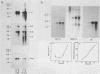
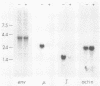
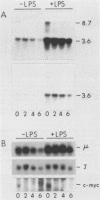
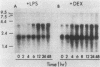
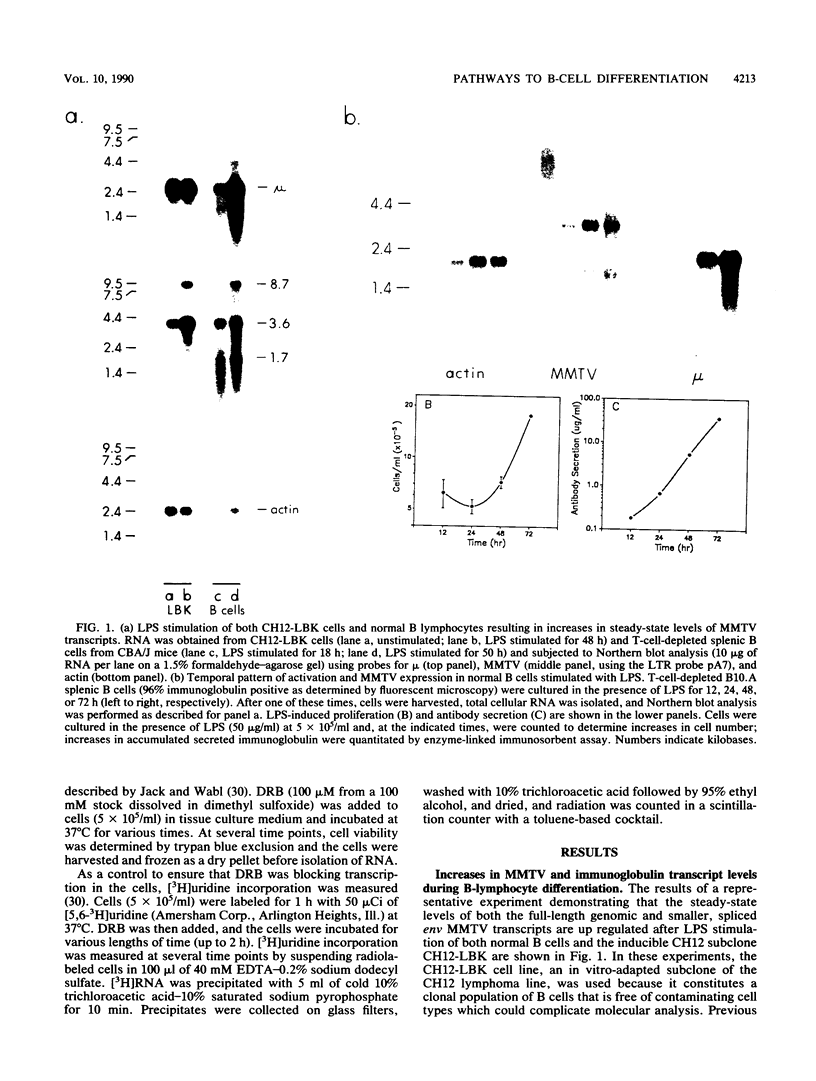
Abstract
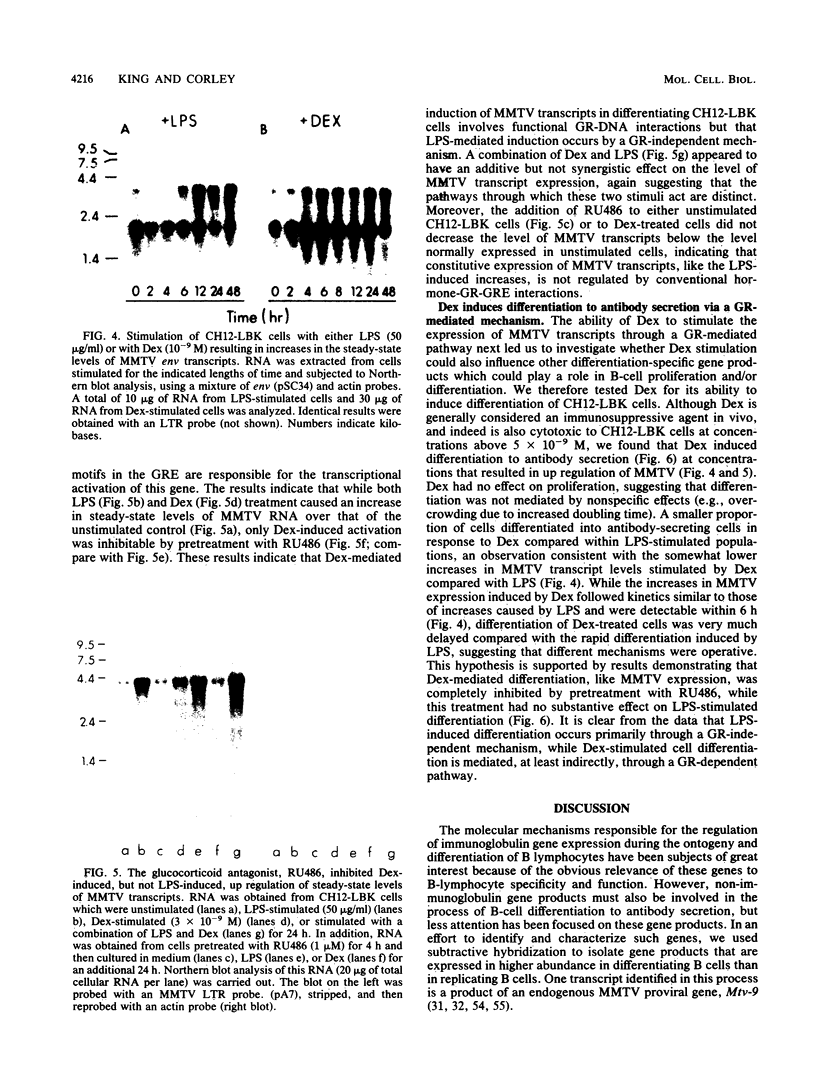
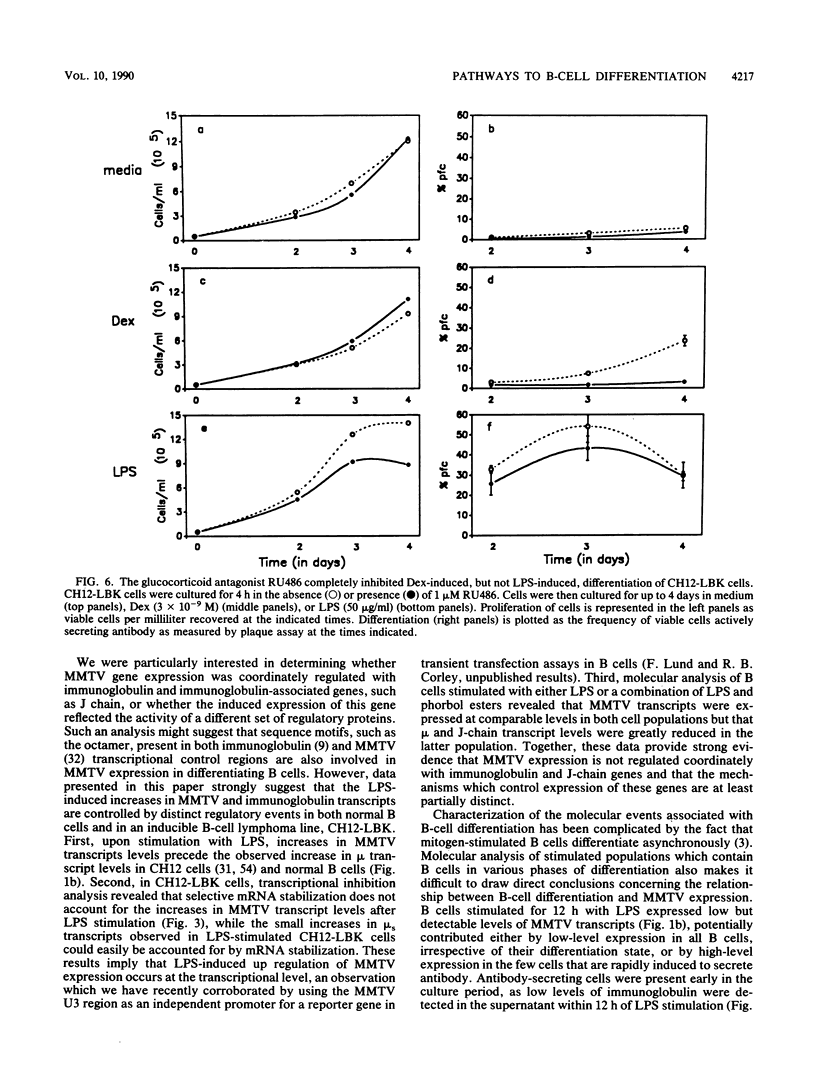
Endogenous mouse mammary tumor virus (MMTV) proviral transcripts are up regulated during the normal course of B-lymphocyte differentiation. We report here that the regulatory mechanisms which lead to increased levels of MMTV transcripts in differentiating, lipopolysaccharide (LPS)-stimulated normal B cells and in the inducible B-cell lymphoma line CH12 are at least partially distinct from those controlling increases in immunoglobulin and J-chain gene expression. In studies designed to characterize the stimulatory pathways leading to MMTV expression in CH12 cells, we found that stimulation with either LPS or dexamethasone (Dex), a transcriptional activator of MMTV genes, induced not only MMTV expression but also differentiation to antibody secretion. Only Dex-induced and not LPS-induced MMTV expression and differentiation were inhibited by the glucocorticoid antagonist RU486, demonstrating that Dex and LPS stimulate B cells by distinct molecular pathways. Therefore, in B cells, MMTV expression can be regulated via either the conventional hormone receptor-dependent pathway or a hormone receptor-independent pathway. Furthermore, these results suggest that steroid stimulation of B cells can lead to alterations in the expression of other results suggest that steroid stimulation of B cells can lead to alterations in the expression of other steroid-responsive genes that can become involved in the process of B-cell differentiation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberto B. P., Callahan L. F., Pincus T. Evidence that retrovirus expression in mouse spleen cells results from B cell differentiation. J Immunol. 1982 Dec;129(6):2768–2772. [PubMed] [Google Scholar]
- Andersson J., Coutinho A., Lernhardt W., Melchers F. Clonal growth and maturation to immunoglobulin secretion in vitro of every growth-inducible B lymphocyte. Cell. 1977 Jan;10(1):27–34. doi: 10.1016/0092-8674(77)90136-2. [DOI] [PubMed] [Google Scholar]
- Ansar Ahmed S., Dauphinée M. J., Montoya A. I., Talal N. Estrogen induces normal murine CD5+ B cells to produce autoantibodies. J Immunol. 1989 Apr 15;142(8):2647–2653. [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Berthois Y., Katzenellenbogen J. A., Katzenellenbogen B. S. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois S., Pfahl M., Baulieu E. E. DNA binding properties of glucocorticosteroid receptors bound to the steroid antagonist RU-486. EMBO J. 1984 Apr;3(4):751–755. doi: 10.1002/j.1460-2075.1984.tb01879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calame K., Eaton S. Transcriptional controlling elements in the immunoglobulin and T cell receptor loci. Adv Immunol. 1988;43:235–275. doi: 10.1016/s0065-2776(08)60367-3. [DOI] [PubMed] [Google Scholar]
- Carr J. K., Traina-Dorge V. L., Cohen J. C. Mouse mammary tumor virus gene expression regulated in trans by Lps locus. Virology. 1985 Nov;147(1):210–213. doi: 10.1016/0042-6822(85)90241-7. [DOI] [PubMed] [Google Scholar]
- Chen-Bettecken U., Wecker E., Schimpl A. Transcriptional control of mu- and kappa-gene expression in resting and bacterial lipopolysaccharide-activated normal B cells. Immunobiology. 1987 Mar;174(2):162–176. doi: 10.1016/s0171-2985(87)80036-0. [DOI] [PubMed] [Google Scholar]
- Conger J. D., Pike B. L., Nossal G. J. Clonal analysis of the anti-DNA repertoire of murine B lymphocytes. Proc Natl Acad Sci U S A. 1987 May;84(9):2931–2935. doi: 10.1073/pnas.84.9.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corley R. B., LoCascio N. J., Ovnic M., Arnold L. W., Pillai P. S., Scott D. W., Haughton G. Three classes of signalling molecules on B-cell membranes. J Cell Biochem. 1985;27(1):1–12. doi: 10.1002/jcb.240270102. [DOI] [PubMed] [Google Scholar]
- Corley R. B., LoCascio N. J., Ovnic M., Haughton G. Two separate functions of class II (Ia) molecules: T-cell stimulation and B-cell excitation. Proc Natl Acad Sci U S A. 1985 Jan;82(2):516–520. doi: 10.1073/pnas.82.2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani C., Blanchard J. M., Piechaczyk M., El Sabouty S., Marty L., Jeanteur P. Extreme instability of myc mRNA in normal and transformed human cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7046–7050. doi: 10.1073/pnas.81.22.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFranco A. L., Gold M. R., Jakway J. P. B-lymphocyte signal transduction in response to anti-immunoglobulin and bacterial lipopolysaccharide. Immunol Rev. 1987 Feb;95:161–176. doi: 10.1111/j.1600-065x.1987.tb00504.x. [DOI] [PubMed] [Google Scholar]
- Dennis G., June C. H., Mizuguchi J., Ohara J., Witherspoon K., Finkelman F. D., McMillan V., Mond J. J. Glucocorticoids suppress calcium mobilization and phospholipid hydrolysis in anti-Ig antibody-stimulated B cells. J Immunol. 1987 Oct 15;139(8):2516–2523. [PubMed] [Google Scholar]
- Dudley J. P., Arfsten A., Hsu C. L., Kozak C., Risser R. Molecular cloning and characterization of mouse mammary tumor proviruses from a T-cell lymphoma. J Virol. 1986 Jan;57(1):385–388. doi: 10.1128/jvi.57.1.385-388.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emilie D., Crevon M. C., Auffredou M. T., Galanaud P. Glucocorticosteroid-dependent synergy between interleukin 1 and interleukin 6 for human B lymphocyte differentiation. Eur J Immunol. 1988 Dec;18(12):2043–2047. doi: 10.1002/eji.1830181226. [DOI] [PubMed] [Google Scholar]
- Ewert D. L., Vainio O., Halpern M. S. Increased endogenous retroviral gene expression is a consequence of lymphocyte activation. J Immunol. 1983 Dec;131(6):3036–3041. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gagne D., Pons M., Philibert D. RU 38486: a potent antiglucocorticoid in vitro and in vivo. J Steroid Biochem. 1985 Sep;23(3):247–251. doi: 10.1016/0022-4731(85)90401-7. [DOI] [PubMed] [Google Scholar]
- Grayson J., Dooley N. J., Koski I. R., Blaese R. M. Immunoglobulin production induced in vitro by glucocorticoid hormones: T cell-dependent stimulation of immunoglobulin production without B cell proliferation in cultures of human peripheral blood lymphocytes. J Clin Invest. 1981 Dec;68(6):1539–1547. doi: 10.1172/JCI110408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberger J. S., Phillips S. M., Stephenson J. R., Aaronson S. A. Induction of mouse type-C RNA virus by lipopolysaccharide. J Immunol. 1975 Jul;115(1):317–320. [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern M. S., Ewert D. L., Flores L. J., Lin K. Y., England J. M. Endogenous retroviral envelope antigen in plasma cells. J Immunol. 1981 Aug;127(2):698–702. [PubMed] [Google Scholar]
- Hsu C. L., Fabritius C., Dudley J. Mouse mammary tumor virus proviruses in T-cell lymphomas lack a negative regulatory element in the long terminal repeat. J Virol. 1988 Dec;62(12):4644–4652. doi: 10.1128/jvi.62.12.4644-4652.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Högbom E., Mårtensson I. L., Leanderson T. Regulation of immunoglobulin transcription rates and mRNA processing in proliferating normal B lymphocytes by activators of protein kinase C. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9135–9139. doi: 10.1073/pnas.84.24.9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäck H. M., Wabl M. Immunoglobulin mRNA stability varies during B lymphocyte differentiation. EMBO J. 1988 Apr;7(4):1041–1046. doi: 10.1002/j.1460-2075.1988.tb02911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King L. B., Corley R. B. Characterization of a presecretory phase in B-cell differentiation. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2814–2818. doi: 10.1073/pnas.86.8.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King L. B., Lund F. E., White D. A., Sharma S., Corley R. B. Molecular events in B lymphocyte differentiation. Inducible expression of the endogenous mouse mammary tumor proviral gene, Mtv-9. J Immunol. 1990 Apr 15;144(8):3218–3227. [PubMed] [Google Scholar]
- Klaus G. G., Bijsterbosch M. K., O'Garra A., Harnett M. M., Rigley K. P. Receptor signalling and crosstalk in B lymphocytes. Immunol Rev. 1987 Oct;99:19–38. doi: 10.1111/j.1600-065x.1987.tb01170.x. [DOI] [PubMed] [Google Scholar]
- Kozak C., Peters G., Pauley R., Morris V., Michalides R., Dudley J., Green M., Davisson M., Prakash O., Vaidya A. A standardized nomenclature for endogenous mouse mammary tumor viruses. J Virol. 1987 May;61(5):1651–1654. doi: 10.1128/jvi.61.5.1651-1654.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhara T., Haughton G., Corley R. B. Antigen-nonspecific T cell-derived factors in B cell activation: differences in the requirements for interleukin 2 in responses of unprimed and primed B cells. Eur J Immunol. 1985 Aug;15(8):787–793. doi: 10.1002/eji.1830150809. [DOI] [PubMed] [Google Scholar]
- Kwon B. S., Weissman S. M. Mouse mammary tumor virus-related sequences in mouse lymphocytes are inducible by 12-O-tetradecanoyl phorbol-13-acetate. J Virol. 1984 Dec;52(3):1000–1004. doi: 10.1128/jvi.52.3.1000-1004.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamson G., Koshland M. E. Changes in J chain and mu chain RNA expression as a function of B cell differentiation. J Exp Med. 1984 Sep 1;160(3):877–892. doi: 10.1084/jem.160.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoCascio N. J., Haughton G., Arnold L. W., Corley R. B. Role of cell surface immunoglobulin in B-lymphocyte activation. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2466–2469. doi: 10.1073/pnas.81.8.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez D. M., Charyulu V., Paul R. D. B cell subsets in spleens of BALB/c mice: identification and isolation of MMTV-expressing and MMTV-responding subpopulations. J Immunol. 1985 Jan;134(1):603–607. [PubMed] [Google Scholar]
- Luster M. I., Germolec D. R., Clark G., Wiegand G., Rosenthal G. J. Selective effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin and corticosteroid on in vitro lymphocyte maturation. J Immunol. 1988 Feb 1;140(3):928–935. [PubMed] [Google Scholar]
- Matthyssens G., Rabbitts T. H. The sequence at the 3' terminus of mouse immunoglobulin secreted mu chain messenger RNA determined from cloned cDNA. Nucleic Acids Res. 1980 Feb 25;8(4):703–713. [PMC free article] [PubMed] [Google Scholar]
- McCairns E., Fahey D., Muscat G. E., Murray M., Rowe P. B. Changes in levels of actin and tubulin mRNAs upon the lectin activation of lymphocytes. Mol Cell Biol. 1984 Sep;4(9):1754–1760. doi: 10.1128/mcb.4.9.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan V. M., Dennis G. J., Glimcher L. H., Finkelman F. D., Mond J. J. Corticosteroid induction of Ig+Ia- B cells in vitro is mediated via interaction with the glucocorticoid cytoplasmic receptor. J Immunol. 1988 Apr 15;140(8):2549–2555. [PubMed] [Google Scholar]
- Morley K. L., Toohey M. G., Peterson D. O. Transcriptional repression of a hormone-responsive promoter. Nucleic Acids Res. 1987 Sep 11;15(17):6973–6989. doi: 10.1093/nar/15.17.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi K., Cohen D. I., Blackman M., Nielsen E., Ohara J., Hamaoka T., Koshland M. E., Paul W. E. Ig RNA expression in normal B cells stimulated with anti-IgM antibody and T cell-derived growth and differentiation factors. J Exp Med. 1984 Dec 1;160(6):1736–1751. doi: 10.1084/jem.160.6.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa K., Armelin H. A., Sato G. Control of ovarian cell growth in culture by serum and pituitary factors. Proc Natl Acad Sci U S A. 1975 Feb;72(2):483–487. doi: 10.1073/pnas.72.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovnic M., Corley R. B. Quantitation of cell surface molecules on a differentiating, Ly-1+ B cell lymphoma. J Immunol. 1987 May 1;138(9):3075–3082. [PubMed] [Google Scholar]
- Plaut M. Lymphocyte hormone receptors. Annu Rev Immunol. 1987;5:621–669. doi: 10.1146/annurev.iy.05.040187.003201. [DOI] [PubMed] [Google Scholar]
- Racevskis J., Beyer H. Amplification of mouse mammary tumor virus genomes in non-mammary tumor cells. J Virol. 1989 Jan;63(1):456–459. doi: 10.1128/jvi.63.1.456-459.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Bishop J. M., Varmus H. E. Glucocorticoid-stimulated accumulation of mouse mammary tumor virus RNA: increased rate of synthesis of viral RNA. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2879–2883. doi: 10.1073/pnas.74.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbele N. R., Van Oudenaren A., Hooijkaas H., Benner R. The effect of corticosteroids upon murine B cells in vivo and in vitro as determined in the LPS-culture system. Immunology. 1987 Oct;62(2):285–290. [PMC free article] [PubMed] [Google Scholar]
- Salmons B., Günzburg W. H. Current perspectives in the biology of mouse mammary tumour virus. Virus Res. 1987 Aug;8(2):81–102. doi: 10.1016/0168-1702(87)90022-0. [DOI] [PubMed] [Google Scholar]
- Schumann G., Moroni C. Mitogen induction of murine C-type viruses. I. Analysis of lymphoid cell subpopulations. J Immunol. 1976 Apr;116(4):1145–1150. [PubMed] [Google Scholar]
- Sharma S., King L. B., Corley R. B. Molecular events during B lymphocyte differentiation. Induction of endogenous mouse mammary tumor proviral envelope transcripts after B cell stimulation. J Immunol. 1988 Oct 1;141(7):2510–2518. [PubMed] [Google Scholar]
- Tax A., Ewert D., Manson L. A. An antigen cross-reactive with gp52 of mammary tumor virus is expressed on a B cell subpopulation of mice. J Immunol. 1983 May;130(5):2368–2371. [PubMed] [Google Scholar]
- Traina-Dorge V. L., Carr J. K., Bailey-Wilson J. E., Elston R. C., Taylor B. A., Cohen J. C. Cellular genes in the mouse regulate in trans the expression of endogenous mouse mammary tumor viruses. Genetics. 1985 Nov;111(3):597–615. doi: 10.1093/genetics/111.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucker D. S., Yamamoto K. R. Early events in the stimulation of mammary tumor virus RNA synthesis by glucocorticoids. Novel assays of transcription rates. J Biol Chem. 1984 Jun 25;259(12):7416–7420. [PubMed] [Google Scholar]
- Wecker E., Schimpl A., Hünig T. Expression of MuLV GP71-like antigen in normal mouse spleen cells induced by antigenic stimulation. Nature. 1977 Oct 13;269(5629):598–600. doi: 10.1038/269598a0. [DOI] [PubMed] [Google Scholar]
- Wellinger R. J., Garcia M., Vessaz A., Diggelmann H. Exogenous mouse mammary tumor virus proviral DNA isolated from a kidney adenocarcinoma cell line contains alterations in the U3 region of the long terminal repeat. J Virol. 1986 Oct;60(1):1–11. doi: 10.1128/jvi.60.1.1-11.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler D. A., Butel J. S., Medina D., Cardiff R. D., Hager G. L. Transcription of mouse mammary tumor virus: identification of a candidate mRNA for the long terminal repeat gene product. J Virol. 1983 Apr;46(1):42–49. doi: 10.1128/jvi.46.1.42-49.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]
- Young H. A., Shih T. Y., Scolnick E. M., Parks W. P. Steroid induction of mouse mammary tumor virus: effect upon synthesis and degradation of viral RNA. J Virol. 1977 Jan;21(1):139–146. doi: 10.1128/jvi.21.1.139-146.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandomeni R., Bunick D., Ackerman S., Mittleman B., Weinmann R. Mechanism of action of DRB. III. Effect on specific in vitro initiation of transcription. J Mol Biol. 1983 Jul 5;167(3):561–574. doi: 10.1016/s0022-2836(83)80098-9. [DOI] [PubMed] [Google Scholar]
- van Ooyen A. J., Michalides R. J., Nusse R. Structural analysis of a 1.7-kilobase mouse mammary tumor virus-specific RNA. J Virol. 1983 May;46(2):362–370. doi: 10.1128/jvi.46.2.362-370.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]