratio ex vivo.
RESEARCH DESIGN AND METHODS
Before the clinical trial, insulin aspart was injected ex vivo into explanted human abdominal skin flaps. The surface-to-volume ratio of the subcutaneous insulin depot was assessed by microfocus computed tomography that compared 1 bolus of 18 IU with 9 dispersed boluses of 2 IU. These two injection strategies were then tested in vivo, in 12 C-peptide–negative type 1 diabetic patients in a euglycemic glucose clamp (glucose target 5.5 ± 1.1 mmol/L) for 8 h after the first insulin administration.
RESULTS
The ex vivo experiment showed a 1.8-fold higher mean surface-to-volume ratio for the dispersed injection strategy. The maximum glucose infusion rates (GIR) were similar for the two strategies (10 ± 4 vs. 9 ± 4; P = 0.5); however, times to reach maximum GIR and 50% and 10% of the maximum GIR were significantly reduced by using the 9 × 2 IU strategy (68 ± 33 vs. 127 ± 93 min; P = 0.01; 38 ± 9 vs. 49 ± 16 min; P < 0.01; 23 ± 6 vs. 30 ± 10 min; P < 0.05). For 9 × 2 IU, the area under the GIR curve was greater during the first 60 min (219 ± 89 vs. 137 ± 75; P < 0.01) and halved until maximum GIR (242 ± 183 vs. 501 ± 396; P < 0.01); however, it was similar across the whole study period (1,361 ± 469 vs. 1,565 ± 527; P = 0.08).
CONCLUSIONS
A dispersed insulin injection strategy enhanced the effect of a fast-acting insulin analog. The increased surface-to-volume ratio of the subcutaneous insulin depot can facilitate insulin absorption into the vascular system.
Fast-acting insulin analogs have been developed to avoid postprandial glucose peaks (1,2). Some studies suggest that postprandial hyperglycemia can contribute to elevated levels of hemoglobin A1c (3,4) and lead to the development of short- and long-term diabetes complications (5,6). Although currently available fast-acting insulin analogs have been designed for a better match with meal-induced glucose excursions, insulin absorption and insulin action still lag behind (7,8). Even bolus administration of fast-acting insulin analogs immediately before meals does not completely avoid postprandial glucose peaks. Modern fast-acting insulin analogs still only insufficiently mimic physiological insulin profiles; however, their effect could be further improved by accelerating insulin absorption from the injection site into the vascular system.
Accelerated insulin absorption in response to an increased blood flow has been described for heated injection sites (9) or coadministered adjuvants such as hyaluronidase (10–12) but also for a larger distribution of the subcutaneous insulin depot achieved with a modified injection strategy. Human insulin absorption has been tested with a “sprinkler needle” that has 14 holes in its walls and a sealed tip, thus dispersing the insulin bolus at the injection site. With the sprinkler needle, insulin was absorbed more rapidly and glucose levels were less raised relative to a regular injection needle (13). A dispersed insulin bolus should have an increased surface-to-volume ratio and could further contribute to even faster insulin absorption of modern already fast-acting insulin analogs.
The aim of our study was to test whether the absorption rate of a fast-acting insulin analog (insulin aspart) could be further accelerated through the dispersion of a single predefined insulin bolus into nine separate insulin injections. We compared the two different injection strategies ex vivo by using microfocus computed tomography (micro-CT) to assess the increase in the surface-to-volume ratio and in vivo by assessing the pharmacokinetic and pharmacodynamic response in a clinical trial.
RESEARCH DESIGN AND METHODS
Insulin administration
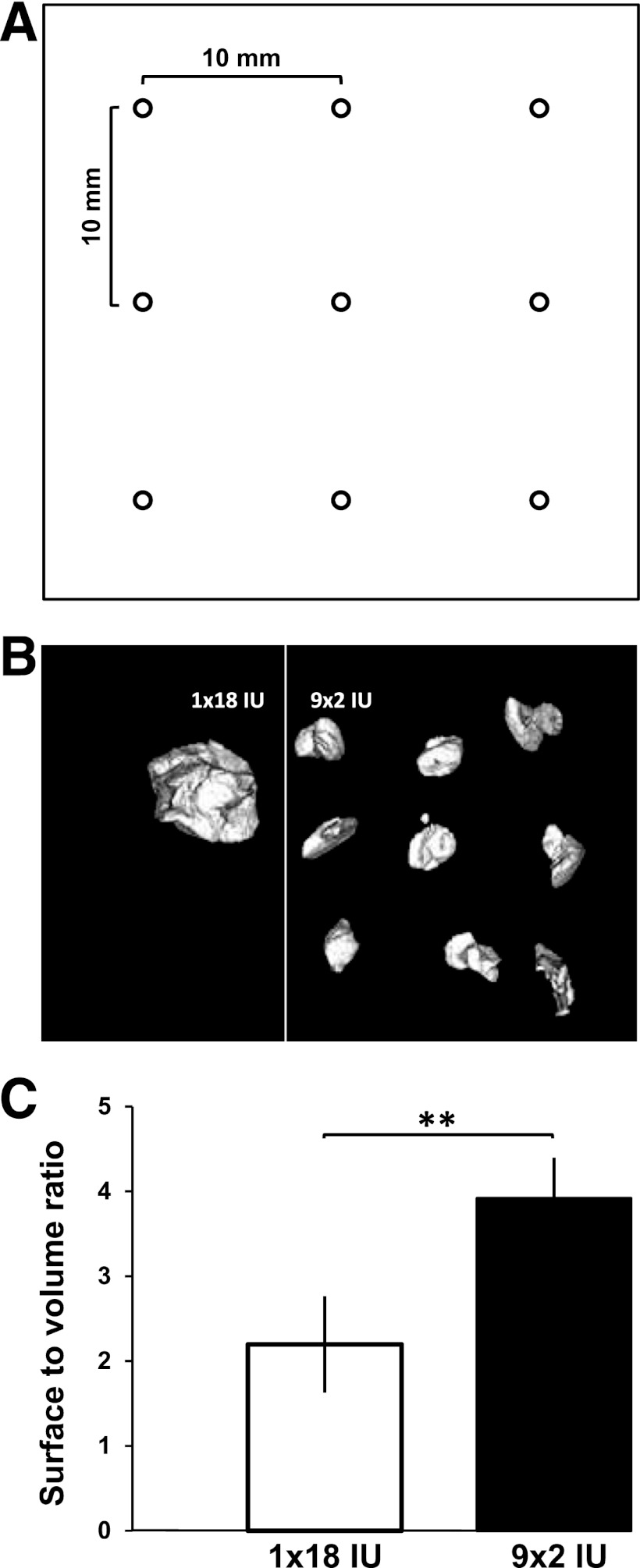
The fast-acting insulin analog aspart (NovoRapid; Novo Nordisk A/S, Baegsvard, Denmark) was administered with a FlexPen with an 8-mm pen needle (NovoFine 30G; Novo Nordisk) ex vivo and in vivo by a trained study nurse. Insulin was administered either as a single bolus of 18 IU or as nine boluses of 2 IU each in a predefined 10-mm grid pattern (Fig. 1A). To assess the dosing accuracy of each insulin pen used in the study, two insulin doses were injected into vials with both application strategies (1 × 18 IU and 9 × 2 IU), and then each dose was weighed with a microbalance (Sartorius, Göttingen, Germany). In total, 36 insulin pens were used (3 per subject to allow insulin injection within 1 min for the dispersed injection strategy). Injected insulin weights were the same for the two injection strategies (18.3 ± 0.4 µg for 1 × 18 IU vs. 18.3 ± 0.3 µg for 9 × 2 IU).
Figure 1.
A: Individual injection sites for the dispersed injection strategy (9 × 2 IU) were separated by 10 mm. B: A 3D reconstruction of the micro-CT measurements. C: Mean surface-to-volume ratios comparing two injection strategies (1 × 18 IU vs. 9 × 2 IU). **P < 0.01.
Micro-CT
The ex vivo micro-CT experiment was performed to compare the surface-to-volume ratio of the two injection strategies before their use in the in vivo clamp study. The two injection strategies were applied to explanted abdominal skin flaps; surface and volume of each resulting subcutaneous liquid depot were measured with micro-CT. To enhance the contrast between the liquid depots and the surrounding adipose tissue, 10% (by weight) of the insulin aspart solution in the FlexPen was replaced with a contrast agent (Iopamiro, iodine 200 mg/mL; Bracco s.p.a., Milan, Italy). The contrast agent was selected for its high iodine content and low viscosity, providing good contrast enhancement at minimal viscosity changes of the insulin solution. Twelve abdominal skin flaps with a minimal size of 50 × 50 mm and at least 20 mm of subcutaneous adipose tissue were collected from BioBank (Medical University of Graz, Austria). All skin samples had been explanted during pendulous abdomen resections after massive weight loss. The time span between explantation and injection of the insulin/contrast agent solution never exceeded 5 h. During this time, skin flaps were kept at room temperature.
Immediately after injection of the insulin and contrast agent solution, skin flaps were scanned with a micro-CT scanner (Inveon Multimodality System, Siemens, Germany) with Siemens Inveon Acquisition Workplace software (version 1.2.2.2). To optimize contrast, acquisition time, and resolution, the scanning sequence was set to a voltage of 50 kV, a current of 300 µA, and an exposure time of 500 ms. A rotation of 210° in 180 rotation steps led to a resolution with an effective pixel size of 53 µm. For three-dimensional (3D) image reconstruction, a downsample factor of 1 was used. The system supports a 3D reconstruction of an acquired data set of two-dimensional projections by using back projection of a filtered projection algorithm. Analysis of the 3D model was performed with Siemens Inveon Research Workplace. The volume of interest was selected by a region-growing algorithm, with a spot of high intensity selected manually as a starting point. Regions with decreasing levels of intensity were added to the volume of interest until the predefined injection volume (180 ± 1.8 µL) was reached. The surface of the selected volume of interest was calculated for both injection strategies (1 × 18 IU and 9 × 2 IU) (Fig. 1B).
Clinical euglycemic clamp study
A monocentric, randomized, controlled, two-period crossover euglycemic clamp study was performed in 12 type 1 diabetic patients at the Clinical Research Centre at the Medical University of Graz between April and June 2011. The study was approved by the local ethics committee and performed in accordance with the Declaration of Helsinki and the principles of Good Clinical Practice. Participants gave written, informed consent after the purpose, nature, and potential risks of the study had been explained and before any study-related activities were started. Participants had to fulfill all inclusion criteria (fasting C-peptide <0.3 nmol/L; BMI 20.0–28.0 kg/m2; hemoglobin A1c <10%) and none of the exclusion criteria (i.e., insulin aspart use, lipodystrophy, smoking). The study consisted of four visits: one screening visit, two clamp visits separated by a washout phase of 5–21 days, and one follow-up visit. To avoid any residual action of their regular insulin regimens, participants were instructed to follow a specific regimen: participants on multiple daily injections administered their last dose of short-acting insulin at least 5 h before the clamp visit. Participants on long-acting insulin analogs were transferred to NPH insulin 2 days before the clamp visit and received the last injection of NPH insulin 20 h before the clamp visit. Participants on continuous subcutaneous insulin infusion stopped their insulin infusion at least 5 h before the clamp visit. Participants abstained from strenuous exercise 24 h before the clamp visits. Participants attended the research facility under fasting conditions and remained fasting throughout each clamp visit. One 18-gauge venous catheter was inserted in each arm: one was used for insulin and glucose infusion; the other was used for blood sampling. The arm used for blood sampling was put under a heating blanket at 55°C to obtain arterialized venous blood. The euglycemic clamp procedure was started by using a variable intravenous human insulin infusion (Actrapid; Novo Nordisk) or glucose infusion to obtain a stable plasma glucose (target 5.5 ± 1.1 mmol/L) during the run-in period (3–7 h). During the last hour of the run-in period, the insulin infusion was tapered and finally switched off. At time 0 (between 11 a.m. and 2 p.m.), a dose of 18 IU insulin aspart was injected into the subcutaneous adipose tissue of the abdominal wall. The insulin dose was administered either as 1 × 18 IU or as 9 × 2 IU. On arrival, the participant number was entered into the computer program Randomizer, which assigned the participant randomly to one of the injection strategies. The other injection strategy was then administered on the second clamp visit. The dosing sequence was also randomly assigned by using Randomizer according to parameters provided by the Institute for Medical Informatics, Statistics and Documentation (Medical University of Graz). For the 9 × 2 IU injection strategy, where administration took <1 min, a transparent grid was used that separated the individual injection sites from each other by exactly 10 mm (Fig. 1A). The euglycemic glucose clamp was continued at a level of 5.5 ± 1.1 mmol/L for 8 h after the insulin bolus administration or until plasma glucose rose above 200 mg/dL. Euglycemia was maintained by variable glucose infusion (glucose 10%; Fresenius Kabi, Bad Homburg, Germany), which was administered with a perfusion pump (B. Braun, Melsungen, Germany). Arterialized venous blood samples were drawn in 5- to 10-min intervals throughout the euglycemic clamp. Plasma glucose was measured in duplicate on site with a Super GL 2 Glucose Analyzer (Müller Gerätebau, Freital, Germany). Plasma samples for insulin determination were taken at baseline (0 min), every 5 min up to 120 min, every 15 min from 120 to 180 min, every 30 min from 180 to 240 min, and then at 60-min intervals until study end (480 min). Plasma samples for insulin determination were deep frozen on site, and insulin measurements were subsequently performed at the laboratories of Novo Nordisk A/S (Maaloev, Denmark) by means of a homogenous immunoassay with analog-specific antibody for aspart determination.
Data analysis
Primary pharmacokinetic and secondary pharmacodynamic study end points were derived from insulin concentration profile and exogenous glucose infusion rate (GIR). Pharmacokinetic data included time to maximum insulin concentration, times to 10% and 50% of maximum insulin concentration, and maximum insulin concentration. Pharmacodynamic data included time to maximum GIR, times to 10% and 50% of maximum GIR, and maximum GIR. Area under the curve (AUC) was calculated for insulin concentration and GIR up to 480 min after insulin administration and for the time to reach maximum insulin concentration and maximum GIR.
All data were tested for normal distribution with a Shapiro-Wilk test. The paired measurements were analyzed with paired t tests or Wilcoxon signed rank tests, depending on whether the paired differences were normally distributed. The unpaired measurements from the micro-CT experiment were analyzed with Mann-Whitney U tests. AUCs were estimated with the trapezoidal rule for defined time points. P < 0.05 was considered to indicate a significant difference. Bonferroni corrections were used to correct for multiple testing of the pharmacokinetic and pharmacodynamic results. Unless otherwise specified, data are reported as mean ± SD. All statistical analyses were performed with the software package R (v.2.10.1).
RESULTS
Ex vivo
To directly compare the calculated surface-to-volume ratios of the subcutaneous insulin depots with each other, the two injection strategies (1 × 18 IU and 9 × 2 IU) were applied with the same total volume. Although the measured mean volume of the dispersed injection strategy was thus similar to that of the single injection strategy (179.6 ± 0.96 mm3 vs. 180.6 ± 0.70 mm3), the mean surface of the dispersed injection strategy was significantly larger than the single injection surface (703.6 ± 83.8 mm2 vs. 396.6 ± 101.1 mm2; P < 0.01). The dispersed injection strategy thus enhanced the surface-to-volume ratio by a factor of 1.8 (3.9 ± 0.48 vs. 2.2 ± 0.57; P < 0.01), which is in line with the expected increase in surface calculated from geometric analysis according to the assumption of spherical liquid depots (Fig. 1C).
Clinical euglycemic clamp study
We included 12 C-peptide–negative type 1 diabetic patients (age 32 ± 9 years; 6 females; BMI 23.9 ± 2.5 kg/m2; hemoglobin A1c 7.3 ± 0.6%; diabetes duration 19 ± 10 years). All participants completed both clamp visits, no clinically relevant adverse events were observed, and all collected data were used for subsequent statistical analysis.
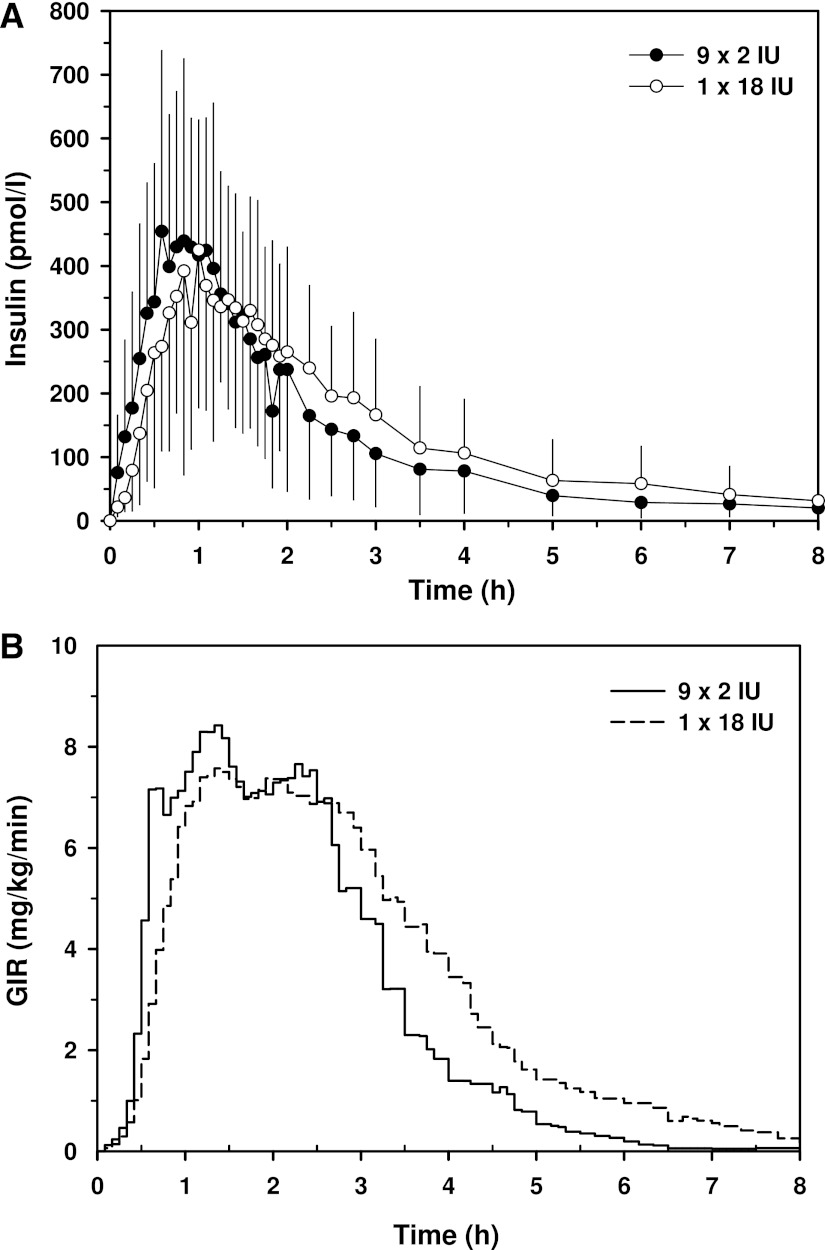
Maximum insulin concentrations were similar for both injection strategies (Table 1). Time to reach maximum insulin concentration was shorter for 9 × 2 IU, although the differences were only significant for the time to reach 10% (P < 0.03) and 50% (P < 0.001) of the maximum insulin concentrations (Fig. 2A).
Table 1.
Pharmacokinetic and pharmacodynamic parameters for the two injection strategies
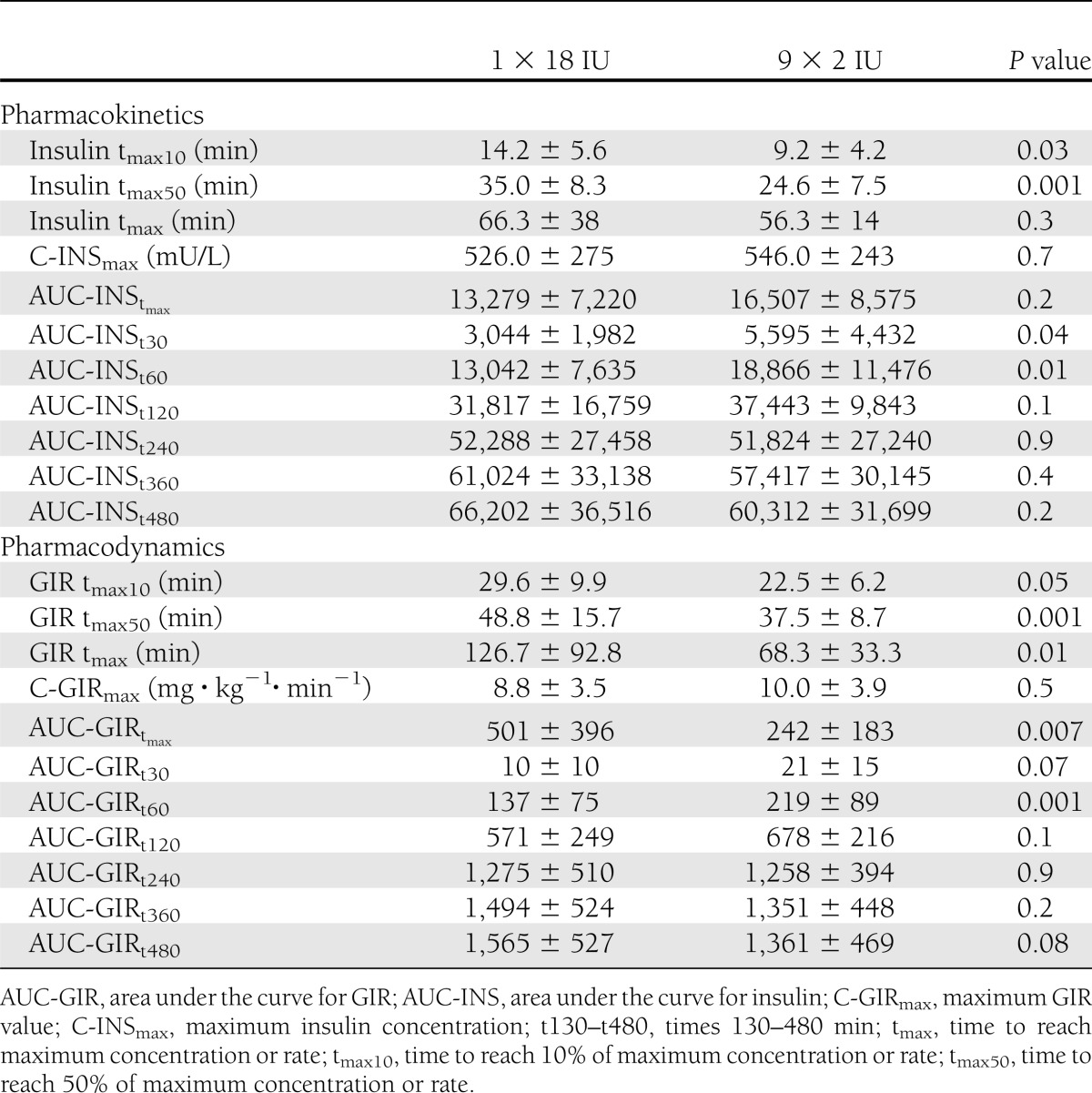
Figure 2.
A: Mean insulin curve for 9 × 2 IU (●) and 1 × 18 IU (○) injections of insulin aspart. Values represent mean ± SD (only one direction shown for clarity). B: Mean glucose infusion rate curve for 9 × 2 IU (solid line) and 1 × 18 IU (dashed line) injections of insulin aspart.
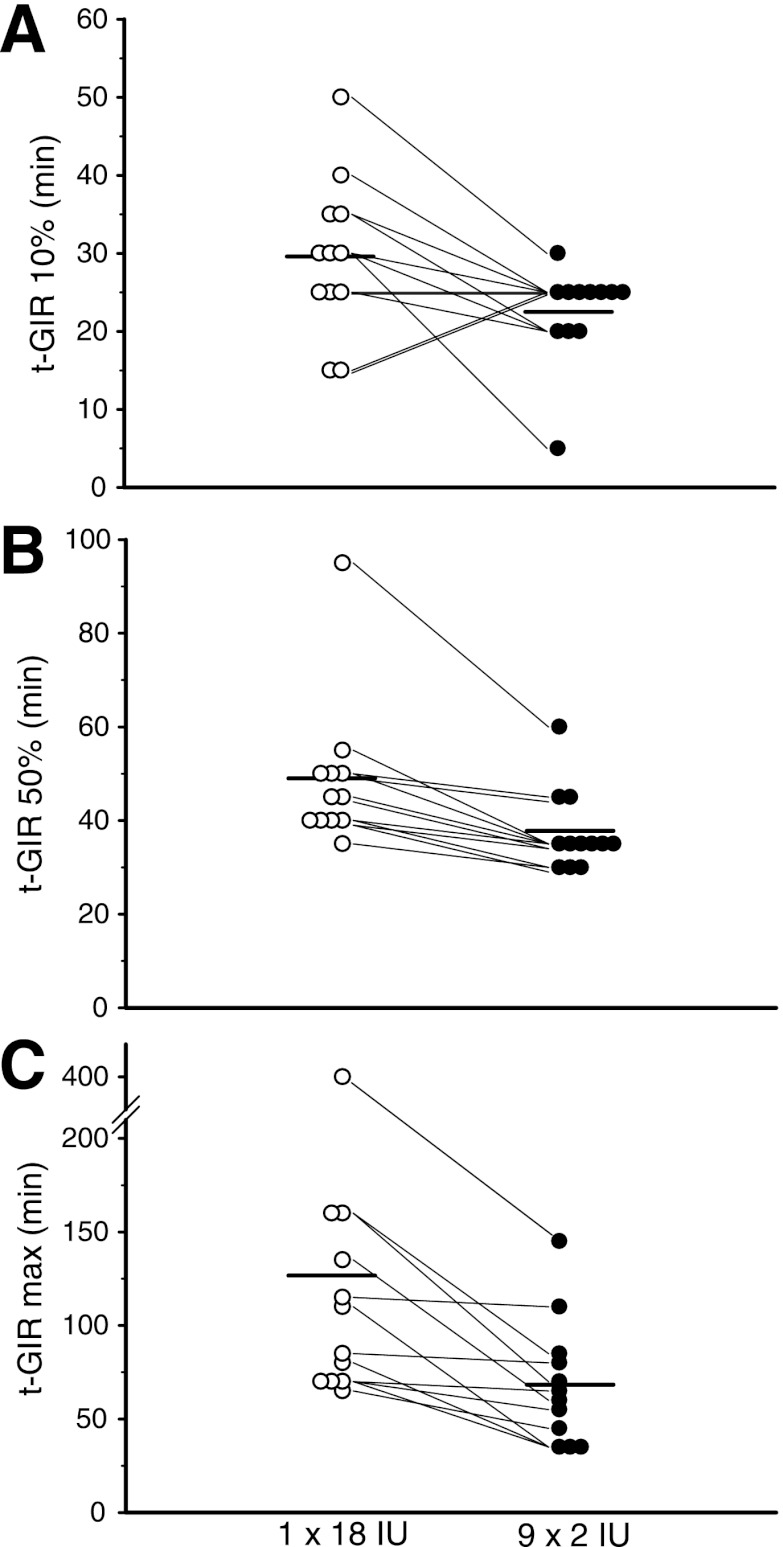
The time to reach maximum GIR was almost 50% shorter for 9 × 2 IU than for 1 × 18 IU (Table 1). The times taken to reach 10% and 50% of maximum GIR were still significantly lower for the dispersed injection strategy (Fig. 2B), whereas the concentrations at maximum insulin action were similar for both injection strategies. Individual GIR data for times to reach 10% of maximum GIR, 50% of maximum GIR, and maximum GIR are indicated in Fig. 3A–C. The AUC for glucose was significantly larger for 9 × 2 IU during the first 60 min, whereas the AUC until time of maximum GIR was smaller for 9 × 2 IU (Table 1). After 120 min, all AUCs for GIR were similar for both injection strategies.
Figure 3.
Individual GIR profile data showing time to 10% of maximum GIR (A), time to 50% of maximum GIR (B), time to maximum GIR (C). Horizontal bars indicate means for the 1 × 18 IU and 9 × 2 IU injection strategies.
CONCLUSIONS
Although fast-acting insulin analogs are designed for a more rapid absorption into the vascular system, it is still difficult to achieve a physiological insulin profile. To accelerate insulin absorption of the fast-acting insulin aspart, we tested whether a dispersed injection strategy would affect insulin absorption in comparison with a regular single-bolus insulin injection by using ex vivo micro-CT in explanted abdominal skin flaps and by assessing pharmacokinetic and pharmacodynamic parameters in vivo in a euglycemic clamp study in type 1 diabetic patients.
After injection, insulin aspart solutions form liquid depots in the tissue. Liquid depots with a higher surface-to-volume ratio can be absorbed faster, because a larger depot surface involves more capillaries and enhances absorption into the vascular system. Enhanced drug absorption with increased surface-to-volume ratio has been demonstrated in a canine model (14), and in humans an increased volume slowed the speed of pharmacokinetics in large versus small skin blisters (15). By using micro-CT, a nondestructive 3D imaging technique, we were able to compare quantitatively the surface-to-volume ratio of subcutaneous insulin depots of a dispersed injection strategy (9 × 2 IU) with that of a single insulin injection (1 × 18 IU). For the dispersed injection strategy, we found a 1.8-fold higher surface-to-volume ratio, which is in line with theoretical calculations from spherical liquid depots. A recently developed technology for morphometric tissue analysis, micro-CT is mainly used in bone and dental structure analysis (16–18). In combination with a CT contrast agent, micro-CT provided excellent resolution, fast analysis, and exact semiautomated volume and surface calculations for each individual insulin depot. Because micro-CT measurements require no tissue fixation and only minimal tissue manipulation, we were able to assess unaltered samples that closely matched in vivo conditions immediately after insulin injection.
Insulin pharmacokinetic and pharmacodynamic data collected during the clinical trial in type 1 diabetic patients supported the micro-CT findings. The lag time between insulin injection and insulin appearance in blood plasma was significantly reduced when the fast-acting insulin aspart was administered with a dispersed injection strategy. Other approaches to accelerate insulin absorption, such as the use of hyaluronidase to facilitate the dissociation of insulin hexamers into more easily absorbed monomers and dimers, have also been associated with faster insulin absorption and an earlier onset of insulin action (12,19). Adding hyaluronidase to a fast-acting insulin analog reduced time to reach maximum insulin concentration by 51% (19) and resulted in an even more pronounced effect in comparison with our dispersed injection strategy (15%). The decrease in the time to reach 50% of maximum insulin concentrations with the dispersed injection strategy (29%) was comparable with the hastening of the onset of insulin action by adding hyaluronidase (34%). Application of local heating to the injection site, which locally increases the blood flow, has also been demonstrated to result in an accelerated insulin absorption and more rapid onset of insulin action (9). Heating the injection site reduced time to maximum insulin concentration by 42% and time to 50% of maximum insulin concentration by 29%. The very similar twice as fast onset of insulin action of a dispersed insulin bolus is most likely caused by an increase in the number of capillaries involved in the absorption of insulin into the vascular system, comparable with the effect of intradermal injections with microneedles that access a more dense capillary network (20). In a study that compared insulin injection of a fast-acting insulin analog using a jet injector, time to maximum insulin concentration and insulin action were halved in comparison to an insulin pen (21). This was attributed to a cone-like dispersion pattern with a large surface area caused by the jet injector, as described earlier by Mitragotri et al. (22).
Use of the dispersed injection strategy not only reduced the lag time but also improved all other pharmacokinetic parameters during the first 60 min after insulin injection. Additionally, duration of insulin action was shorter for the dispersed injection strategy. The shorter duration of insulin action can contribute to reduced late postprandial hypoglycemia, which occurs when the carbohydrate content of a meal is already consumed but insulin action is still present. Although pharmacokinetic data are important for the development of new insulin analogs, pharmacodynamic results from glucose infusion rates provide relevant data for more efficient glycemic control. In our in vivo clamp study in type 1 diabetic patients, the time to reach maximum GIR was almost halved when the insulin bolus was evenly dispersed among nine injection sites compared with a single injection site, indicating a much faster onset of insulin action. In combination with fast-acting insulin analogs, the effect of a dispersed injection strategy can lead to a clinically relevant improvement of glycemic control. Although we have shown that multiple injection sites significantly accelerate the absorption of a fast-acting insulin analog, the simultaneous injection of multiple small boluses is not feasible for routine diabetes care. The use of a sprinkler needle concept is one way to translate our findings into a technology acceptable for patients. Enhanced absorption of human insulin with flatter blood glucose profiles and less pronounced blood glucose peaks has been found with the sprinkler needle relative to a regular injection needle (13).
Although the lag time between insulin injection and insulin appearance is already reduced in fast-acting insulin analogs, an earlier onset of insulin action would enable diabetic patients to inject insulin regularly after meals without having to take into account the delayed action of currently available insulins. This would be of considerable benefit for glycemic control in pediatric diabetic patients, in whom the consumed carbohydrates and the resulting blood glucose are difficult to predict because of highly variable eating habits and activity levels (23) and in geriatric patients with varying levels of appetite depending on their health status. These patient populations always bear an increased risk of postprandial hypoglycemic events when applying premeal insulin boluses without complete consumption of the planned meal.
Maintenance of good glucose control in diabetic pregnancy is also essential to avoid adverse fetal outcomes, such as fetal growth acceleration (24,25). Glucose uptake, endogenous glucose production, and insulin requirements strongly vary throughout pregnancy. Especially in late gestation of type 1 diabetic pregnancies, postprandial glucose control is difficult to manage because of slower glucose disposal (26). Postprandial insulin dosing could contribute to a better glycemic control in this patient group.
Faster insulin absorption can also promote the development of closed-loop artificial pancreas systems that use subcutaneous insulin administration and continuous glucose measurement. The substantial lag time between insulin administration and the onset of insulin action is a major obstacle for the development of algorithms that control closed-loop systems (27). A shortened absorption time of fast-acting insulin analogs can lead to faster control algorithms, and a clinically feasible dispersed injection strategy would be a major step toward the successful implementation of fully closed-loop systems, which do not require a meal announcement followed by a priming bolus.
In summary, this study confirmed acceleration of insulin absorption of a fast-acting insulin analog by using a dispersed insulin bolus. A clinically feasible dispersed injection strategy might better mimic physiological insulin profiles and contribute to improvements in glycemic control. To evaluate the implications for clinical outcomes, such as a reduction of late postprandial hypoglycemia, both reduction of glucose fluctuations and improvement in glycemic control need to be addressed in further clinical trials.
Acknowledgments
This study was partly funded by AP@home (FP7-ICT-2009-4 247138). T.R.P. is a member of the advisory board of Novo Nordisk A/S and has received speaker honoraria from Novo Nordisk A/S. J.K.M. and G.K. received speaker honoraria from Novo Nordisk A/S. S.B. is an employee of Novo Nordisk A/S. No other potential conflicts of interest relevant to this article were reported.
J.K.M. designed and performed the clinical study, interpreted data, and drafted the manuscript. T.B. designed and performed the ex vivo experiment, interpreted data, and drafted the manuscript. S.K., S.D., and G.K. helped to design the study, performed the study, interpreted the data, and critically reviewed the manuscript. S.B. performed the analytical insulin analysis, interpreted the data, and reviewed the manuscript. T.A. designed the study and performed statistical analysis. S.I.M. interpreted data and drafted the manuscript. F.S. contributed to the study design and discussions and critically revised the manuscript. T.R.P. designed the study, supervised the project, contributed to discussions, and critically revised the manuscript. All authors and the AP@home Consortium approved the final version of the manuscript. T.R.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
The authors thank Helmut Schöllnast (Medical University of Graz) for providing information on micro-CT contrast agents; Martina Brunner (Medical University of Graz) for the help with the sample preparation; Martina Urschitz, Michael Wolf, Janka Gerdova, and Harold Kojzar (all, Medical University of Graz) for help with the clamp studies; Bernd Tschapeller (Joanneum Research) for data management; and Novo Nordisk A/S (Maaloev, Denmark) for the insulin measurements performed free of charge.
Footnotes
Clinical trial reg. no. NCT01399346, clinicaltrials.gov.
References
- 1.Lindholm A, McEwen J, Riis AP. Improved postprandial glycemic control with insulin aspart. A randomized double-blind cross-over trial in type 1 diabetes. Diabetes Care 1999;22:801–805 [DOI] [PubMed] [Google Scholar]
- 2.Pettitt DJ, Ospina P, Howard C, Zisser H, Jovanovic L. Efficacy, safety and lack of immunogenicity of insulin aspart compared with regular human insulin for women with gestational diabetes mellitus. Diabet Med 2007;24:1129–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association Standards of medical care in diabetes—2012. Diabetes Care 2012;35(Suppl. 1):S11–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA1c. Diabetes Care 2003;26:881–885 [DOI] [PubMed] [Google Scholar]
- 5.Qiao Q, Tuomilehto J, Borch-Johnsen K. Post-challenge hyperglycaemia is associated with premature death and macrovascular complications. Diabetologia 2003;46(Suppl. 1):M17–M21 [DOI] [PubMed] [Google Scholar]
- 6.Clement S. What are the best options for controlling prandial glycemia? Curr Diab Rep 2009;9:355–359 [DOI] [PubMed] [Google Scholar]
- 7.Plank J, Wutte A, Brunner G, et al. A direct comparison of insulin aspart and insulin lispro in patients with type 1 diabetes. Diabetes Care 2002;25:2053–2057 [DOI] [PubMed] [Google Scholar]
- 8.Hovorka R, Shojaee-Moradie F, Carroll PV, et al. Partitioning glucose distribution/transport, disposal, and endogenous production during IVGTT. Am J Physiol Endocrinol Metab 2002;282:E992–E1007 [DOI] [PubMed] [Google Scholar]
- 9.Raz I, Weiss R, Yegorchikov Y, Bitton G, Nagar R, Pesach B. Effect of a local heating device on insulin and glucose pharmacokinetic profiles in an open-label, randomized, two-period, one-way crossover study in patients with type 1 diabetes using continuous subcutaneous insulin infusion. Clin Ther 2009;31:980–987 [DOI] [PubMed] [Google Scholar]
- 10.Muchmore DB, Vaughn DE. Review of the mechanism of action and clinical efficacy of recombinant human hyaluronidase coadministration with current prandial insulin formulations. J Diabetes Sci Tech 2010;4:419–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hompesch M, Muchmore DB, Morrow L, Vaughn DE. Accelerated insulin pharmacokinetics and improved postprandial glycemic control in patients with type 1 diabetes after coadministration of prandial insulins with hyaluronidase. Diabetes Care 2011;34:666–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaughn DE, Muchmore DB. Use of recombinant human hyaluronidase to accelerate rapid insulin analogue absorption: experience with subcutaneous injection and continuous infusion. Endocr Pract 2011;17:914–921 [DOI] [PubMed] [Google Scholar]
- 13.Edsberg B, Herly D, Hildebrandt P, Kühl C. Insulin bolus given by sprinkler needle: effect on absorption and glycaemic response to a meal. Br Med J (Clin Res Ed) 1987;294:1373–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosin E, Ebert S, Uphoff TS, Evans MH, Schultz-Darken NJ. Penetration of antibiotics into the surgical wound in a canine model. Antimicrob Agents Chemother 1989;33:700–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blaser J, Rieder HL, Lüthy R. Interface-area-to-volume ratio of interstitial fluid in humans determined by pharmacokinetic analysis of netilmicin in small and large skin blisters. Antimicrob Agents Chemother 1991;35:837–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones AC, Milthorpe B, Averdunk H, et al. Analysis of 3D bone ingrowth into polymer scaffolds via micro-computed tomography imaging. Biomaterials 2004;25:4947–4954 [DOI] [PubMed] [Google Scholar]
- 17.Swain MV, Xue J. State of the art of Micro-CT applications in dental research. Int J Oral Sci 2009;1:177–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraus T, Fischerauer SF, Hänzi AC, Uggowitzer PJ, Löffler JF, Weinberg AM. Magnesium alloys for temporary implants in osteosynthesis: in vivo studies of their degradation and interaction with bone. Acta Biomater 2012;8:1230–1238 [DOI] [PubMed] [Google Scholar]
- 19.Vaughn DE, Yocum RC, Muchmore DB, et al. Accelerated pharmacokinetics and glucodynamics of prandial insulins injected with recombinant human hyaluronidase. Diabetes Technol Ther 2009;11:345–352 [DOI] [PubMed] [Google Scholar]
- 20.Pettis RJ, Ginsberg B, Hirsch L, et al. Intradermal microneedle delivery of insulin lispro achieves faster insulin absorption and insulin action than subcutaneous injection. Diabetes Technol Ther 2011;13:435–442 [DOI] [PubMed] [Google Scholar]
- 21.Engwerda EE, Abbink EJ, Tack CJ, de Galan BE. Improved pharmacokinetic and pharmacodynamic profile of rapid-acting insulin using needle-free jet injection technology. Diabetes Care 2011;34:1804–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitragotri S. Current status and future prospects of needle-free liquid jet injectors. Nat Rev Drug Discov 2006;5:543–548 [DOI] [PubMed] [Google Scholar]
- 23.Steck AK, Klingensmith GJ, Fiallo-Scharer R. Recent advances in insulin treatment of children. Pediatr Diabetes 2007;8(Suppl. 6):49–56 [DOI] [PubMed] [Google Scholar]
- 24.Herranz L, Saez-de-Ibarra L, Grande C, Pallardo LF. Non-glycemic-dependent reduction of late pregnancy A1C levels in women with type 1 diabetes. Diabetes Care 2007;30:1579–1580 [DOI] [PubMed] [Google Scholar]
- 25.Kerssen A, de Valk HW, Visser GHA. Increased second trimester maternal glucose levels are related to extremely large-for-gestational-age infants in women with type 1 diabetes. Diabetes Care 2007;30:1069–1074 [DOI] [PubMed] [Google Scholar]
- 26.Murphy HR, Elleri D, Allen JM, et al. Pathophysiology of postprandial hyperglycaemia in women with type 1 diabetes during pregnancy. Diabetologia 2012;55:282–293 [DOI] [PubMed] [Google Scholar]
- 27.Elleri D, Dunger DB, Hovorka R. Closed-loop insulin delivery for treatment of type 1 diabetes. BMC Med 2011;9:120. [DOI] [PMC free article] [PubMed] [Google Scholar]