Abstract
Objective
Fragment Bb is an activator of the alternative pathway of the complement system. Recently, increased first trimester maternal plasma concentrations of this fragment were reported in patients destined to have a spontaneous preterm delivery before 34 weeks of gestation. The aim of this study was to determine whether the amniotic fluid (AF) concentrations of fragment Bb change with gestational age, spontaneous labor (term and preterm), and in the presence of intra-amniotic infection/ inflammation (IAI).
Study design
This cross-sectional study included patients in the following groups: 1) midtrimester (n=64); 2) term in spontaneous labor (n=70); 3) term not in labor (n=43); 4) spontaneous preterm labor (PTL) who delivered at term (n=76); 5) PTL without IAI who delivered preterm (n=73); 6) PTL with IAI (n=76); 7) prelabor rupture of the membranes (preterm PROM) without IAI (n=71); and 8) preterm PROM with IAI (n=71). Fragment Bb concentration in amniotic fluid was determined by an enzyme-linked immunoassay. Non-parametric statistics were used for analyses.
Results
1) Fragment Bb was detected in all AF samples (n=544); 2) The median AF concentration of fragment Bb in patients at term not in labor was significantly higher than that of those in the mid-trimester [2.42 μg/mL, interquartile range (IQR) 1.78-3.22 vs. 1.64 μg/mL, IQR 1.06-3.49; p<0.001]; 3) Among patients with PTL, those with IAI had a higher median AF fragment Bb concentration than that of woman without IAI who delivered preterm (4.82 μg/mL, IQR 3.32-6.08 vs. 3.67 μg/mL, IQR 2.35-4.57; p<0.001) and than that of women with an episode of PTL who delivered at term (3.21 μg/mL, IQR 2.39-4.16; p<0.001); 4) Similarly, among patients with preterm PROM, the median AF fragment Bb concentration was higher in individuals with IAI than in those without IAI (4.24 μg/mL, IQR 2.58-5.79 vs. 2.79 μg/mL, IQR 2.09-3.89; p<0.001). 5) Among patients at term, the median AF fragment Bb concentration did not differ between women with spontaneous labor and those without labor (term in labor: 2.47 μg/mL, IQR 1.86-3.22; p=0.97).
Conclusions
1) Fragment Bb, an activator of the alternative complement pathway, is a physiologic constituent of the amniotic fluid, and its concentration increases with advancing gestational age; 2) Amniotic fluid concentrations of fragment Bb are higher in pregnancies complicated with IAI; and 3) Labor at term is not associated with changes in the amniotic fluid concentrations of fragment Bb. These findings suggest a role for fragment Bb in the host immune response against IAI.
Keywords: alternative pathway, chorioamnionitis, complement, pregnancy, preterm labor, prelabor rupture of membranes, PPROM, microbial invasion of the amniotic cavity
INTRODUCTION
Preterm parturition is syndromic in nature,[1,2] and several mechanisms of disease have been implicated in its pathophysiology, including intrauterine infection, uterine ischemia, uterine overdistension, abnormal allogenic recognition, allergic-like reaction, cervical disease, and endocrine disorders.[2] Nevertheless, intrauterine infection[3-13] is the only pathological process for which a firm causal link with preterm birth has been established and a defined molecular pathophysiology is known.[1] Of note, the pregnancy outcome of patients with spontaneous preterm labor with intra-amniotic inflammation defined by elevated pro-inflammatory cytokines [e.g. interleukin (IL)-6[14-16] and matrix metalloproteinase (MMP) 8[17]], is similar to that of those with microbiologically-proven intra-amniotic infection.[16] Both, intra-amniotic infection and inflammation are associated with development of a fetal inflammatory response syndrome (FIRS),[18-20] which is a risk factor for fetal injury.[14,21-29]
The complement system is an important component of innate immunity, and plays a pivotal role in the process of recognition of foreign antigens and pathogens. In addition, the complement system participates in the inflammatory response elicited against infection, and has a role in activating the adaptive immune system.[30-32] Three different pathways can trigger complement activation: the “classical,” “lectin,” and “alternative.” Of note, these pathways converge at the point of C3 convertase generation.[32,33]
The classical and the lectin pathways are initiated by the binding of recognition proteins to specific targets (protein-to-protein and protein-to-carbohydrate interactions, respectively).[32] In contrast, the alternative pathway does not depend on binding of a protein to a pathogen, but is capable of auto-activation by spontaneous hydrolysis of C3 in the plasma generating C3(H2O).[32,34] The later is able to bind factor B, allowing its cleavage by factor D into fragments Ba and Bb. The C3(H2O)Bb complex can cleave additional C3 molecules, generating C3b that, in turn, associates with factor B to generate more C3-convertase.[32] The alternative pathway can also be activated through an “amplification loop,” in which fixed C3b generated by the classical or lectin pathways binds factor B.[31]
During pregnancy there is a physiologic activation of the complement system in the maternal circulation, which has been proposed to be a compensatory mechanism aimed to protect the host against infection.[35] However, several pregnancy complications including spontaneous pregnancy losses,[36-41] preeclampsia,[42,43] pyelonephritis,[44] fetal death,[45] as well as preterm birth,[13] have been associated with an excessive systemic maternal complement activation.
Increased activation of components of the complement system in maternal blood (C3a and C5a) [13] and the amniotic fluid (C3)[46] has been reported in patients with preterm labor and intact membranes. Recently, increased maternal plasma concentrations of fragment Bb in the first trimester were reported in patients who subsequently had a spontaneous preterm delivery before 34 weeks of gestation.[47] However, to date, there is limited information regarding the concentration of fragment Bb in amniotic fluid (AF).
This study was conducted to determine whether the amniotic fluid concentration of fragment Bb changes with advancing gestational age, spontaneous labor at term, and in the presence of intra-amniotic infection/inflammation (IAI) in patients with spontaneous preterm labor (PTL) and intact membranes, as well as in women with preterm prelabor rupture of membranes (preterm PROM).
MATERIALS AND METHODS
Study design and population
A cross-sectional study, was conducted by searching our clinical database and bank of biological specimens, and consisted of patients in the following groups: 1) women in the mid-trimester of pregnancy (14-18 weeks) who underwent amniocentesis for genetic indications and delivered a normal neonate at term (n=64); 2) normal pregnant women at term with spontaneous labor (n=70); 3) normal pregnant women at term not in labor (n=43); 4) women with an episode of preterm labor (PTL) and intact membranes who delivered at term (n=76); 5) PTL without IAI who delivered preterm (<37 weeks gestation) (n=73); 6) PTL with IAI (n=76); 7) women with preterm PROM without IAI (n=71); and 8) preterm PROM with IAI (n=71).
All women involved in the study provided written informed consent prior to the collection of amniotic fluid. The collection of amniotic fluid and its utilization for research purposes were approved by the Institutional Review Boards of participating institutions and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD/NIH/DHHS). Many of these samples have previously been used to study the biology of inflammation, haemostasis, angiogenesis regulation, and growth factor concentrations in normal pregnant women and those with pregnancy complications.
Definitions
Patients were considered to have a normal pregnancy outcome if they did not have obstetrical complications and delivered a term neonate (≥37 weeks) of appropriate birthweight for gestational age[48,49] without complications. Spontaneous preterm labor was defined by the presence of regular uterine contractions occurring at a frequency of at least two every 10 minutes associated with cervical changes before 37 completed weeks of gestation that required hospitalization. Preterm PROM was diagnosed by sterile speculum examination confirming pooling of amniotic fluid in the vagina in association with nitrazine and ferning tests when necessary, before 37 weeks of gestation and in the absence of labor. Women at term not in labor underwent amniocentesis for the assessment of fetal lung maturity prior to cesarean section. Women at term in labor consisted of women who were suspected to have preterm labor because of uncertain dates and had an amniocentesis for the assessment of fetal lung maturity and microbial invasion of the amniotic cavity. If analysis of amniotic fluid was consistent with maturity, tocolysis was not used. In addition, if the women delivered a baby heavier than 2500 grams without complications of prematurity, they were considered to represent patients in spontaneous labor at term. Intra-amniotic infection was defined as a positive amniotic fluid culture for microorganisms. Intra-amniotic inflammation was diagnosed in the presence of an amniotic fluid interleukin (IL)-6 concentration ≥2.6 ng/mL.[16] Acute histologic chorioamnionitis was diagnosed based on the presence of inflammatory cells in the chorionic plate and/or chorioamniotic membranes. Acute funisitis was defined by the presence of neutrophils in the wall of the umbilical vessels and/or Wharton’s jelly using the criteria previously described.[50]
Sample collection
Amniotic fluid samples were obtained from transabdominal amniocenteses performed for evaluation of microbial status of the amniotic cavity and/or assessment of fetal lung maturity. Sample of amniotic fluid was transported to the laboratory in a sterile capped syringe, and cultured for aerobic/anaerobic bacteria and genital Mycoplasmas. White blood cell (WBC) count, glucose concentration as well as Gram stain were also performed shortly after collection. The results of these tests were used for subsequent clinical management. Amniotic fluid not required for clinical assessment was centrifuged for 10 minutes at 4°C, and the supernatant was aliquoted and stored at −70°C until analysis. Midtrimester samples were not evaluated for infection. However, all had an amniotic fluid IL-6 concentration <2.6 ng/mL.
Among patients with spontaneous PTL with intact membranes who delivered within 72 hours of amniocentesis, placenta, umbilical cord and chorioamniotic membranes were collected, and the presence or absence of histologic chorioamnionitis and/or funisitis was assessed. The 72-hour interval was chosen to preserve a meaningful temporal relationship between amniotic fluid fragment Bb concentration and placental histopathologic findings.
Determination of fragment Bb concentration in amniotic fluid
Amniotic fluid concentration of human fragment Bb was determined by sensitive enzyme-linked immunoassays (Quidel Corporation, San Diego, CA, USA). Fragment Bb immunoassay was validated for human amniotic fluid in our laboratory, prior to the conduction of this study. Validation included spike and recovery experiments which produced parallel curves, indicating that amniotic fluid constituents did not interfere with antigen-antibody binding in this assay. Immunoassays were carried out according to the manufacturer’s recommendations. Amniotic fluid samples were incubated in duplicate wells of the micro titer plates that were pre-coated with an antibody specific for the analyte (fragment Bb). During this incubation, the analyte present in the standards or amniotic fluid samples was bound by the immobilized antibodies in the respective assay plates. After repeated washing and aspiration to remove all unbound substances, an enzyme-linked polyclonal antibody specific for the analyte was added to the wells of the assay plates. Unbound enzyme conjugate was removed by repeated washing and a substrate solution was added to the wells of the assay plates. Color developed in proportion to the amount of the analyte bound in the initial step, and this development was stopped with the addition of an acid solution. The intensity of color was read using a programmable spectrophotometer (SpectraMax M2, Molecular Devices, Sunnyvale, CA, USA). The concentrations of fragment Bb in amniotic fluid samples were determined by interpolation from individual standard curves. The calculated inter- and intra-assay coefficients of variation for fragment Bb immunoassays in our laboratory were 3.1% and 2.4%, respectively, and the sensitivity was 0.015 μg/mL.
Statistical analysis
Shapiro-Wilk and Kolmogorov-Smirnov tests were used to test for normal distribution of the data. Since amniotic fluid fragment Bb concentrations were not normally distributed, non parametric tests were used for analyses. Correlations between continuous variables were assessed by the Spearman’s rank correlation test. Comparisons between proportions were performed with Chi-square or Fisher’s exact tests. Kruskal-Wallis with post-hoc test (Mann-Whitney U tests) was used for continuous variables. Multiple linear regression analysis was performed to determine the relationship between amniotic fluid concentration of fragment Bb and the following variables: maternal age, gestational age at amniocentesis, and sample storage time. Among patients with PTL and intact membranes, receiver-operating characteristic (ROC) curve analysis was performed to determine cutoffs for the amniotic fluid fragment Bb concentrations for the identification of patients who had IAI. A p-value of <0.05 was considered statistically significant. The statistical analyses were performed with SPSS package version 12 (SPSS Inc, Chicago, IL, USA).
RESULTS
Fragment Bb was detected in all the amniotic fluid samples tested (n=544). The demographic and clinical characteristics of patients with a normal pregnancy (mid-trimester, term not in labor and term in labor), with spontaneous preterm labor and intact membranes and with preterm PROM are displayed in Tables 1, 2 and 3, respectively. The median gestational age at amniocentesis was significantly lower among patients with PTL with IAI than that of the other two subgroups of PTL (Table 2), and among patients with preterm PROM with IAI compared to those with preterm PROM without IAI (Table 3).
Table 1.
Demographic and clinical characteristics of patients with a normal pregnancy in the mid-trimester and those at term, with and without spontaneous labor.
| Mid-trimester (n=64) |
p a | Term not in labor (n=43) |
p b | Term in labor (n=70) |
p c | |
|---|---|---|---|---|---|---|
| Maternal age (years) † | 37 (35-38) | <0.001 | 27 (21-32) | <0.001 | 22 (20-27) | <0.001 |
| GA at amniocentesis (weeks)† |
16 (16-17) | <0.001 | 39 (38-40) | NS | 39 (38-40) | <0.001 |
| GA at delivery (weeks)†† | 39.5 (38-40) | NS | 39 (38-40) | NS | 39 (38-40) | NS |
| Birth weight (grams)†† | 3343 (3015-3616) | NS | 3250 (3030- 3580) |
NS | 3330 (3100- 3680) |
NS |
Values expressed as median (interquartile range)
GA: gestational age; NS: not significant.
p between mid-trimester and term not in labor
p between term not in labor and term in labor
p mid-trimester and term in labor
p<0.001,
p=NS, Kruskal-Wallis test with Bonferroni correction.
Table 2.
Demographic and clinical characteristics of patients presenting with spontaneous preterm labor (PTL) and intact membranes.
| PTL without IAI Term delivery (n=76) |
p a | PTL without IAI Preterm delivery (n=73) |
p b | PTL with IAI Preterm delivery (n=76) |
p c | |
|---|---|---|---|---|---|---|
| Maternal age (years) | 23 (20-27) | NS | 23 (20-29.5) | NS | 23 (20-28.2) | NS |
| GA at amniocentesis (weeks)† |
30.7 (28.6-32.3) | NS | 29.3 (26.4-32.4) | <0.001 | 27.4 (24.7-32.5) | <0.001 |
| GA at delivery (weeks)†† | 38.7 (37.9-39.9) | <0.001 | 33.0 (28.9-34.8) | <0.001 | 27.6 (25.0-32.6) | <0.001 |
| Birth weight (grams)†† | 3100 (2778-3387) | <0.001 | 1956 (1080-2940) | <0.001 | 1068 (647-1990) | <0.001 |
Values expressed as median (interquartile range)
GA: gestational age; PTL: preterm labor; IAI: intra-amniotic infection/inflammation; NS: not significant.
p between PTL without IAI who delivered at term and PTL without IAI who delivered preterm
p between PTL without IAI who delivered preterm and PTL with IAI who delivered preterm
p between PTL without IAI who delivered at term and PTL with IAI who delivered preterm
p=0.006,
p<0.001, Kruskal-Wallis test with Bonferroni correction.
Table 3.
Demographic and clinical characteristics of patients presenting with preterm prelabor rupture of membranes (preterm PROM).
| Preterm PROM without IAI (n=71) |
Preterm PROM with IAI (n=71) |
p * | |
|---|---|---|---|
| Maternal age (years) | 24 (20-31) | 28 (22-33) | 0.03 |
| GA at amniocentesis (weeks) | 31.4 (27.6-32.7) | 29.7 (26.6-32.0) | 0.03 |
| GA at delivery (weeks) | 32.6 (30.3-33.7) | 30.1 (28.0-32.1) | <0.001 |
| Birth weight (grams) | 1835 (1435-2160) | 1480 (1134-1810) | <0.001 |
Values expressed as median (interquartile range)
GA: gestational age; IAI: intra-amniotic infection/inflammation; PROM: prelabor rupture of the membranes
Mann-Whitney U-test
Amniotic fluid concentration of fragment Bb in normal pregnancies
Women with a normal pregnancy at term not in labor had a higher median amniotic fluid concentration of fragment Bb than those in the mid-trimester [2.42 μg/mL, interquartile range (IQR) 1.78-3.22 vs. 1.64 μg/mL, IQR 1.06-3.49; p<0.001] (Figure 1). Among women at term, the median amniotic fluid fragment Bb concentration did not differ significantly between patients with spontaneous labor and those without labor (term in labor: 2.47 μg/mL, IQR 1.86-3.22 vs. term not in labor: 2.42 μg/mL, IQR 1.78-3.22; p=0.97) (Figure 1).
Figure 1. Amniotic fluid concentration of fragment Bb in normal pregnancies in the mid-trimester and at term, with and without spontaneous labor.
The median amniotic fluid concentration of fragment Bb was higher in women at term not in labor than in those in the mid-trimester [term not in labor: 2.42 μg/mL, interquartile range (IQR) 1.78-3.22 vs. midtrimester: 1.64 μg/mL, IQR 1.06-3.49; p<0.001]. Among women at term, the median amniotic fluid fragment Bb concentration did not differ between patients with spontaneous labor and those not in labor (term in labor: 2.47 μg/mL, IQR 1.86-3.22; p=0.97).
Amniotic fluid concentration of fragment Bb in women with spontaneous preterm labor and intact membranes and those with preterm PROM
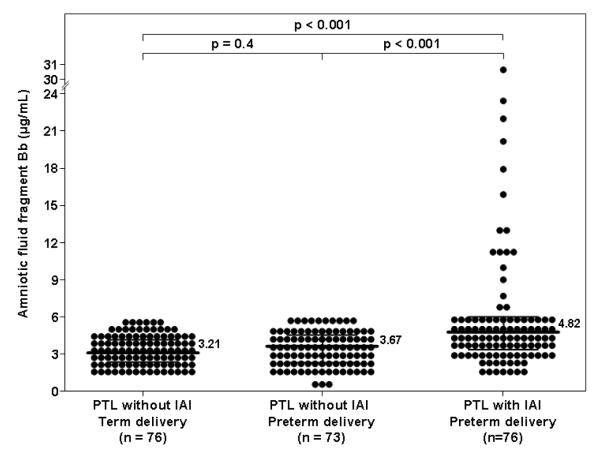
Among women with PTL, the median amniotic fluid concentration of fragment Bb was higher in patients with IAI than in those without IAI who delivered preterm (4.82 μg/mL, IQR 3.32-6.08 vs. 3.67 μg/mL, IQR 2.35-4.57; p<0.001) or at term (3.21 μg/mL, IQR 2.39-4.16; p<0.001) (Figure 2). Among women with PTL without IAI, there was no significant difference in the median amniotic fluid concentration of fragment Bb between those who delivered preterm and the ones who delivered at term (p=0.4; Figure 2).
Figure 2. Amniotic fluid concentration of fragment Bb among women with spontaneous preterm labor and intact membranes.
The median amniotic fluid concentration of fragment Bb was higher in patients with intra-amniotic infection/inflammation (IAI) than in those without IAI who delivered preterm (PTL: 4.82 μg/mL, IQR 3.32-6.08 vs. 3.67 μg/mL, IQR 2.35-4.57; p<0.001), as well as than that of those who delivered at term (3.21 μg/mL, IQR 2.39-4.16; p<0.001). Among women with PTL without IAI, there was no significant difference in the median amniotic fluid fragment Bb concentration between patients who delivered preterm and those who delivered at term (p=0.4).
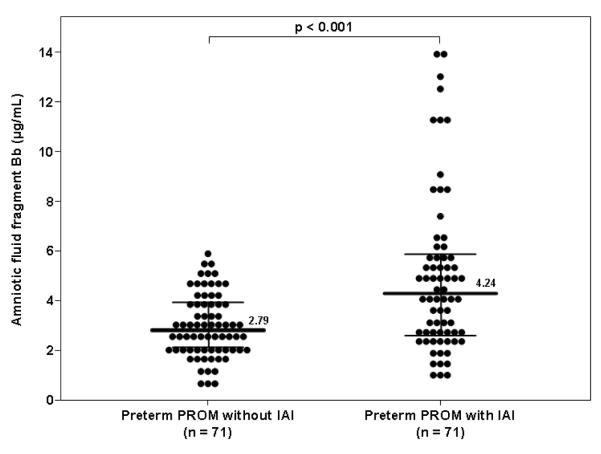
Among women with preterm PROM, the median amniotic fluid concentration of fragment Bb was higher in patients with IAI than in those without IAI (4.24 μg/mL, IQR 2.58-5.79 vs. 2.79 μg/mL, IQR 2.09-3.89; p<0.001) (Figure 3).
Figure 3. Amniotic fluid concentration of fragment Bb in women with preterm prelabor rupture of membranes (preterm PROM).
median amniotic fluid concentration of fragment Bb was higher in patients with IAI than in those without IAI (4.24 μg/mL, IQR 2.58-5.79 vs. 2.79 μg/mL, IQR 2.09-3.89; p<0.001).
Among patients with PTL and those with preterm PROM, amniotic fluid fragment Bb concentrations were positively correlated with amniotic fluid WBC count (Spearman rho coefficient: r=0.27, p<0.001) and IL-6 concentrations (r=0.4, p<0.001) and were negatively correlated with amniotic fluid glucose concentrations (r= −0.14, p=0.006).
In order to examine the association between amniotic fluid fragment Bb concentrations, IAI, and possible confounding factors, a multiple regression analysis was performed adjusting for maternal age, gestational age at amniocentesis, and sample storage time. The model demonstrated that the presence of IAI was independently associated with increased amniotic fluid fragment Bb concentrations (p<0.001).
The ROC curve of amniotic fluid fragment Bb concentration for the identification of IAI among patients with PTL with intact membranes is displayed in Figure 4 (area under the curve (AUC) 0.72, p<0.001). An optimized cutoff value (sensitivity and specificity sharing an equal importance) of amniotic fluid fragment Bb concentration of ≥4.31 μg/mL in patients with PTL and intact membranes had a sensitivity of 61.8% and a specificity of 75.8% for identification of IAI (Table 4). Due to the relative poor diagnostic performance of this cutoff, a cutoff value of ≥6.2 μg/mL, which has a specificity of 100%, however, a low sensitivity of 23.7% for the detection of IAI in patients with PTL and intact membranes, has been chosen for the definition of an elevated fragment Bb concentration (Figure 4).
Figure 4.
Receiver operating characteristic (ROC) curve of amniotic fluid fragment Bb concentration of patients with PTL and intact membranes for the identification of intra-amniotic infection/inflammation (n=225, area under the curve 0.72, p<0.001).
Table 4.
Diagnostic indices and likelihood ratios of amniotic fluid fragment Bb concentration for the detection of intra-amniotic infection/inflammation in patients presenting with spontaneous preterm labor with intact membranes (n=225).
| Cutoff (μg/mL) |
OR (95% CI) |
Sensitivity (95% CI) |
Specificity (95% CI) |
+LR (95% CI) |
−LR (95% CI) |
|---|---|---|---|---|---|
| ≥ 4.31 | 5.1 (2.8-9.2) |
61.8% (53.0-69.8) |
75.8% (71.3-79.9) |
2.56 (1.85-3.48) |
0.50 (0.38-0.66) |
When this cutoff was tested on the entire study population, we found that 21.8% of the women with IAI (32/147) had an elevated fragment Bb concentration (≥6.2 μg/mL), while none of those without IAI reached the cutoff. (0/397, p<0.001).
Amniotic fluid concentration of fragment Bb and placental histopathologic findings
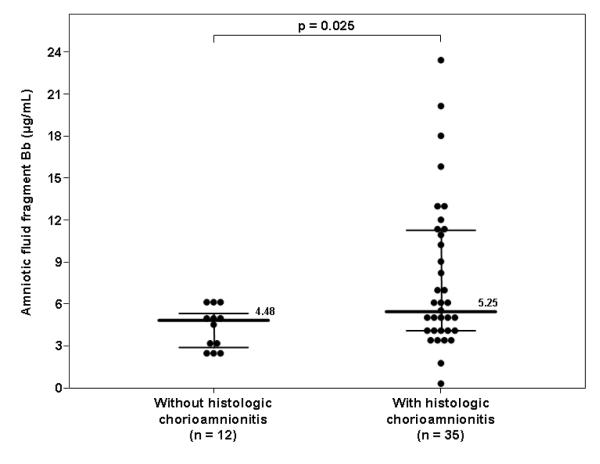
Among patients with spontaneous PTL and intact membranes with IAI, 79% (60/76) delivered within 72 hours of amniocentesis, and placental histopathologic diagnoses were available in 78% (47/60) of these individuals. The median amniotic fluid concentration of fragment Bb in patients with histologic chorioamnionitis was higher than that of women without it (histologic chorioamnionitis: 5.25 μg/mL, IQR 3.97-11.27 vs. no histologic chorioamnionitis: 4.48 μg/mL, IQR 2.80-5.29; p=0.025) (Figure 5). Moreover, none of the patients without histologic chorioamnionitis (0/12) had an elevated fragment Bb concentration (≥6.2 μg/mL), while 40% (14/35) of those with histologic chorioamnionitis had an elevated amniotic fluid fragment Bb concentration (Fisher’s exact, p=0.009).
Figure 5. Amniotic fluid concentration of fragment Bb in women with spontaneous PTL, intact membranes and IAI who delivered within 72 hours of amniocentesis and have placental histopathologic diagnosis.
Among women with IAI, the median amniotic fluid concentration of fragment Bb was higher in patients with histologic chorioamnionitis than in those without (5.25 μg/mL, IQR 3.97-11.27 vs. 4.48 μg/mL, IQR 2.80-5.29; p=0.025).
COMMENT
Principal findings of this study
1) Fragment Bb was detectable in all amniotic fluid samples and seems to be a physiologic constituent of the amniotic fluid; 2) patients with intra-amniotic infection/inflammation, regardless of the membranes status, had a higher median amniotic fluid concentration of fragment Bb than that of women without IAI; 3) amniotic fluid fragment Bb concentrations increase with advancing gestation and do not change with labor at term; and 4) elevated amniotic fluid fragment Bb concentrations were independently associated with IAI.
What is fragment Bb?
The complement system, an important component of the innate immunity, plays a pivotal role in the process of recognition of foreign antigens and pathogens. In addition, the complement system mediates the inflammatory response elicited against infection, and has a role in activating the adaptive immune system.[30-32] More than 30 proteins and cell membrane receptors encompass the complement system which can be activated by three different pathways: the “classical,” the “lectin,” and the “alternative.” Despite the different pathways, all three converge at the point of C3 convertase formation.[33] C3 convertase cleaves C3 to C3a and C3b and the latter participates in the formation of the C5 convertase, which cleaves C5 to C5a and C5b. C3a and C5a, termed anaphylatoxins, are pleiotropic inflammatory mediators.[32]
The classical pathway is initiated by the binding of C1q to antigen-antibody complexes, whereas the lectin pathway begins with the binding of mannose-binding lectin (MBL) to sugars present on the bacterial cell wall. Increased concentrations of C4 can be regarded as a marker of complement activation by either the classical or lectin pathways. In contrast, the alternative pathway is capable of auto-activation by a process termed “tickover” of C3.[34] Tickover occurs spontaneously in the absence of a recognizable trigger at a rate of approximately 1% of total C3 per hour, generating a conformationally altered C3, namely C3(H2O).[34] The latter is capable of binding factor B, a unique component of the alternative pathway, which can then be cleaved by factor D at a single Arg-Lys bond,[51-53] generating two unequal fragments, an N-terminal Ba fragment and a C-terminal Bb fragment that carries the active site of factor B.[54] Fragment Bb remains associated with C3(H2O) and the C3(H2O)Bb complex, through its own serine protease domain, can cleave additional C3 molecules, generating C3b that associates with factor B to generate more C3-convertase.[32] The alternative pathway can also be initiated as an “amplification loop” when fixed C3b, generated by the classical or lectin pathways, binds factor B resulting in conformational changes in factor B that allow factor D to cleave it similarly to the tickover process.[31] Thus, activation of the alternative pathway is characterized by increased production of fragment Bb.
The complement system in normal and complicated pregnancies
The complement system is an effector arm of the innate immune system, which is important in the host defense against infections. An excessive or inappropriate activation of the complement system has been implicated in the pathophysiology of many disorders such as rheumatoid arthritis,[55,56] systemic lupus erythematosus[57] and stroke.[58,59] In addition, perturbation of the complement system homeostasis following exposure of healthy volunteers to stressful conditions has been reported.[60-62]
Pregnancy, characterized by physiologic activation of the complement system in the maternal blood, has been proposed to be compensatory mechanism aimed to protect the host against infection.[35] However, several pregnancy complications, such as spontaneous pregnancy losses,[36-41] preeclampsia,[42,43,63] pyelonephritis,[44] fetal death[45] as well as preterm birth[13] have been associated with excessive systemic maternal complement activation. Components of the complement system have been detected in placenta,[64-66] chorioamniotic membranes,[67-69] fetal tissues (i.e. liver, spleen and thymus),[64,70-73] cord blood,[74-80] and amniotic fluid.[46,77,81,82]
In 1988, Stabile et al.[77] measured complement factors (C3, C4, C5, Factor B, H and I) in maternal and fetal circulations as well as in amniotic fluid obtained between 15-28 weeks of gestation from 55 women with a retrospectively defined normal pregnancy who underwent diagnostic fetoscopy or cordocentesis for the exclusion of hematological disorders or chromosomal defects. Concentrations of these proteins were 10 times higher in the maternal than that in the fetal circulation which, in turn, were 10 times higher than that in the amniotic fluid. Fetal concentrations of C3, C4 and factor H and amniotic fluid concentrations of C3 and factor B increased with advancing gestation, but this was not observed in maternal blood. Thus, the authors concluded that the fetus is independently synthesizing proteins of the complement system.[77]
Increased activation of components of the complement system has been reported in the amniotic fluid from patients with preterm labor.[46,83] Studying the amniotic fluid of 104 women with preterm labor with and without intra-amniotic infection, Elimian et al.[46] found a significantly higher median C3 concentration in the culture-positive group. In addition, our group reported that among 129 patients with preterm labor, those with intra-amniotic infection had higher median amniotic fluid concentrations of the anaphylatoxins, C3a, C4a and C5a.[83]
Although there are numerous reports regarding activation of the complement system in the maternal and fetal compartments during normal gestation and complications of pregnancy,[13,35-42,44-46,81,83] data concerning fragment Bb during pregnancy are scarce.[43,47,84] Recently, Lynch et al.[43,47] were the first to report the association between elevated maternal plasma fragment Bb concentrations and pregnancy complications. Elevated maternal plasma concentrations of fragment Bb in early pregnancy (before 20 weeks of gestation) were associated with an increased risk for later development of preeclampsia.[43] In a subsequent report, an association between increased maternal plasma concentrations of fragment Bb in early pregnancy and a later spontaneous preterm birth before 34 weeks has been proposed.[47]
Fragment Bb during normal pregnancy
The study presented herein reports, for the first time, the presence of fragment Bb in the amniotic fluid. Fragment Bb, an activation product of the alternative pathway, was detected in all samples included in this study, suggesting that it is a physiologic constituent of the amniotic fluid. In addition, its concentration in amniotic fluid increases with gestational age and does not change during labor at term or preterm, suggesting increasing physiologic activation of the complement system through the alternative pathway with advancing gestation, but without further activation during labor. These results are consistent with the findings of Stabile et al.,[77] who reported higher amniotic fluid concentration of factor B with advancing gestational age, and with a previous study from our group[83] that did not find an association between spontaneous labor at term and changes in the amniotic fluid concentration of other complement activation products (C3a, C4a and C5a).
Spontaneous labor at term is regarded as an inflammatory process.[85] Supporting this view are its associations with inflammatory cells infiltration in the cervix,[86-88] myometrium,[88,89] and chorioamniotic membranes,[88,90] and increased production of proinflammatory cytokines[85,91] (i.e. IL-1ß,[88,92-94] IL-6,[88,94,95] TNF-α,[93,94] and IL-8[88,95-97]) and chemokines[85,91] (i.e. GRO-α,[98] G-CSF,[95] MCP-1[99-101]). However, the results presented herein and those reported by Soto et al.,[83] suggest that spontaneous labor at term is not associated with activation of the complement system in the amniotic fluid.
Fragment Bb in intra-amniotic infection/inflammation
The finding that IAI is associated with a higher median amniotic fluid concentration of fragment Bb in patients with intact as well as ruptured membranes is novel and supports the concept of activation of the complement system as part of the fetal inflammatory response to microbial invasion of the amniotic cavity.[18,20] Evidence in support of this view comes from a report by Hogasen et al.,[84] who analyzed the complement activation products in cord blood of neonates born after preterm PROM, and reported a significantly increased concentration of Bb in the cord blood of these neonates when compared to healthy controls. Moreover, higher amniotic fluid concentration of C3[46] as well as of C3a, C4a and C5a[83] have been found in women with preterm labor and microbial invasion of the amniotic cavity compared to those with a negative amniotic fluid culture. Of interest, patients with PTL and IAI who delivered within 72 hours of amniocentesis and had histologic evidence of placental inflammation had a higher median amniotic fluid fragment Bb concentration than women without it. Most cases (29/35) with a positive placental pathology for inflammation showed evidence of fetal involvement manifested as funisitis, which is considered the histological counterpart of the fetal inflammatory response syndrome.[50] Moreover, the association between elevated amniotic fluid fragment Bb concentrations and IAI is independent of confounding factors, such as gestational age at amniocentesis.
An interesting finding of the present study is that only women with IAI had an elevated amniotic fluid fragment Bb concentration (≥6.2 μg/mL). All women with a normal pregnancy (mid-trimester, term with or without labor) as well as those with PTL who delivered preterm or at term and those with preterm PROM without IAI had amniotic fluid fragment Bb concentrations below 6.2 μg/mL. Thus, although a cutoff of ≥6.2 μg/mL has a very low sensitivity for the identification of IAI, its specificity is as high as 100%. In other words, if a fragment Bb concentration of ≥6.2 μg/mL is found in the amniotic fluid the chance of intra-amniotic infection and/or inflammation is 100%. Moreover, among patients with PTL and intact membranes with IAI who delivered within 72 hours from amniocentesis, only those with histologic chorioamnionitis had an elevated amniotic fluid fragment Bb concentration (Figure 5). This suggests that amniotic fluid fragment Bb concentrations above ≥6.2 μg/mL may be almost exclusively attributed to intra-amniotic infection and/or inflammation with histologic chorioamnionitis.
The fetal inflammatory response syndrome (FIRS), originally defined in fetuses with preterm labor and preterm PROM by an elevated of the fetal plasma IL-6 concentration,[18] is characterized by systemic activation of the fetal innate immune system.[18,20] In the presence of invading microorganisms, an inflammatory process is initiated in order to deliver cells and molecules to suppress the infection, to generate a barrier to the spread of the infection and to support repair of the injured tissue.[32] This process involves: 1) the attraction of cells to the site of injury, including macrophages, neutrophils, and lymphocytes; and 2) the release of antimicrobial peptides, cytokines, chemokines, and other inflammatory mediators such as prostaglandins, leukotrienes, and complement.[32] Some of these molecules are capable of changing the state of activation of macrophages and neutrophils. Indeed, fragment Bb has the ability to induce rapid macrophage spreading[102] and is an effective inhibitor of macrophage migration in a dose-dependent manner.[103] All the while, the complement component C5 has an opposite effect, causing enhancement of macrophage migration where the outcome of the reaction will depend on the predominant peptide.[103] Thus, a role for the alternative pathway of the complement system in the localization of mononuclear phagocytes to areas of inflammation has been suggested.[102]
In conclusion, our study demonstrates that fragment Bb can be detected in the amniotic fluid of all pregnant women, that intra-amniotic infection and/or inflammation is associated with increased amniotic fluid concentration of fragment Bb, regardless of the membranes status, and that the amniotic fluid fragment Bb concentration does not change with term or preterm labor. Collectively, these findings suggest that activation of the alternative pathway of the complement system may be part of the fetal innate immune response to intra-amniotic infection/inflammation.
Acknowledgment
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann.N.Y.Acad.Sci. 1994;734:414–29. doi: 10.1111/j.1749-6632.1994.tb21771.x. 414-429. [DOI] [PubMed] [Google Scholar]
- 2.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. BJOG. 2006;113(Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. 17-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naeye RL, Ross SM. Amniotic fluid infection syndrome. Clin.Obstet.Gynaecol. 1982;9:593–607. [PubMed] [Google Scholar]
- 4.Minkoff H. Prematurity: infection as an etiologic factor. Obstet Gynecol. 1983;62:137–144. [PubMed] [Google Scholar]
- 5.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, Hobbins JC. Infection in the pathogenesis of preterm labor. Semin.Perinatol. 1988;12:262–279. [PubMed] [Google Scholar]
- 6.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis AP, Hobbins JC. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161:817–824. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 7.Ledger WJ. Infection and premature labor. Am.J.Perinatol. 1989;6:234–236. doi: 10.1055/s-2007-999583. [DOI] [PubMed] [Google Scholar]
- 8.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am.J.Obstet.Gynecol. 1992;166:1515–1528. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- 9.Brocklehurst P. Infection and preterm delivery. BMJ. 1999;318:548–549. doi: 10.1136/bmj.318.7183.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N.Engl.J.Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 11.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment.Retard.Dev.Disabil.Res.Rev. 2002;8:3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 12.Hirsch E, Wang H. The molecular pathophysiology of bacterially induced preterm labor: insights from the murine model. J Soc.Gynecol Investig. 2005;12:145–155. doi: 10.1016/j.jsgi.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Soto E, Romero R, Richani K, Espinoza J, Nien JK, Chaiworapongsa T, Santolaya-Forgas J, Edwin SS, Mazor M. Anaphylatoxins in preterm and term labor. J Perinat.Med. 2005;33:306–313. doi: 10.1515/JPM.2005.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, Syn HC. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am.J.Obstet.Gynecol. 1995;172:960–970. doi: 10.1016/0002-9378(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 15.Wenstrom KD, Andrews WW, Hauth JC, Goldenberg RL, DuBard MB, Cliver SP. Elevated second-trimester amniotic fluid interleukin-6 levels predict preterm delivery. Am J Obstet Gynecol. 1998;178:546–550. doi: 10.1016/s0002-9378(98)70436-3. [DOI] [PubMed] [Google Scholar]
- 16.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, Jun JK. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet.Gynecol. 2001;185:1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 17.Yoon BH, Oh SY, Romero R, Shim SS, Han SY, Park JS, Jun JK. An elevated amniotic fluid matrix metalloproteinase-8 level at the time of mid-trimester genetic amniocentesis is a risk factor for spontaneous preterm delivery. Am.J.Obstet.Gynecol. 2001;185:1162–1167. doi: 10.1067/mob.2001.117678. [DOI] [PubMed] [Google Scholar]
- 18.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am.J.Obstet.Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 19.Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Edwin SS, Berry SM. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am.J.Obstet.Gynecol. 1998;179:186–193. doi: 10.1016/s0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- 20.Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O, Espinoza J, Hassan SS. The fetal inflammatory response syndrome. Clin.Obstet Gynecol. 2007;50:652–683. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- 21.Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr.Res. 1997;42:1–8. doi: 10.1203/00006450-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Yoon BH, Romero R, Jun JK, Park KH, Park JD, Ghezzi F, Kim BI. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am.J.Obstet.Gynecol. 1997;177:825–830. doi: 10.1016/s0002-9378(97)70276-x. [DOI] [PubMed] [Google Scholar]
- 23.Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, Kim IO. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177:19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- 24.Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, Han TR. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am.J.Obstet.Gynecol. 2000;182:675–681. doi: 10.1067/mob.2000.104207. [DOI] [PubMed] [Google Scholar]
- 25.Dammann O, Leviton A. Role of the fetus in perinatal infection and neonatal brain damage. Curr.Opin.Pediatr. 2000;12:99–104. doi: 10.1097/00008480-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Gibbs RS. The relationship between infections and adverse pregnancy outcomes: an overview. Ann.Periodontol. 2001;6:153–163. doi: 10.1902/annals.2001.6.1.153. [DOI] [PubMed] [Google Scholar]
- 27.Patrick LA, Smith GN. Proinflammatory cytokines: a link between chorioamnionitis and fetal brain injury. J.Obstet.Gynaecol.Can. 2002;24:705–709. doi: 10.1016/s1701-2163(16)30325-5. [DOI] [PubMed] [Google Scholar]
- 28.Hagberg H, Mallard C, Jacobsson B. Role of cytokines in preterm labour and brain injury. BJOG. 2005;112(Suppl 1):16–18. doi: 10.1111/j.1471-0528.2005.00578.x. [DOI] [PubMed] [Google Scholar]
- 29.Bashiri A, Burstein E, Mazor M. Cerebral palsy and fetal inflammatory response syndrome: a review. J Perinat.Med. 2006;34:5–12. doi: 10.1515/JPM.2006.001. [DOI] [PubMed] [Google Scholar]
- 30.Holers VM. The complement system as a therapeutic target in autoimmunity. Clin.Immunol. 2003;107:140–151. doi: 10.1016/s1521-6616(03)00034-2. [DOI] [PubMed] [Google Scholar]
- 31.Thurman JM, Holers VM. The central role of the alternative complement pathway in human disease. J Immunol. 2006;176:1305–1310. doi: 10.4049/jimmunol.176.3.1305. [DOI] [PubMed] [Google Scholar]
- 32.Murphy K, Travers P, Walport M. Innate immunity. 2008;Seventh:39–103. [Google Scholar]
- 33.Walport MJ. Complement. First of two parts. N.Engl.J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 34.Muller-Eberhard HJ. Molecular organization and function of the complement system. Annu.Rev.Biochem. 1988;57:321–47. doi: 10.1146/annurev.bi.57.070188.001541. 321-347. [DOI] [PubMed] [Google Scholar]
- 35.Richani K, Soto E, Romero R, Espinoza J, Chaiworapongsa T, Nien JK, Edwin S, Kim YM, Hong JS, Mazor M. Normal pregnancy is characterized by systemic activation of the complement system. J Matern.Fetal Neonatal Med. 2005;17:239–245. doi: 10.1080/14767050500072722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tichenor JR, Bledsoe LB, Opsahl MS, Cunningham DS. Activation of complement in humans with a first-trimester pregnancy loss. Gynecol Obstet Invest. 1995;39:79–82. doi: 10.1159/000292384. [DOI] [PubMed] [Google Scholar]
- 37.Mellor AL, Sivakumar J, Chandler P, Smith K, Molina H, Mao D, Munn DH. Prevention of T cell-driven complement activation and inflammation by tryptophan catabolism during pregnancy. Nat.Immunol. 2001;2:64–68. doi: 10.1038/83183. [DOI] [PubMed] [Google Scholar]
- 38.Holers VM, Girardi G, Mo L, Guthridge JM, Molina H, Pierangeli SS, Espinola R, Xiaowei LE, Mao D, Vialpando CG, et al. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. J Exp.Med. 2002;195:211–220. doi: 10.1084/jem.200116116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caucheteux SM, Kanellopoulos-Langevin C, Ojcius DM. At the innate frontiers between mother and fetus: linking abortion with complement activation. Immunity. 2003;18:169–172. doi: 10.1016/s1074-7613(03)00028-1. [DOI] [PubMed] [Google Scholar]
- 40.Girardi G, Salmon JB. The role of complement in pregnancy and fetal loss. Autoimmunity. 2003;36:19–26. doi: 10.1080/0891693031000067322. [DOI] [PubMed] [Google Scholar]
- 41.Girardi G. Complement inhibition keeps mothers calm and avoids fetal rejection. Immunol.Invest. 2008;37:645–659. doi: 10.1080/08820130802191615. [DOI] [PubMed] [Google Scholar]
- 42.Richani K, Soto E, Romero R, Espinoza J, Chaiworapongsa T, Nien JK, Edwin S, Kim YM, Hong JS, Goncalves L, et al. Preeclampsia and SGA differ in the maternal plasma complememt split products profile. J Soc Gynecol Investig. 2005;12(Suppl 2):148A. [Google Scholar]
- 43.Lynch AM, Murphy JR, Byers T, Gibbs RS, Neville MC, Giclas PC, Salmon JE, Holers VM. Alternative complement pathway activation fragment Bb in early pregnancy as a predictor of preeclampsia. Am J Obstet Gynecol. 2008;198:385–389. doi: 10.1016/j.ajog.2007.10.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soto E, Richani K, Romero R, Espinoza J, Chaiworapongsa T, Nien JK, Edwin S, Kim YM, Hong JS, Goncalves L, et al. Increased concentration of the complement split product C5a in acute pyelonephritis during pregnancy. J Matern.Fetal Neonatal Med. 2005;17:247–252. doi: 10.1080/14767050500072805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richani K, Romero R, Soto E, Espinoza J, Nien JK, Chaiworapongsa T, Refuerzo J, Blackwell S, Edwin SS, Santolaya-Forgas J, et al. Unexplained intrauterine fetal death is accompanied by activation of complement. J Perinat.Med. 2005;33:296–305. doi: 10.1515/JPM.2005.052. [DOI] [PubMed] [Google Scholar]
- 46.Elimian A, Figueroa R, Canterino J, Verma U, guero-Rosenfeld M, Tejani N. Amniotic fluid complement C3 as a marker of intra-amniotic infection. Obstet Gynecol. 1998;92:72–76. doi: 10.1016/s0029-7844(98)00123-9. [DOI] [PubMed] [Google Scholar]
- 47.Lynch AM, Gibbs RS, Murphy JR, Byers T, Neville MC, Giclas PC, Salmon JE, Van Hecke TM, Holers VM. Complement activation fragment Bb in early pregnancy and spontaneous preterm birth. Am J Obstet Gynecol. 2008;199:354–358. doi: 10.1016/j.ajog.2008.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alexander GR, Kogan M, Martin J, Papiernik E. What are the fetal growth patterns of singletons, twins, and triplets in the United States? Clin.Obstet.Gynecol. 1998;41:114–125. doi: 10.1097/00003081-199803000-00017. [DOI] [PubMed] [Google Scholar]
- 49.Gonzalez RP, Gomez RM, Castro RS, Nien JK, Merino PO, Etchegaray AB, Carstens MR, Medina LH, Viviani PG, Rojas IT. [A national birth weight distribution curve according to gestational age in Chile from 1993 to 2000] Rev.Med.Chil. 2004;132:1155–1165. doi: 10.4067/s0034-98872004001000001. [DOI] [PubMed] [Google Scholar]
- 50.Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, Ghezzi F, Berry SM, Qureshi F, Jacques SM, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern.Fetal Neonatal Med. 2002;11:18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 51.Lesavre PH, Hugli TE, Esser AF, Muller-Eberhard HJ. The alternative pathway C3/C5 convertase: chemical basis of factor B activation. J Immunol. 1979;123:529–534. [PubMed] [Google Scholar]
- 52.Kerr MA. Limited proteolysis of complement components C2 and factor B. Structural analogy and limited sequence homology. Biochem.J. 1979;183:615–622. doi: 10.1042/bj1830615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niemann MA, Volanakis JE, Mole JE. Amino-terminal sequence of human factor B of the alternative complement pathway and its cleavage fragments, Ba and Bb. Biochemistry. 1980;19:1576–1583. doi: 10.1021/bi00549a007. [DOI] [PubMed] [Google Scholar]
- 54.Gotze O, Muller-Eberhard HJ. The C3-activator system: an alternate pathway of complement activation. J Exp.Med. 1971;134:90s–108s. [PubMed] [Google Scholar]
- 55.Nakagawa K, Sakiyama H, Tsuchida T, Yamaguchi K, Toyoguchi T, Masuda R, Moriya H. Complement C1s activation in degenerating articular cartilage of rheumatoid arthritis patients: immunohistochemical studies with an active form specific antibody. Ann.Rheum.Dis. 1999;58:175–181. doi: 10.1136/ard.58.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neumann E, Barnum SR, Tarner IH, Echols J, Fleck M, Judex M, Kullmann F, Mountz JD, Scholmerich J, Gay S, et al. Local production of complement proteins in rheumatoid arthritis synovium. Arthritis Rheum. 2002;46:934–945. doi: 10.1002/art.10183. [DOI] [PubMed] [Google Scholar]
- 57.Walport MJ. Complement and systemic lupus erythematosus. Arthritis Res. 2002;4(Suppl 3):S279–93. doi: 10.1186/ar586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.del Zoppo GJ. In stroke, complement will get you nowhere. Nat.Med. 1999;5:995–996. doi: 10.1038/12431. [DOI] [PubMed] [Google Scholar]
- 59.Huang J, Kim LJ, Mealey R, Marsh HC, Jr., Zhang Y, Tenner AJ, Connolly ES, Jr., Pinsky DJ. Neuronal protection in stroke by an sLex-glycosylated complement inhibitory protein. Science. 1999;285:595–599. doi: 10.1126/science.285.5427.595. [DOI] [PubMed] [Google Scholar]
- 60.Endresen IM, Relling GB, Tonder O, Myking O, Walther BT, Ursin H. Brief uncontrollable stress and psychological parameters influence human plasma concentrations of IgM and complement component C3. Behav.Med. 1991;17:167–176. doi: 10.1080/08964289.1991.9935168. [DOI] [PubMed] [Google Scholar]
- 61.Maes M, Hendriks D, Van GA, Demedts P, Wauters A, Neels H, Janca A, Scharpe S. Effects of psychological stress on serum immunoglobulin, complement and acute phase protein concentrations in normal volunteers. Psychoneuroendocrinology. 1997;22:397–409. doi: 10.1016/s0306-4530(97)00042-5. [DOI] [PubMed] [Google Scholar]
- 62.Burns VE, Edwards KM, Ring C, Drayson M, Carroll D. Complement cascade activation after an acute psychological stress task. Psychosom.Med. 2008;70:387–396. doi: 10.1097/PSY.0b013e31816ded22. [DOI] [PubMed] [Google Scholar]
- 63.Haeger M, Bengtson A, Karlsson K, Heideman M. Complement activation and anaphylatoxin (C3a and C5a) formation in preeclampsia and by amniotic fluid. Obstet Gynecol. 1989;73:551–556. [PubMed] [Google Scholar]
- 64.Kohler PF. Maturation of the human complement system. I. Onset time and sites of fetal C1q, C4, C3, and C5 synthesis. J Clin.Invest. 1973;52:671–677. doi: 10.1172/JCI107228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Holmes CH, Simpson KL. Complement and pregnancy: new insights into the immunobiology of the fetomaternal relationship. Baillieres Clin.Obstet.Gynaecol. 1992;6:439–460. doi: 10.1016/s0950-3552(05)80005-7. [DOI] [PubMed] [Google Scholar]
- 66.Goldberg M, Luknar-Gabor N, Keidar R, Katz Y. Synthesis of complement proteins in the human chorion is differentially regulated by cytokines. Mol.Immunol. 2007;44:1737–1742. doi: 10.1016/j.molimm.2006.07.298. [DOI] [PubMed] [Google Scholar]
- 67.Holmes CH, Simpson KL, Okada H, Okada N, Wainwright SD, Purcell DF, Houlihan JM. Complement regulatory proteins at the feto-maternal interface during human placental development: distribution of CD59 by comparison with membrane cofactor protein (CD46) and decay accelerating factor (CD55) Eur J Immunol. 1992;22:1579–1585. doi: 10.1002/eji.1830220635. [DOI] [PubMed] [Google Scholar]
- 68.Vanderpuye OA, Labarrere CA, McIntyre JA. Expression of CD59, a human complement system regulatory protein, in extraembryonic membranes. Int.Arch.Allergy Immunol. 1993;101:376–384. doi: 10.1159/000236480. [DOI] [PubMed] [Google Scholar]
- 69.Richani K, Soto E, Romero R, Han Y, Pineles B, Kim YM, CE, Yoon BH, Kusanovic J, Kim CJ. Decreased mRNA expression of complement regulatory proteins in chorioamnionitis. Am.J.Obstet.Gynecol. 2006;195(Suppl 1):S71. [Google Scholar]
- 70.Adinolfi M, Gardner B, Wood CB. Ontogenesis of two components of human complement: beta1E and beta1C-1A globulins. Nature. 1968;219:189–191. doi: 10.1038/219189a0. [DOI] [PubMed] [Google Scholar]
- 71.Colten HR, Gordon JM, Borsos T, Rapp HJ. Synthesis of the first component of human complement in vitro. J.Exp.Med. 1968;128:595–604. doi: 10.1084/jem.128.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gitlin D, Biasucci A. Development of gamma G, gamma A, gamma M, beta IC-beta IA, C 1 esterase inhibitor, ceruloplasmin, transferrin, hemopexin, haptoglobin, fibrinogen, plasminogen, alpha 1-antitrypsin, orosomucoid, beta-lipoprotein, alpha 2-macroglobulin, and prealbumin in the human conceptus. J.Clin.Invest. 1969;48:1433–1446. doi: 10.1172/JCI106109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Colten HR. Ontogeny of the human complement system: in vitro biosynthesis of individual complement components by fetal tissues. J.Clin.Invest. 1972;51:725–730. doi: 10.1172/JCI106866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fireman P, Zuchowski DA, Taylor PM. Development of human complement system. J.Immunol. 1969;103:25–31. [PubMed] [Google Scholar]
- 75.Ballow M, Fang F, Good RA, Day NK. Developmental aspects of complement components in the newborn. The presence of complement components and C3 proactivator (properdin factor B) in human colostrum. Clin.Exp.Immunol. 1974;18:257–266. [PMC free article] [PubMed] [Google Scholar]
- 76.Miyano A, Nakayama M, Fujita T, Kitajima H, Imai S, Shimizu A. Complement activation in fetuses: assessment by the levels of complement components and split products in cord blood. Diagn.Clin.Immunol. 1987;5:86–90. [PubMed] [Google Scholar]
- 77.Stabile I, Nicolaides KH, Bach A, Teisner B, Rodeck C, Westergaard JG, Grudzinskas JG. Complement factors in fetal and maternal blood and amniotic fluid during the second trimester of normal pregnancy. Br.J Obstet Gynaecol. 1988;95:281–285. doi: 10.1111/j.1471-0528.1988.tb06870.x. [DOI] [PubMed] [Google Scholar]
- 78.Zilow G, Zilow EP, Burger R, Linderkamp O. Complement activation in newborn infants with early onset infection. Pediatr.Res. 1993;34:199–203. doi: 10.1203/00006450-199308000-00020. [DOI] [PubMed] [Google Scholar]
- 79.Enskog A, Bengtsson A, Bengtson JP, Heideman M, Andreasson S, Larsson L. Complement anaphylatoxin C3a and C5a formation in premature children with respiratory distress. Eur.J.Pediatr. 1996;155:41–45. doi: 10.1007/BF02115625. [DOI] [PubMed] [Google Scholar]
- 80.Sonntag J, Brandenburg U, Polzehl D, Strauss E, Vogel M, Dudenhausen JW, Obladen M. Complement system in healthy term newborns: reference values in umbilical cord blood. Pediatr.Dev.Pathol. 1998;1:131–135. doi: 10.1007/s100249900016. [DOI] [PubMed] [Google Scholar]
- 81.Sharma A, Prabhakar P, Sharma DP, Jayasinghe RG. Immunoglobulin and C3 levels in normal human amniotic fluid. West Indian Med.J. 1983;32:140–146. [PubMed] [Google Scholar]
- 82.Huffaker J, Witkin SS, Cutler L, Druzin ML, Ledger WJ. Total complement activity in maternal sera, amniotic fluids and cord sera in women with premature labor, premature rupture of membranes or chorioamnionitis. Surg.Gynecol.Obstet. 1989;168:397–401. [PubMed] [Google Scholar]
- 83.Soto E, Romero R, Richani K, Chaiworapongsa T, Yoon BH, Nien JK, Edwin S, Kusanovic JP, Espinoza J. Evidence for complement activation in premature labor associated with intra-amniotic infection. Am J Obstet Gynecol. 2006;195(Suppl 1):S74. [Google Scholar]
- 84.Hogasen AK, Overlie I, Hansen TW, Abrahamsen TG, Finne PH, Hogasen K. The analysis of the complement activation product SC5 b-9 is applicable in neonates in spite of their profound C9 deficiency. J Perinat.Med. 2000;28:39–48. doi: 10.1515/JPM.2000.006. [DOI] [PubMed] [Google Scholar]
- 85.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin.Fetal Neonatal Med. 2006;11:317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Junqueira LC, Zugaib M, Montes GS, Toledo OM, Krisztan RM, Shigihara KM. Morphologic and histochemical evidence for the occurrence of collagenolysis and for the role of neutrophilic polymorphonuclear leukocytes during cervical dilation. Am J Obstet Gynecol. 1980;138:273–281. doi: 10.1016/0002-9378(80)90248-3. [DOI] [PubMed] [Google Scholar]
- 87.Liggins GC. Cervical ripening as an inflammatory reaction. In: Ellwood DA, Anderson AMB, editors. The Cervix in Pregnancy and Labor, Clinical and Biochemical Investigation. Churchill Livingstone; Edinburgh: 1981. pp. 1–9. [Google Scholar]
- 88.Osman I, Young A, Ledingham MA, Thomson AJ, Jordan F, Greer IA, Norman JE. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol.Hum.Reprod. 2003;9:41–45. doi: 10.1093/molehr/gag001. [DOI] [PubMed] [Google Scholar]
- 89.Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, Greer IA, Norman JE. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum.Reprod. 1999;14:229–236. [PubMed] [Google Scholar]
- 90.Keski-Nisula L, Aalto ML, Katila ML, Kirkinen P. Intrauterine inflammation at term: a histopathologic study. Hum.Pathol. 2000;31:841–846. doi: 10.1053/hupa.2000.8449. [DOI] [PubMed] [Google Scholar]
- 91.Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition--a review. Placenta. 2003;24(Suppl A):S33–46. doi: 10.1053/plac.2002.0948. S33-S46. [DOI] [PubMed] [Google Scholar]
- 92.Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, Durum SK. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989;160:1117–1123. doi: 10.1016/0002-9378(89)90172-5. [DOI] [PubMed] [Google Scholar]
- 93.Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, Williams J. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol. 1992;166:1576–1587. doi: 10.1016/0002-9378(92)91636-o. [DOI] [PubMed] [Google Scholar]
- 94.Opsjln SL, Wathen NC, Tingulstad S, Wiedswang G, Sundan A, Waage A, Austgulen R. Tumor necrosis factor, interleukin-1, and interleukin-6 in normal human pregnancy. Am J Obstet Gynecol. 1993;169:397–404. doi: 10.1016/0002-9378(93)90096-2. [DOI] [PubMed] [Google Scholar]
- 95.Saito S, Kasahara T, Kato Y, Ishihara Y, Ichijo M. Elevation of amniotic fluid interleukin 6 (IL-6), IL-8 and granulocyte colony stimulating factor (G-CSF) in term and preterm parturition. Cytokine. 1993;5:81–88. doi: 10.1016/1043-4666(93)90027-3. [DOI] [PubMed] [Google Scholar]
- 96.Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol. 1991;165:813–820. doi: 10.1016/0002-9378(91)90422-n. [DOI] [PubMed] [Google Scholar]
- 97.Osmers RG, Blaser J, Kuhn W, Tschesche H. Interleukin-8 synthesis and the onset of labor. Obstet Gynecol. 1995;86:223–229. doi: 10.1016/0029-7844(95)93704-4. [DOI] [PubMed] [Google Scholar]
- 98.Cohen J, Ghezzi F, Romero R, Ghidini A, Mazor M, Tolosa JE, Goncalves LF, Gomez R. GRO alpha in the fetomaternal and amniotic fluid compartments during pregnancy and parturition. Am J Reprod.Immunol. 1996;35:23–29. doi: 10.1111/j.1600-0897.1996.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 99.Esplin MS, Romero R, Chaiworapongsa T, Kim YM, Edwin S, Gomez R, Gonzalez R, Adashi EY. Amniotic fluid levels of immunoreactive monocyte chemotactic protein-1 increase during term parturition. J Matern.Fetal Neonatal Med. 2003;14:51–56. doi: 10.1080/jmf.14.1.51.56. [DOI] [PubMed] [Google Scholar]
- 100.Esplin MS, Peltier MR, Hamblin S, Smith S, Fausett MB, Dildy GA, Branch DW, Silver RM, Adashi EY. Monocyte chemotactic protein-1 expression is increased in human gestational tissues during term and preterm labor. Placenta. 2005;26:661–671. doi: 10.1016/j.placenta.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 101.Shynlova O, Tsui P, Dorogin A, Lye SJ. Monocyte chemoattractant protein-1 (CCL-2) integrates mechanical and endocrine signals that mediate term and preterm labor. J Immunol. 2008;181:1470–1479. doi: 10.4049/jimmunol.181.2.1470. [DOI] [PubMed] [Google Scholar]
- 102.Gotze O, Bianco C, Cohn ZA. The induction of macrophage spreading by factor B of the properdin system. J Exp.Med. 1979;149:372–386. doi: 10.1084/jem.149.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bianco C, Gotze O, Cohn ZA. Regulation of macrophage migration by products of the complement system. Proc.Natl.Acad.Sci.U.S.A. 1979;76:888–891. doi: 10.1073/pnas.76.2.888. [DOI] [PMC free article] [PubMed] [Google Scholar]