Abstract
OBJECTIVE
Patients with type 2 diabetes mellitus (T2DM) are at increased risk of developing cardiovascular disease, largely as a result of defective production of cardioprotective nitric oxide and a concomitant rise in oxidative stress. Dietary interventions that could reverse this trend would be extremely beneficial. Here we investigated whether dietary n-3 polyunsaturated fatty acid (n-3 PUFA) supplementation positively affected platelet nitroso-redox imbalance.
RESEARCH DESIGN AND METHODS
We randomized hypertensive T2DM patients (T2DM HT; n = 22) and age-and-sex matched hypertensive study participants without diabetes (HT alone; n = 23) in a double-blind, crossover fashion to receive 8 weeks of n-3 PUFAs (1.8 g eicosapentaenoic acid and 1.5 g docosahexaenoic acid) or identical olive oil capsules (placebo), with an intervening 8-week washout period. Platelet nitrite and superoxide were measured and compared before and after treatment; 8-isoprostane was determined by ELISA and subcellular compartmentalization of the NAD(P)H oxidase subunit p47-phox examined by Western blotting.
RESULTS
The n-3 PUFA supplementation reduced 8-isoprostane and superoxide levels in platelets from T2DM HT, but not HT alone, participants, without effect on nitrite production. This coincided with a significant decrease in p47-phox membrane localization and a similar reduction in superoxide to that achieved with apocynin. At baseline, a subcohort of T2DM HT and HT alone participants showed evidence of nitric oxide synthase (NOS)–derived superoxide production, indicating defective enzymatic activity. This was reversed significantly in T2DM HT participants after treatment, demonstrating improved NOS function.
CONCLUSIONS
Our finding that n-3 PUFAs diminish platelet superoxide production in T2DM HT patients in vivo suggests a therapeutic role for these agents in reducing the vascular-derived oxidative stress associated with diabetes.
Dietary interventions that could reduce the risk of development of cardiovascular disease (CVD) in patients with type 2 diabetes mellitus (T2DM) would clearly be advantageous instead of expensive pharmacological treatments. There is now considerable evidence demonstrating an association between enhanced oxidative stress and the incidence of CVD. In healthy vasculature, oxidative stress is kept in check by nitric oxide (NO) derived from endothelial NO synthase (NOS). Endothelial- and platelet-derived NO plays a pivotal role in maintaining normal vascular homeostasis and has atheroprotective effects, inhibiting smooth muscle cell proliferation, leukocyte adhesion, and aggregation of platelets (1–3). Diabetic vascular disease, in contrast, is characterized by altered NO bioavailability resulting from an increase in the amount of the NO scavenger superoxide (O2−), derived predominantly from NAD(P)H oxidase or from uncoupled endothelial NOS (1,4). Together, these alterations lead to endothelial dysfunction, enhanced platelet activity, and an increased risk of thrombosis (5–7). Despite a large body of evidence linking dysregulated NO production and enhanced oxidative stress to vascular disease, however, clinical studies with well-characterized antioxidant vitamins, such as vitamins C and E, have proved disappointing (8). The inability of antioxidants to be delivered and retained in the vascular bed may explain these results. Moreover, with increasing evidence of the importance of free radicals in normal physiological function, a new paradigm is emerging that favors controlled free radical production. In this model, a therapeutically beneficial rebalancing of NO relative to O2− production is sought, rather than the complete suppression of free radical production, such as occurs after antioxidant administration (9,10). In this regard, we have recently shown that n-3 polyunsaturated fatty acids (n-3 PUFAs), the major constituents of oily fish, can modulate the nitroso-redox balance by increasing vasculoprotective NO and decreasing O2− production in endothelial cells (11). This finding may be extremely valuable for diabetic patients whose disease manifests as a high oxidative status. Furthermore, fish oil–derived n-3 PUFAs may also offer several other advantages. Importantly, they are easily absorbed and retained within cellular membranes, where they can act as a readily available supply of free radical–modifying agents. This bioavailability, coupled with their ability to specifically modulate the function of enzymes producing reactive oxygen species (ROS) and reactive nitrogen species, such as NAD(P)H and NOS, suggests that, far from simply being free radical scavengers like other antioxidants, n-3 PUFAs may be therapeutically superior.
The health benefits of high oily fish consumption were first recognized in the Greenland Inuit, in whom n-3 PUFA consumption was correlated with a lower CVD mortality rate. Since that discovery, a substantial amount of evidence has accumulated to show that the high content of n-3 PUFAs, particularly docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), are responsible for these health benefits. The n-3 PUFAs are believed to exert their therapeutic benefits through a variety of mechanisms, including a reduction in antithrombotic and antiarrhythmic actions and improved endothelial function (12–14). Few studies, however, have investigated the potential benefits of n-3 PUFA treatment for diabetic patients (15–17).
Platelets contain all the components of a functioning NO-producing pathway, including the enzyme necessary for the production of the cofactor tetrahydrobiopterin (18,19), and are therefore capable of producing NO in response to platelet-specific agonists (20,21). In addition, they also contain all the subunits required for the assembly of the NAD(P)H oxidase system (7,22). Platelets thus represent an accessible cellular model for functional studies to explore free radical activity in relation to changes in NAD(P)H oxidase and NOS activity (7,20,22). Accordingly, in the current study we examined platelets to investigate the effects of n-3 PUFAs on NO and O2− bioactivities and on oxidative stress in a diabetic population.
RESEARCH DESIGN AND METHODS
Materials and antibodies
We obtained all reagents from Sigma (St. Louis, MO) unless stated otherwise.
Study design
We recruited T2DM patients with hypertension (T2DM HT group; n = 22; duration of diabetes 6 ± 1 years) and age- and sex-matched control study participants with hypertension (HT alone group; n = 23). A cohort of healthy individuals without treatment (control), collected as an adjunct to this study, was also used to provide baseline reference values and is included for illustrative purposes. We identified diabetic study participants eligible for the trial from clinical registers. Previous T2DM diagnosis was defined as a fasting plasma glucose level of >7.0 mmol/L or plasma venous sample level of >11.1 mmol/L 2 h after consuming a 75-g oral glucose load (23). Patients were eligible for the study if their records provided evidence of stable control of diabetes, defined as HbA1c levels of 6.5–10%. For the purposes of the study, we used fasting glucose levels throughout as a surrogate marker for diabetes status. We excluded participants if they had any history of overt CVD or established microcomplications and macrocomplications of T2DM or hypertension, such as retinopathy or nephropathy, as well as excluding those receiving insulin therapy. All study participants adhered to their regular diets and medications throughout and gave written, informed consent for all procedures. This study was approved by the local ethical committee of Queen's University Belfast. Data from previous intervention studies revealed an SD of 0.39 pmol/108 platelets (after log transformation) for measurements of NO production. Thus to detect a 40% differential change in NO at a 5% significance level with a two-tailed hypothesis test, our study required 20 volunteers per group to achieve 90% power.
This was a randomized, double-blind, crossover study of 8 weeks of n-3 PUFA or placebo, with an intervening 8-week washout period. In brief, after randomization, study participants received daily for 8 weeks either four fish oil capsules (Omacor) containing 0.46 g EPA and 0.38 g DHA (Solvay Pharma) or four identical olive oil–containing capsules. We chose olive oil as the placebo to provide a calorie intake similar to that of the fish oil capsule, as described previously (17,24). After an 8-week washout period in which study participants did not receive any treatment, participants were then allocated the oil supplement they had not previously received. We performed studies before treatment (baseline 1), after 8 weeks of treatment, after the 8-week washout (baseline 2), and after a further 8 weeks of treatment. This was in line with previous studies confirming that an 8-week treatment phase permits sufficient time for the incorporation of the fatty acids into cellular membranes (17).
Platelet isolation
Study participants attended all trial appointments after having fasted overnight, and they refrained from taking their prescribed medications until after the assessment. After a 30-min rest period, we withdrew 120 mL of venous blood from the left antecubital fossa with a 19-gauge butterfly needle. Three aliquots were processed for routine biochemical assays. The remainder of the blood in 9.0-mL aliquots was run into each of 10 polypropylene tubes containing 1.0 mL 3.18% sodium citrate and gently mixed. Care was taken with this collection process to avoid activation of the platelets. The platelet-rich plasma was separated from erythrocytes by centrifugation (200g for 30 min at room temperature), and platelets were concentrated by centrifugation at 850g for 30 min. The resulting pellets were washed twice with Tyrode buffer. Two pellets were flash frozen in liquid nitrogen and stored at −80°C for subsequent platelet 8-isoprostane and membrane fatty acid analysis. A third pellet was separated into cytosol and membrane fractions for subsequent p47 content assessment and stored at −80°C. Finally, we resuspended the remaining two pellets in fresh Tyrode buffer in preparation for O2− or nitrite measurement. Estimation of platelet purity verified that the number of contaminating polymorphic nuclear cells was consistently below 0.05/108 platelets. Samples containing >1 erythrocyte/1000 platelets were discarded.
Fatty acid composition of platelet membranes
Fatty acid analysis was determined by gas chromatography as described previously (17,24).
Assay of total 8-isoprostane in platelets
Total 8-isoprostane (free and esterified) was extracted and purified before quantification with a commercially available competitive enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI). All values were expressed per milligram of cellular protein.
Measurement of O2− production
Released O2− was measured in freshly isolated platelets by enhanced chemiluminescence detection with lucigenin as a luminescent substrate. Our group has previously validated this assay, described in detail elsewhere, to show that luminescence reflects O2− production as indicated by inhibition with superoxide dismutase and diphenyliodonium (21,25). Briefly, lucigenin was added to an aliquot of platelet suspension, and O2− production was measured before and after the addition of the stimulant phorbal 12-myristate 13-acetate (PMA; 10 µmol/L). We considered a stable baseline as indicative of the fact that that sample preparation had no effect on ROS production. Assays were carried out in the presence or absence of l-NG-nitro-l-arginine methyl ester (l-NAME; 45 µmol/L) or apocynin (4-hydroxy-3-methoxyacetophenone 30 μmol/L; Fluka Chemie, Neu-Ulm, Germany).
Nitrite assay
Freshly isolated platelets were stimulated with ADP (5 μmol/L) in the presence of Ca2+/Mg2+ (2 mmol/L), and fibrinogen (300 μg/μL) for 15 min at 37°C. The reaction was stopped by the addition of an equal volume of Tris buffer (6 mol/L; pH 9.3), and the supernatant was stored at −20°C until use. Nitrite concentration was measured with an NO-specific assay as described previously (21).
Determination of cytosolic and membrane-associated p47
Freshly isolated platelets were separated into cytosolic and membrane fractions by ultracentrifugation and analyzed by Western blotting as described previously (25).
Statistical analysis
The study was designed as a two-period crossover study with double blinding to the order of randomization to fish oil or placebo. To confirm that the washout period was sufficient and that there was no carry-over or residual effect that would confound the effect of the second treatment, we analyzed the clinical data by Hills and Armitage method and paired t test (26). The data were parametric and processed as the actual difference in value. The nitrite and O2− assay results underwent logarithmic transformation to produce a normal distribution before statistical analysis and compared by paired t test. We compared results before and after l-NAME treatment by repeated measures ANOVA followed by post hoc paired t test. Results are presented as mean ± SEM unless otherwise stated. Statistical comparisons between healthy controls and HT alone or T2DM HT baseline levels were made with one-way ANOVA followed by Bonferroni post hoc test. We used the χ2 test to compare medication between groups, and P < 0.05 was considered significant.
RESULTS
Platelet phospholipid fatty acid profile
We confirmed compliance with fish oil capsule intake and incorporation of both DHA and EPA into platelet membranes by assessment of platelet phospholipid fatty acid composition. Percentages of membrane EPA (HT alone, 1.07 ± 0.17 to 1.572 ± 0.12; P < 0.001; T2DM HT, 0.654 ± 0.06 to 1.725 ± 0.22; P < 0.001) and DHA (HT alone, 1.92 ± 0.14 to 2.3 ± 0.17; P < 0.01; T2DM HT, 1.36 ± 0.15 to 2.01 ± 0.13; P < 0.001) increased. There was also a small decrease in arachidonic acid (AA) content after fish oil supplementation. Fatty acid compositions of EPA and DHA were unchanged after placebo supplementation.
Clinical characteristics and lipid profile
We found baseline differences in diabetes-related indices such as fasting glucose, BMI, and elevated heart rate (Table 1). The blood pressure measurements in the T2DM HT group were lower than those in the HT alone group, reflecting the stricter clinical control of hypertension in the former group. LDL levels were also significantly increased in the HT alone group and were unchanged by the trial interventions. There were no other significant differences in the physical characteristics, including sex, between the two groups. Also, with the exception of antidiabetic medication, statistical comparison showed no significant difference in treatments between participant groups, including statin and aspirin intake. Importantly, the crossover study design, whereby every individual acted as his or her own control, largely controlled for these baseline differences and minimized the confounding influences of medications and interindividual variability observed in larger studies.
Table 1.
Physical and clinical characteristics before and after fish oil or olive oil supplementation
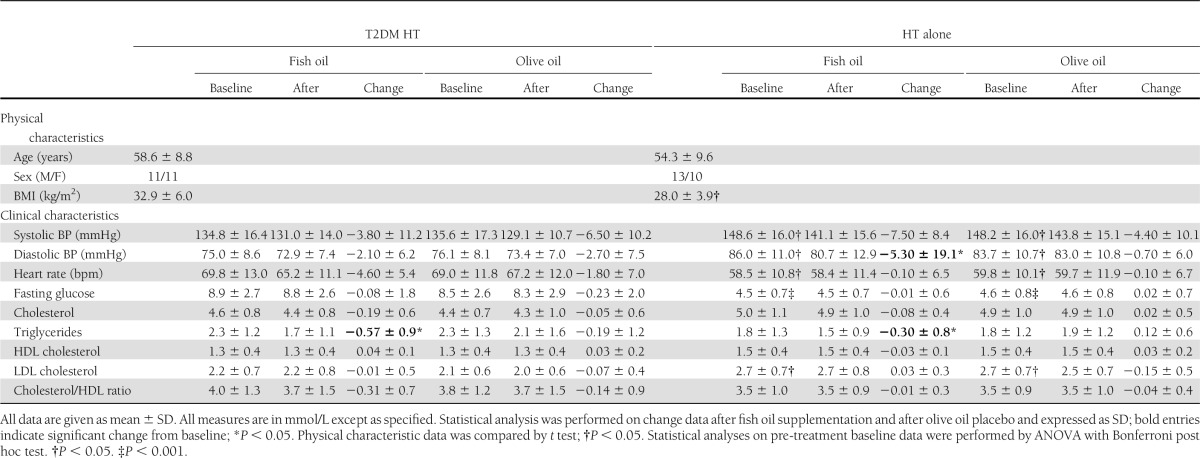
Unsurprisingly, we observed a significant fish oil–mediated lowering of triglycerides in both subject groups (Table 1). All other parameters were unchanged, apart from a decrease in diastolic blood pressure after fish oil supplementation in the HT alone group. No period effects or treatment-by-period interactions were apparent for any measurement variable, confirming the adequacy of the 8-week washout period.
Total 8-isoprostane in platelets before and after supplementation
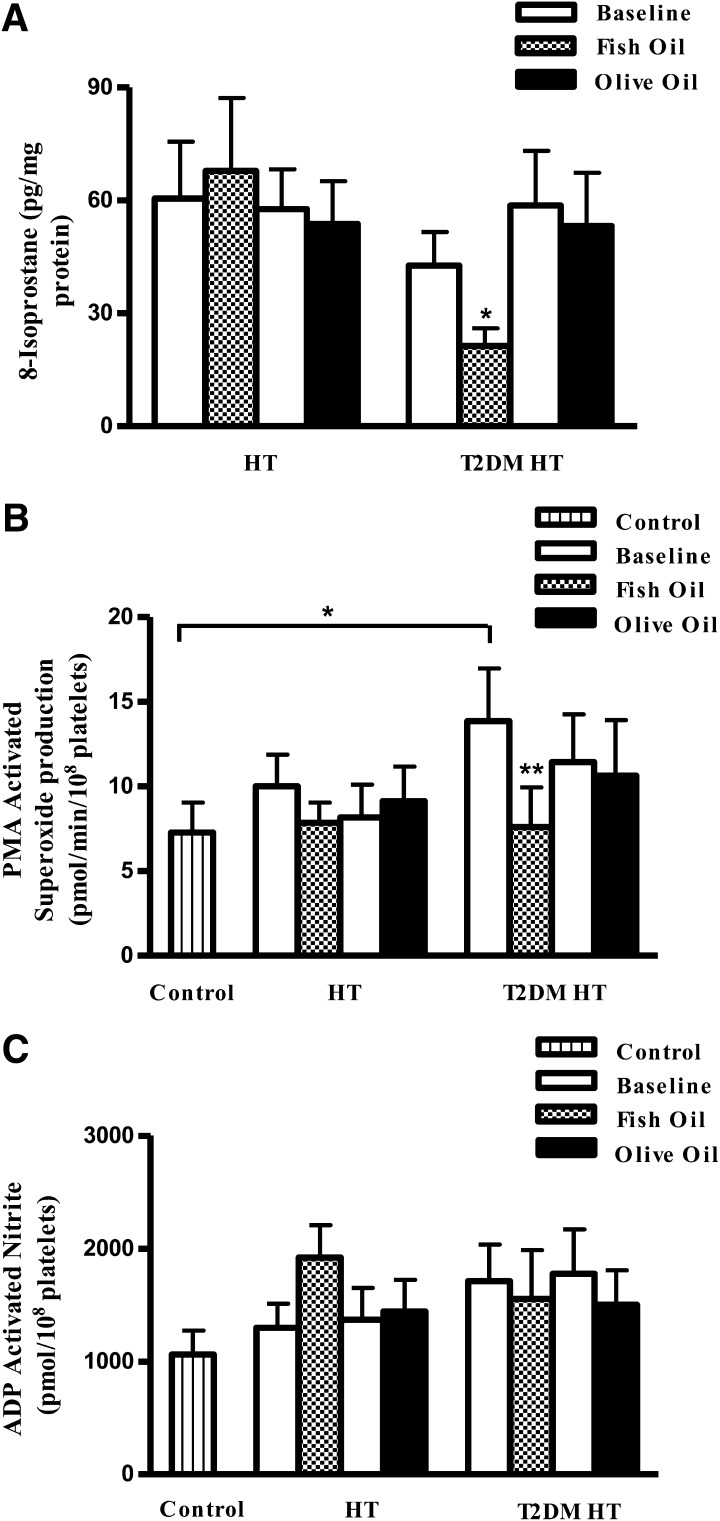
Supplementation with n-3 PUFAs significantly reduced 8-isoprostane levels in platelets isolated from T2DM HT study participants (Fig. 1A).
Figure 1.
Determination of total 8-isoprostane, O2−, and nitrite in platelets before and after dietary fish oil or olive oil supplementation. A: Supplementation with n-3 PUFAs significantly reduced 8-isoprostane levels in platelets isolated from T2DM HT study participants. HT alone, n = 14; T2DM HT, n = 19. B: PMA-stimulated O2− production was measured by lucigenin chemiluminescence. Fish oil treatment significantly reduced PMA-stimulated O2− production in the T2DM HT group. We observed no significant difference for HT alone participants receiving either fish oil or olive oil supplementation. HT alone, n = 20; T2DM HT, n = 21. C: Fish oil treatment did not significantly alter ADP-stimulated nitrite production. HT, n = 19; T2DM HT, n = 21. Data represented are mean ± SEM. *P < 0.05; ** P < 0.01.
Reduced PMA-stimulated O2− production in platelets
PMA-stimulated O2− was increased markedly in the T2DM HT group compared with controls and HT alone participants (Fig. 1B) and was significantly reduced after fish oil supplementation. In contrast, olive oil produced no significant change in PMA-stimulated O2− in T2DM HT study participants, verifying the specificity of the n-3 PUFA–mediated effect.
Effect on nitrite production
At baseline, in comparison with controls, both T2DM HT and HT alone groups exhibited an overall trend toward increased nitrite production in response to the calcium-dependent agonist ADP (Fig. 1C). ADP-stimulated nitrite production was unchanged after treatment.
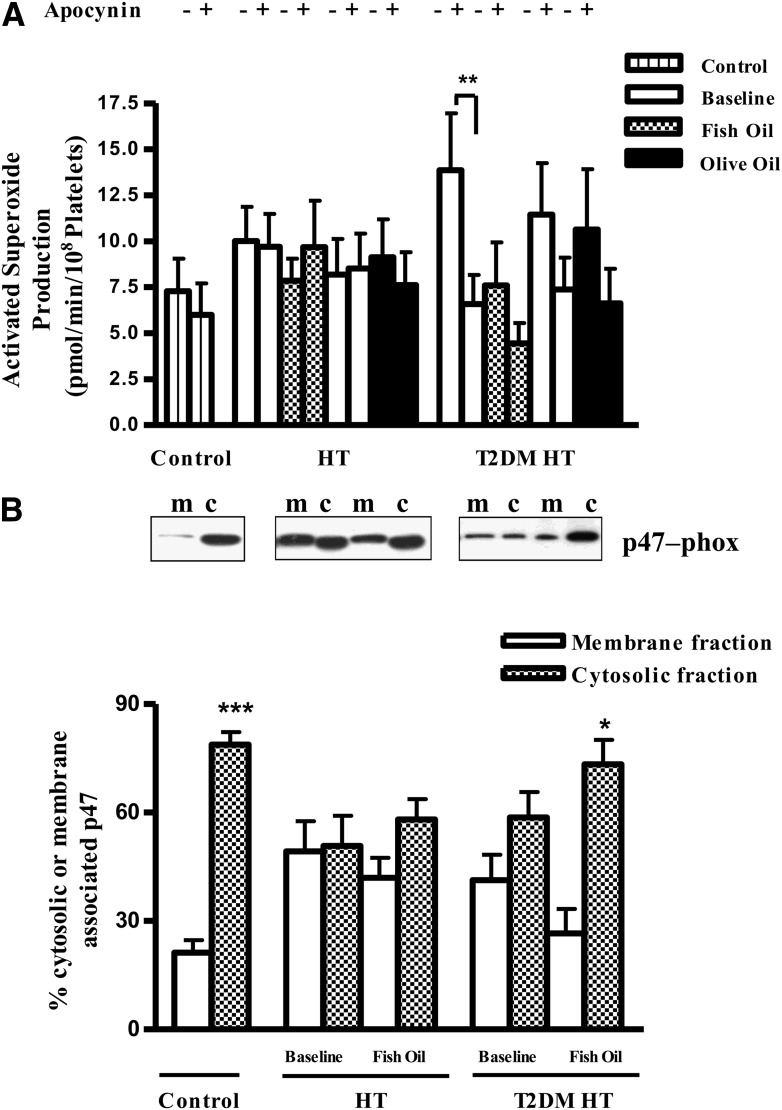
Reduced NAD(P)H-dependent O2− production
Elevated levels of O2− in the baseline T2DM HT state were significantly inhibited by the addition of apocynin, an inhibitor that, depending on the cellular environment, can act as an inhibitor of the NAD(P)H oxidase or function as a O2− scavenger (Fig. 2A). In contrast, after fish oil treatment, we found no significance inhibition of O2− production by apocynin in T2DM HT participants, suggesting that O2− production was already maximally decreased. p47-phox is a subunit of the NAD(P)H oxidase that must be associated with the plasma membrane for the enzyme to be activated, so its subcellular compartmentalization is a useful surrogate marker of NAD(P)H oxidase enzymatic activity. Western blotting indicated that in controls the presence of p47-phox predominated significantly in cytosolic fractions versus membrane fractions. In contrast, at baseline in T2DM HT and HT alone study participants, p47-phox localization was shifted from the cytosolic to the membrane fraction (Fig. 2B). After fish oil supplementation in the T2DM HT group, there was a significant reduction in membrane-associated p47-phox, such that the membrane/cytosolic localization ratio became normalized to that found in controls. This change in p47-phox subcellular localization was not observed in fish oil–treated HT alone participants.
Figure 2.
Inhibition of platelet-derived ROS production by apocynin and p47-phox subcellular localization within T2DM HT platelets. A PMA-activated platelet production of O2− measured by lucigenin-enhanced chemiluminescence was inhibited in the presence of 30 μmol/L apocynin. Fish oil supplementation abolished this inhibition in T2DM HT study participants. B: T2DM HT and HT alone platelets were isolated before and after treatment. Platelet extracts (40 μg), membrane (m), and cytosol (c) prepared from control, T2DM HT, or HT alone blood were separated on 12% SDS-PAGE, electrophoretically transferred to PVDF membrane, and analyzed with specific antiserum raised against p47-phox protein. Shown is a representative blot. The p47-phox expression was analyzed by Western blotting and quantified by measuring integrated optical density. Platelets from T2DM HT study participants showed a reduction in p47-phox membrane localization after exposure to fish oil. Data present are mean ± SEM; n = 5–9. *P < 0.05; **P < 0.01; ***P < 0.001.
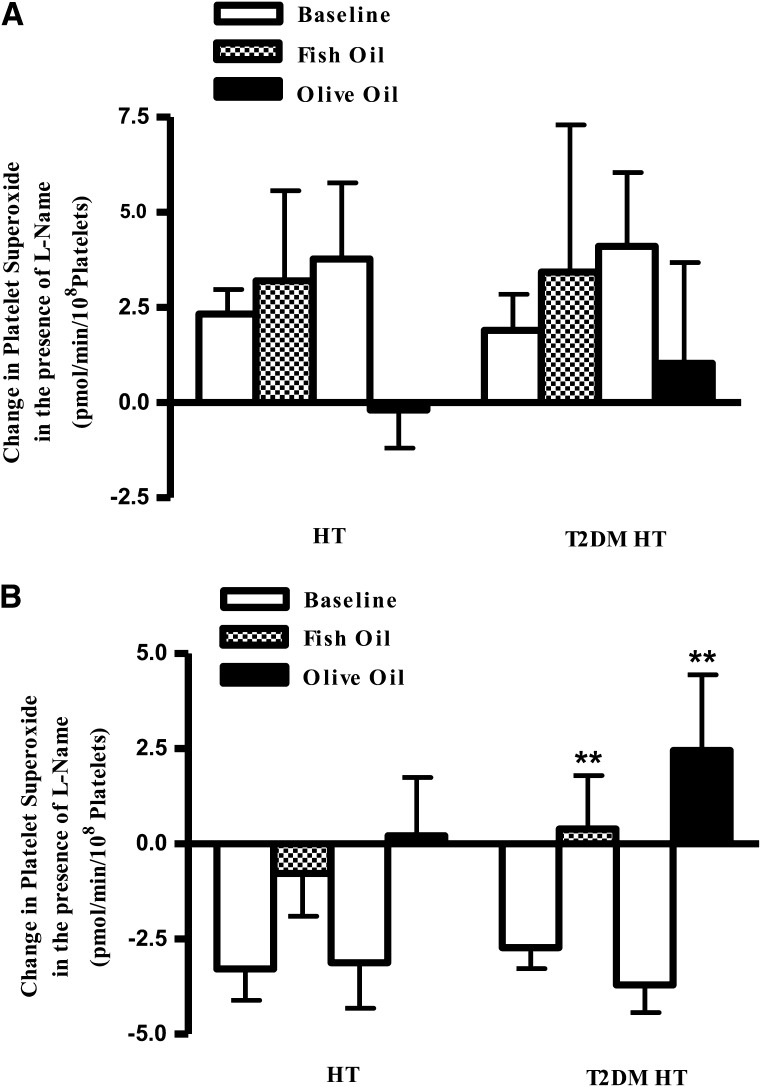
Decreased NOS uncoupling
We have previously noted that patients can be subcategorized according to their response to l-NAME (21), and this was used to assign T2DM HT and HT alone study participants at baseline into coupled and uncoupled NOS groups (Fig. 3). In all, 41% of the HT alone group and 33% of the T2DM HT group exhibited an increase in O2− in response to l-NAME, demonstrating an inhibition in NO production (Fig. 3A). In the remaining participants (59% and 67% of the HT alone and T2DM HT groups, respectively), incorporation of l-NAME reduced O2− detection in accordance with l-NAME inhibition of NOS-mediated O2− production, suggesting the enzymatic uncoupling of NOS (Fig. 3B).
Figure 3.
Change in platelet O2− production after inhibition with l-NAME. PMA-stimulated O2− production in T2DM HT study participants before (baseline) and after fish oil or olive oil treatment was measured by lucigenin-enhanced chemiluminescence. For each participant sample, O2− levels were determined before and after the addition of l-NAME and the corresponding change in chemiluminescence was calculated. A: Coupled NOS group incorporation of l-NAME increases O2− detection as less NO is produced to scavenge O2−. Coupled group HT alone, n = 7; T2DM HT, n = 9. B, Uncoupled NOS acts as a source of O2−, so O2− production is inhibited by l-NAME. Fish oil and olive oil reverse this trend and improve NOS function. Uncoupled HT alone, n = 15; uncoupled T2DM HT, n = 14. Data represented are means ± SEM. **P < 0.01.
After both fish oil and olive oil treatments, the coupled T2DM HT and HT alone study participants exhibited an increase in O2− in response to l-NAME similar to that observed at baseline (Fig. 3A), indicating that NOS status remained unchanged. Significantly, in the uncoupled HT alone and T2DM HT cohorts, after both fish oil and olive oil supplementations inhibition of O2− production by l-NAME was reduced compared with baseline, although only in the latter participant group did this effect reach statistical significance, indicating that fatty acid supplementation reduced NOS-derived O2− production (Fig. 3B). These results suggest that fish oil and olive oil exerted beneficial effects on NOS function in both participant groups, with a greater magnitude of effect being observed in the T2DM HTcohort.
CONCLUSIONS
Dietary interventions that could decrease ROS production while also maintaining cardioprotective NO levels would be of major health benefit to a growing number of T2DM patients. Because we have previously shown that DHA and EPA have such properties in normal endothelial cells, the aim of the current study was to investigate the efficacy of fish oil supplementation in a disease characterized by a high oxidative status (11).
Because of the high susceptibility of PUFAs to oxidative damage, it is important to ensure that supplementation is not damaging to vascular health (24,27). Isoprostanes are the nonenzymatic products of AA oxidation (28). They are chemically similar to prostaglandins but with opposing biological function; they contribute toward the increased platelet activation observed in diabetes (29) and are therefore useful indicators of lipid peroxidation and oxidative stress. After fish oil supplementation, isoprostane levels were unaltered in HT alone study participants, perhaps as a result of the efficacy of conventional antihypertensive therapies in use by this group. In contrast, fish oil treatment significantly decreased isoprostane levels in the T2DM HT group, suggesting that n-3 PUFAs offer an additional therapeutic benefit. These results are in agreement with those of Mori et al. (27), who also demonstrated an n-3 PUFA–mediated decrease in isoprostane levels in T2DM patients.
Assessment of O2− production provided further evidence for a fish oil–mediated decrease in oxidative stress. Our current study demonstrated a significant elevation in O2− production in platelets isolated from diabetic study participants at baseline, suggesting that, despite treatment for blood glucose reduction, these participants still showed evidence of dysfunctional ROS signaling. This dysfunction was normalized by n-3 PUFA supplementation, suggesting that these agents provide additional benefit beyond their conventional drug regimen.
We have previously shown that NOS and NAD(P)H are the two most significant enzymatic sources of O2− production in platelets. With subcellular compartmentalization of p47-phox as a surrogate indicator of NAD(P)H oxidase activity, the results of this study indicate that, compared with healthy individuals, membrane-associated levels of p47 were elevated in both HT alone and T2DM HT study participants, which is in agreement with observations in experimental models (30). Fish oil supplementation markedly reduced this membrane association, suggesting lowered NAD(P)H oxidase activity.
Measurement of NO levels in vascular disease settings is often complicated by a paradoxical increase in NOS expression. The additional protein, however, is dysfunctional and acts as a source of oxidative free radicals along with NO, resulting in elevated nitrite and concomitant O2− accumulation (31). Thus an accurate assessment of altered NO bioavailability requires an assessment of the functional status of NOS. Although the current study did not demonstrate a measurable effect on nitrite production, evidence for a change in the functional status of NOS, in terms of its ability to produce NO or O2−, was found. In the T2DM HT study participants classified as the uncoupled NOS group, fish oil and olive oil supplements both decreased NOS-derived O2− production significantly. These results indicate that both oils reduce the proportion of uncoupled NOS present. Together, these findings suggest that the ability to restore normal NOS function is common to both n-3 PUFA and olive oil, whereas the effects of n-3 PUFA treatment on decreased ROS production from NAD(P)H oxidase are unique to the fish oils. In summary, these findings indicate an overall n-3 PUFA–mediated improvement in global platelet redox status. Indeed, this is in agreement with previous research in our group showing that supplementation with n-3 PUFAs improved forearm blood flow in diabetic patients (17).
Mechanistically, there are several ways in which fish oils may mediate the effects observed in this study. First, the presence of the unsaturated double bonds increases lipid bilayer fluidity, thereby preventing the sequestering of signaling molecules to cholesterol-rich plasma membrane microdomains, such as caveolae and lipid rafts where endothelial NOS and NAD(P)H reside. For endothelial NOS, such a shift leads to a physical separation from its inhibitory partner caveolin-1, leading to an increase in basal NO production (32,33). This effect on membrane fluidity is a feature shared, albeit to a lower extent, by monounsaturated fatty acids like oleic acid, the major n-9 PUFA constituent in olive oil. This may explain the olive oil–mediated improvement in NOS function that we saw.
Second, the incorporation of PUFA displaces AA from the plasma membrane to accommodate the influx of EPA and DHA, effectively reducing AA concentrations. Any reduction in AA, an activator of NAD(P)H oxidase, inhibits NAD(P)H activity (34).
Third, n-3 PUFAs also have a high affinity for eicosanoid-synthesizing enzymes such as cyclooxygenase and lipooxygenase and can be metabolized to specific EPA- or DHA-derived eicosanoids that are less inflammatory and thrombogenic than their n-6 PUFA–derived counterparts (6). The production of eicosanoids is dependent on an n-6 unsaturated bond, which is absent from oleic acid. Thus it is possible that the fish oil–specific effects we observed are due in part to a decrease in n-6 PUFA–derived metabolites in conjunction with a concomitant increase in their n-3 PUFA–derived counterparts (35).
Raised triglyceride levels are a major risk factor for CVD in diabetic patients. Thus the finding that Omacor, currently prescribed for hyperlipidemia, had the ability to decrease triglyceride levels in our study population is clearly advantageous. Interestingly, the decrease in triglyceride levels was more marked in the T2DM HT participants and correlated with decreased ROS production. Mechanistically, this may be the result of a decrease in levels of oxidized LDL, which is known to inhibit NOS function by increasing its association with caveolin-1 (36). Furthermore, diabetes is a chronic inflammatory disease in which enhanced levels of circulating inflammatory cytokines act to exacerbate endothelial dysfunction. In this regard, n-3 PUFAs are known to modulate proinflammatory cytokine expression and also reduce cytokine signaling events by displacement of cytokine receptors from their downstream signaling partners, suggesting a further means by which fish oil may indirectly alter redox mechanisms (37,38).
Much of the advice for diabetic patients regarding fish consumption is based on the higher incidence of CVD in T2DM and is extrapolated from studies on the non-T2DM population or derived from prospective studies (39). T2DM patients have an enhanced oxidative burden compared with non-T2DM patients; therefore, studies confirming efficacy of fish oil supplementation for the former are extremely important. One of the few large scale studies to focus on individuals with T2DM recently reported no significant benefit of fish oils with respect to reducing cardiovascular mortality (40). In common with our study, all study participants received a similar ratio of EPA to DHA. In contrast, however, they received a lower dose (1 g/day) than we used here (3 g/day). Perhaps more importantly, the underlying disease pattern was markedly different, with patients only enrolled if they had already had a cardiovascular event. In contrast, our study focused on study participants without advanced disease and accordingly investigated markers known to be elevated in the early stages of endothelial dysfunction. Future large scale studies, such as ASCEND (A Study of Cardiovascular Events in Diabetes) should clarify whether fish oil supplementation in T2DM patients with no previous CVD would be beneficial in preventing disease progression.
In summary, by using well-characterized markers of platelet and vascular dysfunction, our findings support a model whereby supplementation with n-3 PUFAs decreases ROS production and oxidative stress and improves NOS function in T2DM study participants. These favorable effects on redox state in platelets support a cardioprotective role for n-3 PUFAs in patients with T2DM.
Acknowledgments
This work was supported by funding from the R & D Office Northern Ireland.
No potential conflicts of interest relevant to this article were reported.
D.M.M. codirected research, analyzed data, and wrote the manuscript. F.O. recruited study participants and analyzed data. M.M. and A.B.D. performed research and analyzed data. G.E.M. directed research and reviewed the manuscript. G.E.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Chris Patterson, Epidemiology Research Group, Queen’s University Belfast, for statistical advice.
References
- 1.Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 2006;113:1708–1714 [DOI] [PubMed] [Google Scholar]
- 2.Loscalzo J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ Res 2001;88:756–762 [DOI] [PubMed] [Google Scholar]
- 3.Freedman JE, Sauter R, Battinelli EM, et al. Deficient platelet-derived nitric oxide and enhanced hemostasis in mice lacking the NOSIII gene. Circ Res 1999;84:1416–1421 [DOI] [PubMed] [Google Scholar]
- 4.Kojda G, Harrison D. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res 1999;43:562–571 [DOI] [PubMed] [Google Scholar]
- 5.Schäfer A, Alp NJ, Cai S, et al. Reduced vascular NO bioavailability in diabetes increases platelet activation in vivo. Arterioscler Thromb Vasc Biol 2004;24:1720–1726 [DOI] [PubMed] [Google Scholar]
- 6.Davì G, Catalano I, Averna M, et al. Thromboxane biosynthesis and platelet function in type II diabetes mellitus. N Engl J Med 1990;322:1769–1774 [DOI] [PubMed] [Google Scholar]
- 7.Krötz F, Sohn HY, Gloe T, et al. NAD(P)H oxidase-dependent platelet superoxide anion release increases platelet recruitment. Blood 2002;100:917–924 [DOI] [PubMed] [Google Scholar]
- 8.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:23–3312114037 [Google Scholar]
- 9.Guzik TJ, Harrison DG. Vascular NADPH oxidases as drug targets for novel antioxidant strategies. Drug Discov Today 2006;11:524–533 [DOI] [PubMed] [Google Scholar]
- 10.Ceriello A, Testa R. Antioxidant anti-inflammatory treatment in type 2 diabetes. Diabetes Care 2009;32(Suppl. 2):S232–S236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matesanz N, Park G, McAllister H, et al. Docosahexaenoic acid improves the nitroso-redox balance and reduces VEGF-mediated angiogenic signaling in microvascular endothelial cells. Invest Ophthalmol Vis Sci 2010;51:6815–6825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thies F, Garry JMC, Yaqoob P, et al. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet 2003;361:477–485 [DOI] [PubMed] [Google Scholar]
- 13.Wang C, Harris WS, Chung M, et al. n-3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am J Clin Nutr 2006;84:5–17 [DOI] [PubMed] [Google Scholar]
- 14.Yamagishi K, Iso H, Date C, et al. Japan Collaborative Cohort Study for Evaluation of Cancer Risk Study Group Fish, omega-3 polyunsaturated fatty acids, and mortality from cardiovascular diseases in a nationwide community-based cohort of Japanese men and women the JACC (Japan Collaborative Cohort Study for Evaluation of Cancer Risk) Study. J Am Coll Cardiol 2008;52:988–996 [DOI] [PubMed] [Google Scholar]
- 15.Nettleton JA, Katz R. n-3 Long-chain polyunsaturated fatty acids in type 2 diabetes: a review. J Am Diet Assoc 2005;105:428–440 [DOI] [PubMed] [Google Scholar]
- 16.Kris-Etherton PM, Harris WS, Appel LJ, Nutrition Committee Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Arterioscler Thromb Vasc Biol 2003;23:e20–e30 [DOI] [PubMed] [Google Scholar]
- 17.McVeigh GE, Brennan GM, Johnston GD, et al. Dietary fish oil augments nitric oxide production or release in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 1993;36:33–38 [DOI] [PubMed] [Google Scholar]
- 18.Franscini N, Bachli EB, Blau N, et al. Functional tetrahydrobiopterin synthesis in human platelets. Circulation 2004;110:186–192 [DOI] [PubMed] [Google Scholar]
- 19.Ji Y, Ferracci G, Warley A, et al. beta-Actin regulates platelet nitric oxide synthase 3 activity through interaction with heat shock protein 90. Proc Natl Acad Sci U S A 2007;104:8839–8844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radomski MW, Palmer RM, Moncada S. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc Natl Acad Sci U S A 1990;87:5193–5197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dixon LJ, Morgan DR, Hughes SM, et al. Functional consequences of endothelial nitric oxide synthase uncoupling in congestive cardiac failure. Circulation 2003;107:1725–1728 [DOI] [PubMed] [Google Scholar]
- 22.Seno T, Inoue N, Gao D, et al. Involvement of NADH/NADPH oxidase in human platelet ROS production. Thromb Res 2001;103:399–409 [DOI] [PubMed] [Google Scholar]
- 23.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539–553 [DOI] [PubMed] [Google Scholar]
- 24.McGrath LT, Brennan GM, Donnelly JP, Johnston GD, Hayes JR, McVeigh GE. Effect of dietary fish oil supplementation on peroxidation of serum lipids in patients with non-insulin dependent diabetes mellitus. Atherosclerosis 1996;121:275–283 [DOI] [PubMed] [Google Scholar]
- 25.Hamilton PK, Hughes SMT, Plumb RD, et al. Statins have beneficial effects on platelet free radical activity and intracellular distribution of GTPases in hyperlipidaemia. Clin Sci (Lond) 2010;118:359–366 [DOI] [PubMed] [Google Scholar]
- 26.Hills MAP, Armitage P. The two-period cross-over clinical trial. Br J Clin Pharmacol 1979;8:7–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mori TA, Woodman RJ, Burke V, Puddey IB, Croft KD, Beilin LJ. Effect of eicosapentaenoic acid and docosahexaenoic acid on oxidative stress and inflammatory markers in treated-hypertensive type 2 diabetic subjects. Free Radic Biol Med 2003;35:772–781 [DOI] [PubMed] [Google Scholar]
- 28.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ., 2nd A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A 1990;87:9383–9387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davì G, Ciabattoni G, Consoli A, et al. In vivo formation of 8-iso-prostaglandin f2alpha and platelet activation in diabetes mellitus: effects of improved metabolic control and vitamin E supplementation. Circulation 1999;99:224–229 [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Zalewski A, Liu Y, et al. Diabetes-induced oxidative stress and low-grade inflammation in porcine coronary arteries. Circulation 2003;108:472–478 [DOI] [PubMed] [Google Scholar]
- 31.Cosentino F, Hishikawa K, Katusic ZS, Lüscher TF. High glucose increases nitric oxide synthase expression and superoxide anion generation in human aortic endothelial cells. Circulation 1997;96:25–28 [DOI] [PubMed] [Google Scholar]
- 32.Li Q, Zhang Q, Wang M, et al. Eicosapentaenoic acid modifies lipid composition in caveolae and induces translocation of endothelial nitric oxide synthase. Biochimie 2007;89:169–177 [DOI] [PubMed] [Google Scholar]
- 33.Yang B, Rizzo V. TNF-alpha potentiates protein-tyrosine nitration through activation of NADPH oxidase and eNOS localized in membrane rafts and caveolae of bovine aortic endothelial cells. Am J Physiol Heart Circ Physiol 2007;292:H954–H962 [DOI] [PubMed] [Google Scholar]
- 34.Massaro M, Habib A, Lubrano L, et al. The omega-3 fatty acid docosahexaenoate attenuates endothelial cyclooxygenase-2 induction through both NADP(H) oxidase and PKC epsilon inhibition. Proc Natl Acad Sci U S A 2006;103:15184–15189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jump DB. The biochemistry of n-3 polyunsaturated fatty acids. J Biol Chem 2002;277:8755–8758 [DOI] [PubMed] [Google Scholar]
- 36.Blair A, Shaul PW, Yuhanna IS, Conrad PA, Smart EJ. Oxidized low density lipoprotein displaces endothelial nitric-oxide synthase (eNOS) from plasmalemmal caveolae and impairs eNOS activation. J Biol Chem 1999;274:32512–32519 [DOI] [PubMed] [Google Scholar]
- 37.Chen W, Esselman WJ, Jump DB, Busik JV. Anti-inflammatory effect of docosahexaenoic acid on cytokine-induced adhesion molecule expression in human retinal vascular endothelial cells. Invest Ophthalmol Vis Sci 2005;46:4342–4347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeyda M, Staffler G, Horejsi V, Waldhausl W, Stulnig TM. LAT displacement from lipid rafts as a molecular mechanism for the inhibition of T cell signaling by polyunsaturated fatty acids. J Biol Chem 2002;277:28418–28423 [DOI] [PubMed] [Google Scholar]
- 39.Hu FB, Cho E, Rexrode KM, Albert CM, Manson JE. Fish and long-chain omega-3 fatty acid intake and risk of coronary heart disease and total mortality in diabetic women. Circulation 2003;107:1852–1857 [DOI] [PubMed] [Google Scholar]
- 40.Bosch J, Gerstein HC, Dagenais GR, et al. ORIGIN Trial Investigators n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med 2012;367:309–318 [DOI] [PubMed] [Google Scholar]