Abstract
Based on evidence that patients with type 2 diabetes (T2DM), obese insulin-resistant individuals, and lean insulin-resistant offspring of parents with T2DM have ∼30% less mitochondria in their muscles than lean control subjects, it appears to be widely accepted that mitochondrial “deficiency” is responsible for insulin resistance. The proposed mechanism for this effect is an impaired ability to oxidize fat, resulting in lipid accumulation in muscle. The purpose of this counterpoint article is to review the evidence against the mitochondrial deficiency concept. This evidence includes the findings that 1) development of insulin resistance in laboratory rodents fed high-fat diets occurs despite a concomitant increase in muscle mitochondria; 2) mitochondrial deficiency severe enough to impair fat oxidation in resting muscle causes an increase, not a decrease, in insulin action; and 3) most of the studies comparing fat oxidation in insulin-sensitive and insulin-resistant individuals have shown that fat oxidation is higher in T2DM patients and obese insulin-resistant individuals than in insulin-sensitive control subjects. In conclusion, it seems clear, based on this evidence, that the 30% reduction in muscle content of mitochondria in patients with T2DM is not responsible for insulin resistance.
In a series of studies, Kelley and colleagues (1–4) measured the levels of activity of mitochondrial marker enzymes in skeletal muscles from patients with T2DM or obese insulin-resistant individuals and found that they were lower than in normal, healthy individuals of the same age. In these studies, the enzymes that were measured were citrate synthase (1,3), cytochrome oxidase (2,3), NADH2 oxidoreductase (1,3), carnitine palmitoyl transferase (2), and succinate dehydrogenase (4). The activities of these enzymes were 20–40% lower in the diabetic patients than in normal control subjects. The mitochondria in diabetic muscle were also smaller than normal (1). They referred to these findings as “mitochondrial dysfunction,” although no measurements of function were made; and although this phenomenon is sometimes referred to as mitochondrial dysfunction, studies in which mitochondrial function was evaluated found that the remaining mitochondria in diabetic muscle have normal function (5–7). There is evidence suggesting that accumulation of lipids in muscle plays a role in mediating insulin resistance, and Kelley and colleagues hypothesized that the reduction in muscle mitochondria in T2DM impairs the ability of muscle to oxidize fatty acids, resulting in muscle lipid accumulation and, as a result, insulin resistance.
These articles were followed by publication of a number of studies showing that patients with T2DM, obese insulin-resistant individuals, and lean insulin-resistant offspring of diabetic parents have a ∼30% reduction in muscle mitochondrial content (8–11), suggesting that the only abnormality is a ∼30% decrease in size or number of mitochondria. The mechanism responsible for the reduction in mitochondrial content of diabetic skeletal muscle is not known. One possibility that has been suggested is that the decrease in mitochondria is due to impaired insulin action (12). A second is that it is mediated by oxidative stress (13). A third is that it is due to low physical activity. Another possibility is that it is genetically determined, i.e., that it is a genetic trait that is linked to the genetic predisposition to develop insulin resistance and T2DM. This third possibility is suggested by the findings that reversal of T2DM by weight loss does not result in normalization of muscle mitochondrial content (14), and that some lean offspring of diabetic parents are insulin resistant and have a reduced muscle content of mitochondria (10).
As a result of the many studies showing that T2DM patients, insulin-resistant obese people, and insulin resistant offspring of diabetic parents generally have a ∼30% reduction in muscle mitochondria, the hypothesis that insulin resistance is mediated by a deficiency of muscle mitochondria appears to have gained considerable acceptance (15,16). Assuming that mitochondrial deficiency causes insulin resistance because these two phenomena occur together, i.e., with this, therefore, because of this, is a logical fallacy. Correlation provides no information regarding causality. This raises the question, is there any scientific evidence in support of the hypothesis? As reviewed in the three following sections, the answer is no, the available experimental evidence shows that a decrease in muscle mitochondria does not cause insulin resistance.
DOES A REDUCTION IN MITOCHONDRIA PRECEDE THE DEVELOPMENT OF INSULIN RESISTANCE?
If mitochondrial deficiency causes insulin resistance it must occur before the onset of the insulin resistance. It does not seem possible to answer the question of whether or not mitochondrial deficiency precedes insulin resistance in humans, because T2DM patients and obese insulin resistant individuals are insulin resistant for many years before they are diagnosed. Therefore, available evidence comes from studies on laboratory rodents, which develop muscle insulin resistance within a few weeks after being started on a high-fat diet. If the high-fat diet is continued, the rodents become obese and develop the rodent equivalent of the visceral obesity/metabolic syndrome and/or T2DM (17,18). In a number of early studies, high-fat diets were found to induce an increase in the levels of mitochondrial marker enzymes, such as betahydroxybutyrate dehydrogenase and citrate synthase in muscle (19,20). More recently, it was found that feeding rats high-fat diets (21,22) or intermittently increasing plasma fatty acids to high levels (23) induces an increase in mitochondrial biogenesis in skeletal muscle. This is evidenced by increases in mitochondrial enzyme proteins, in the capacity to oxidize fatty acids, and in mitochondrial DNA copy number. This increase in mitochondria occurs concomitantly with development of muscle insulin resistance. The increase in mitochondria appears to be an early, transient event that is lost as the insulin resistance and obesity progress (24).
The fatty acid-induced increase in muscle mitochondria appears to be mediated by the nuclear receptor peroxisome proliferator–activated receptor-β (PPAR-β; also referred to as PPAR-δ). PPAR-β is activated by fatty acids, which are its natural, endogenous ligands. PPAR-β is a transcription factor for the genes encoding the enzymes of the mitochondrial fatty acid oxidation pathway. Previous studies had shown that overexpression or activation of PPAR-β in muscle induces an increase in mitochondrial biogenesis (25,26). The investigators who performed these studies concluded that the increase in mitochondrial biogenesis was mediated directly by PPAR-β, because overexpression of PPAR-β in muscle did not result in an increase in peroxisome proliferator–activated receptor γ coactivator-1α (PGC-1α) mRNA. However, this did not seem possible, because PPAR-β regulates expression of only a subset of mitochondrial proteins and, therefore, cannot mediate mitochondrial biogenesis, which requires the coordinated activation of numerous transcription factors, a process that is mediated by the transcription coactivator PGC-1α (27). It seemed probable, therefore, that an increase in PPAR-β induces an increase in mitochondria by mediating a posttranscriptional increase in PGC-1α protein. This turned out to be the case, as both overexpression of PPAR-β or activation of PPAR-β by raising plasma fatty acids resulted in an increase in PGC-1α (22).
In light of these findings, it seems clear that mitochondrial deficiency is not necessary for the development of insulin resistance and that, at least in the fat-fed rat model, insulin resistance develops despite an increase in muscle mitochondria. That insulin resistance is also not due to mitochondrial deficiency in humans is evidenced by the finding that insulin-resistant Asian Indians with T2DM have a muscle mitochondrial capacity for oxidative metabolism similar to that of nondiabetic Indians and higher than that of healthy North Americans of Northern European ancestry (28).
DOES A REDUCTION IN MUSCLE MITOCHONDRIA CAUSE INSULIN RESISTANCE?
Various transgenic models have been used to test the hypothesis that a deficiency of mitochondria causes muscle insulin resistance. Wredenberg et al. (29) studied transgenic mice in which mitochondrial transcription factor A (Tfam) was knocked out in skeletal muscle. Tfam is a transcription factor that mediates transcription of genes encoded in the mitochondrial genome, which includes a number of key mitochondrial respiratory chain proteins. There was a progressive, severe deterioration of respiratory chain function in the muscles of the Tfam knockout mice. However, rather than causing insulin resistance, the marked decrease in mitochondrial oxidative capacity resulted in increases in insulin action and glucose tolerance, as well as enhanced basal and insulin-stimulated 2-deoxyglucose uptake in isolated muscles. In another study, Pospisilik et al. (30) examined the effect of deletion of mitochondrial apoptosis-inducing factor, which is required for maintenance of a functional mitochondrial respiratory chain. Skeletal muscle apoptosis-inducing factor knockout mice had reduced levels of mitochondrial respiratory chain protein complexes I and IV with an associated decrease in the capacity for substrate oxidation. This reduction in mitochondrial respiratory capacity resulted in improved insulin sensitivity and glucose tolerance, and enhanced insulin-stimulated muscle glucose transport activity. In a third study, Kelly and colleagues (31) generated mice that had combined deficiency of PGC-1α and PGC-1β in skeletal muscle. These totally PGC-1α–deficient mice had markedly reduced levels of mitochondrial enzymes and ∼90% decrease in the capacity of muscle to oxidize fat (31). This severe mitochondrial deficiency resulted in an improvement in glucose tolerance.
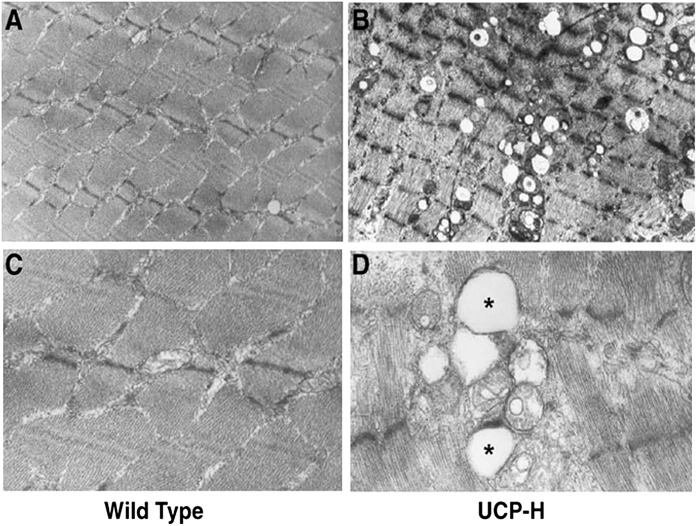
In an earlier study by our group, we generated transgenic mice that expressed uncoupling protein-1 (UCP-1) in their skeletal muscles (32). The original purpose of this study was to determine the effect of a modest reduction of steady-state ATP concentration on mitochondrial biogenesis. However, the amount of UCP-1 expressed in the muscles of the transgenic mice was too high and resulted in disruption of mitochondrial structure (Fig. 1), and caused large decreases in the levels of key mitochondrial enzyme proteins. The mechanism by which a high content of UCP-1 damages muscle mitochondria is not known. Despite the severe reduction in mitochondrial function, and the associated accumulation of large amounts of intramuscular lipid (Fig. 1), there were large increases in basal and insulin-stimulated muscle glucose transport activity (32).
FIG 1.
Electron microscopic images of muscle from wild-type mice (A and C) and from muscles of mice with severely disrupted mitochondria caused by high overexpression of uncoupling protein 1 (UCP-H) (B, low magnification; D, high magnification). *Large lipid droplets surrounding abnormal mitochondria. Reprinted with permission from Han et al. (32).
In 2010, Kelley and colleagues (33) published an article in which they concluded that insulin resistance in T2DM is mediated by a deficiency of the electron transport chain (ETC) enzymes with no deficiency of the citrate cycle or fatty acid oxidation pathway enzymes. They postulated that this results in an imbalance between the ETC and the citrate cycle and fatty acid oxidation pathways, and that this imbalance causes insulin resistance. This claim is puzzling, both because their initial reports that T2DM patients have “mitochondrial dysfunction” were largely based on the finding of decreases in citrate synthase (1,3), and carnitine palmityl transferase (2), and because the studies by Pospisilik et al. (30) and Wredenberg et al. (29) had shown that severe ETC enzyme deficiency results in improved insulin action.
To further test the hypothesis that a selective decrease in components of the mitochondrial ETC, that results in an imbalance between the ETC and the fatty acid oxidation pathway and citric acid cycle is responsible for insulin resistance, rats were made severely iron deficient by means of an iron-deficient diet (34). Iron deficiency results in decreases in the iron-containing mitochondrial respiratory chain proteins without affecting the noniron-containing enzymes of the fatty acid oxidation pathway and citrate cycle (35). Some of the iron-deficient rats were fed a high-fat diet. Iron deficiency resulted in large decreases in iron-containing mitochondrial respiratory chain proteins in muscle (34). Citrate synthase and long-chain fatty acyl-CoA dehydrogenase, used as markers for the citrate cycle and fatty acid oxidation pathways, were unaffected by the iron deficiency. Oleate oxidation by muscle homogenates was increased by high-fat feeding and markedly decreased by iron deficiency despite a high-fat diet. The high-fat diet also increased long-chain acyl-CoA dehydrogenase expression. As a result, there was a ∼fivefold increase in the ratio of long-chain acyl-CoA dehydrogenase to cytochrome c, indicating a large imbalance between the fatty acid oxidation pathway and respiratory chain. The high-fat diet resulted in severe muscle insulin resistance. The iron deficiency completely protected against the high-fat diet–induced insulin resistance, and also resulted in a higher insulin-stimulated muscle glucose transport activity compared with animals in the low-fat diet, normal iron group (34).
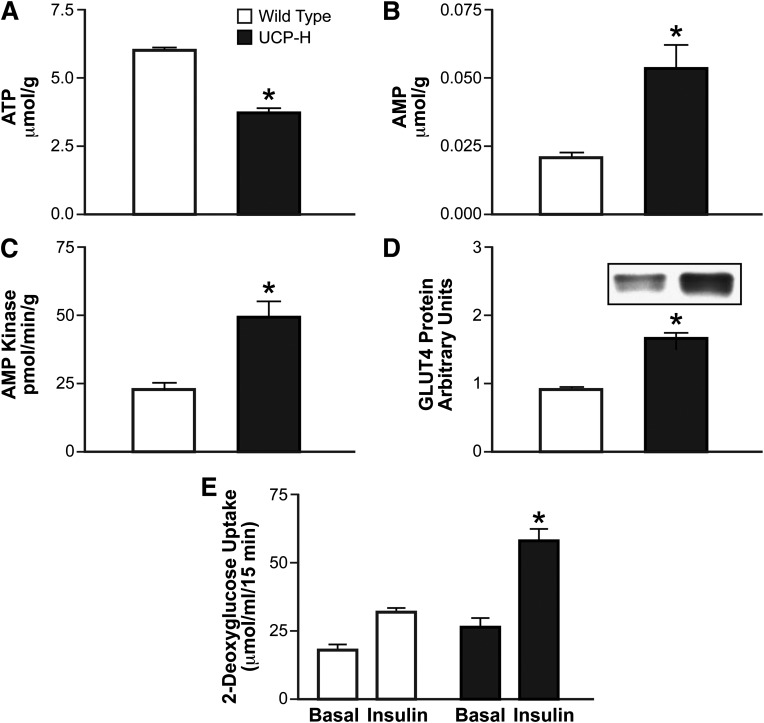
These studies on rodents showing that mitochondrial deficiency/dysfunction causes improvements in insulin action and glucose tolerance raise the question, why are T2DM patients insulin resistant despite a reduction in muscle mitochondria? The answer to this question relates to the severity of the mitochondrial deficiency. The hypothesis that was tested in the studies on rodents reviewed above was, does mitochondrial deficiency/dysfunction severe enough to limit fat oxidation in resting muscle cause insulin resistance? To test this hypothesis, an extremely severe reduction in functional mitochondria is necessary. The rate of substrate oxidation is determined by the demand for energy and is regulated by the rate of ATP breakdown/ADP production. The energy requirement of resting muscle is determined by housekeeping functions such as maintenance of electrochemical gradients and protein synthesis and is very low relative to the maximum capacity for substrate oxidation. Therefore, a very severe reduction in functional mitochondria is necessary in order to result in limitation of resting substrate oxidation in resting muscle. In the various animal models in which mitochondrial deficiency/dysfunction resulted in improved insulin action and glucose tolerance, substrate oxidative capacity was so low that it resulted in a decrease in steady-state ATP concentration and an increase in AMP (Fig. 2). The increase in AMP resulted in activation of AMP kinase, which directly stimulates glucose transport and also induces an increase in the GLUT4 (Fig. 2), thus inducing an increase in insulin responsiveness (29,32,34). The increase in AMP kinase activity explains the improvement in insulin action, glucose tolerance, and muscle glucose uptake in rodent muscle with severe mitochondrial deficiency/dysfunction.
FIG. 2.
ATP (A), AMP (B), AMP kinase activity (C), GLUT4 protein (D), and basal and insulin-stimulated 2-deoxyglucose transport rates (E) in muscle of control mice and mice with high overexpression of uncoupling protein 1 (UCP-H) and severe disruption of mitochondria. *P < 0.05 vs. wild type. Adapted from Han et al. (32).
In contrast to the rodent models of mitochondrial deficiency/dysfunction and improved insulin action in which the decrease in muscle respiratory capacity is so severe they can’t exercise, T2DM patients, despite a low VO2max of ∼26 mL O2 ⋅ kg−1 ⋅ min−1 (6), are able to exercise sufficiently vigorously to increase their whole body rates of O2 uptake/substrate oxidation ∼8–9-fold. Since the muscle mass involved in the exercise makes up ∼20% of body weight, this means muscles of T2DM patients have a sufficiently high respiratory capacity to increase substrate oxidation ∼40-fold. In light of this huge reserve capacity, it is clear that a ∼30% lower than normal content of mitochondria is not sufficient to limit resting substrate oxidation in muscles of T2DM patients or, therefore, to cause a decrease in ATP or an increase in AMP and AMP kinase activity.
It is clear from the many studies showing that mitochondrial deficiency/dysfunction severe enough to limit fat oxidation in resting muscle increases insulin responsiveness, that mitochondrial deficiency plays no role in mediating insulin resistance.
DOES MITOCHONDRIAL DEFICIENCY DECREASE FAT OXIDATION IN T2DM PATIENTS AND INSULIN-RESISTANT OBESE INDIVIDUALS?
Studies by Kelley and colleagues have supported the concept that fat oxidation is reduced in insulin-resistant individuals. In one of these studies, fat oxidation was decreased and glucose oxidation was increased in the resting, postabsorptive state in 11 T2DM patients compared with 9 nondiabetic control subjects (36). In another, fat oxidation by muscle was lower in 40 obese men than in lean control subjects (2). However, in a subsequent study on obese men, Kelley and colleagues in collaboration with Wolfe (37) found that obese men derive a greater proportion of energy from fat oxidation than lean men (43 vs. 31%) during mild exercise. In another study, Kelley and colleagues found that fat oxidation was not reduced in obese insulin-resistant individuals or T2DM patients, with fat providing 37% of total energy in the lean control subjects, 47% of energy in the obese insulin-resistant individuals, and 43% in the T2DM patients (38). There have also been reports that plasma fatty acid oxidation was reduced in insulin-resistant individuals, giving the impression that fat oxidation was impaired (39,40). However, in these studies, nonplasma fatty acid oxidation (probably from intramuscular fat) was increased, so that total fat oxidation was not significantly different between the control and insulin-resistant groups. For example, in a study by Thyfault et al. (40), total fat oxidation averaged 0.92 ± 0.08 μmol ⋅ kg−1 ⋅ min−1 in the control subjects and 1.07 ± 0.18 μmol ⋅ kg−1 ⋅ min in the obese group. Most of the studies by other investigators comparing obese insulin-resistant individuals and/or patients with T2DM have, however, found that insulin resistant individuals have increased fat oxidation (7,37,41–47).
A hypothesis that used to be rather widely accepted is that insulin resistance is mediated by increased fat oxidation. This hypothesis is based on the finding by Randle et al. (48) that fat oxidation inhibits glucose uptake and metabolism and is, therefore, referred to as the “Randle glucose fatty acid cycle.” Evidence for the Randle effect in insulin resistant humans was provided by DeFronzo and colleagues (41), who found that fat oxidation is increased in patients with T2DM and in obese insulin-resistant individuals compared with normal control subjects. Most of the other studies comparing fat oxidation in normal controls with T2DM patients and/or obese insulin-resistant individuals have also shown that fat oxidation is increased in insulin-resistant obese individuals and patients with T2DM (7,37,41–47).
In conclusion, the answer to the three questions addressed in this counterpoint article is clearly no. Rather than a decrease in mitochondria, an increase in mitochondrial biogenesis occurs in skeletal muscle concomitantly with the development of muscle insulin resistance (21,22). Rather than causing insulin resistance, a decrease in mitochondria or disruption of mitochondrial function sufficiently severe to limit fat oxidation in resting muscle results in increases in basal and insulin stimulated glucose transport into skeletal muscle and an improvement glucose tolerance (29–32,34). Rather than a reduction in fat oxidation in muscle, the majority of studies show that fat oxidation is increased in obese, insulin resistant individuals and patients with T2DM (7,37,41–47).
ACKNOWLEDGMENTS
The author’s research is supported by National Institutes of Health Research Grant AG00425 from the National Institute on Aging and a grant from the Longer Life Foundation.
No potential conflicts of interest relevant to this article were reported.
The author is grateful to Victoria Reckamp for help with preparation of the manuscript.
Footnotes
REFERENCES
- 1.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 2002;51:2944–2950 [DOI] [PubMed] [Google Scholar]
- 2.Kelley DE, Goodpaster B, Wing RR, Simoneau J-A. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol 1999;277:E1130–E1141 [DOI] [PubMed] [Google Scholar]
- 3.Simoneau J-A, Veerkamp JH, Turcotte LP, Kelley DE. Markers of capacity to utilize fatty acids in human skeletal muscle: relation to insulin resistance and obesity and effects of weight loss. FASEB J 1999;13:2051–2060 [DOI] [PubMed] [Google Scholar]
- 4.He J, Watkins S, Kelley DE. Skeletal muscle lipid content and oxidative enzyme activity in relation to muscle fiber type in type 2 diabetes and obesity. Diabetes 2001;50:817–823 [DOI] [PubMed] [Google Scholar]
- 5.Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsøe R, Dela F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia 2007;50:790–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsen S, Ara I, Rabøl R, et al. Are substrate use during exercise and mitochondrial respiratory capacity decreased in arm and leg muscle in type 2 diabetes? Diabetologia 2009;52:1400–1408 [DOI] [PubMed] [Google Scholar]
- 7.Ara I, Larsen S, Stallknecht B, et al. Normal mitochondrial function and increased fat oxidation capacity in leg and arm muscles in obese humans. Int J Obes (Lond) 2011;35:99–108 [DOI] [PubMed] [Google Scholar]
- 8.Mootha VK, Lindgren CM, Eriksson K-F, et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 2003;34:267–273 [DOI] [PubMed] [Google Scholar]
- 9.Patti M-E, Butte AJ, Crunkhorn S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 2003;100:8466–8471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morino K, Petersen KF, Dufour S, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest 2005;115:3587–3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Befroy DE, Petersen KF, Dufour S, et al. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes 2007;56:1376–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asmann YW, Stump CS, Short KR, et al. Skeletal muscle mitochondrial functions, mitochondrial DNA copy numbers, and gene transcript profiles in type 2 diabetic and nondiabetic subjects at equal levels of low or high insulin and euglycemia. Diabetes 2006;55:3309–3319 [DOI] [PubMed] [Google Scholar]
- 13.Bonnard C, Durand A, Peyrol S, et al. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest 2008;118:789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berggren JR, Boyle KE, Chapman WH, Houmard JA. Skeletal muscle lipid oxidation and obesity: influence of weight loss and exercise. Am J Physiol Endocrinol Metab 2008;294:E726–E732 [DOI] [PubMed] [Google Scholar]
- 15.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science 2005;307:384–387 [DOI] [PubMed] [Google Scholar]
- 16.Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 2006;55(Suppl. 2):S9–S15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han D-H, Hansen PA, Host HH, Holloszy JO. Insulin resistance of muscle glucose transport in rats fed a high-fat diet: a reevaluation. Diabetes 1997;46:1761–1767 [DOI] [PubMed] [Google Scholar]
- 18.Storlien LH, James DE, Burleigh KM, Chisholm DJ, Kraegen EW. Fat feeding causes widespread in vivo insulin resistance, decreased energy expenditure, and obesity in rats. Am J Physiol 1986;251:E576–E583 [DOI] [PubMed] [Google Scholar]
- 19.Miller WC, Bryce GR, Conlee RK. Adaptations to a high-fat diet that increase exercise endurance in male rats. J Appl Physiol 1984;56:78–83 [DOI] [PubMed] [Google Scholar]
- 20.McAinch AJ, Lee J-S, Bruce CR, Tunstall RJ, Hawley JA, Cameron-Smith D. Dietary regulation of fat oxidative gene expression in different skeletal muscle fiber types. Obes Res 2003;11:1471–1479 [DOI] [PubMed] [Google Scholar]
- 21.Turner N, Bruce CR, Beale SM, et al. Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes 2007;56:2085–2092 [DOI] [PubMed] [Google Scholar]
- 22.Hancock CR, Han D-H, Chen M, et al. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci USA 2008;105:7815–7820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Roves PM, Huss JM, Han D-H, et al. Raising plasma fatty acid concentration induces increased biogenesis of mitochondria in skeletal muscle. Proc Natl Acad Sci USA 2007;104:10709–10713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sreekumar R, Unnikrishnan J, Fu A, et al. Impact of high-fat diet and antioxidant supplement on mitochondrial functions and gene transcripts in rat muscle. Am J Physiol Endocrinol Metab 2002;282:E1055–E1061 [DOI] [PubMed] [Google Scholar]
- 25.Wang Y-X, Zhang C-L, Yu RT, et al. Regulation of muscle fiber type and running endurance by PPARδ. PLoS Biol 2004;2:e294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luquet S, Lopez-Soriano J, Holst D, et al. Peroxisome proliferator-activated receptor δ controls muscle development and oxidative capability. FASEB J 2003;17:2299–2301 [DOI] [PubMed] [Google Scholar]
- 27.Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev 2006;27:728–735 [DOI] [PubMed] [Google Scholar]
- 28.Nair KS, Bigelow ML, Asmann YW, et al. Asian Indians have enhanced skeletal muscle mitochondrial capacity to produce ATP in association with severe insulin resistance. Diabetes 2008;57:1166–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wredenberg A, Freyer C, Sandström ME, et al. Respiratory chain dysfunction in skeletal muscle does not cause insulin resistance. Biochem Biophys Res Commun 2006;350:202–207 [DOI] [PubMed] [Google Scholar]
- 30.Pospisilik JA, Knauf C, Joza N, et al. Targeted deletion of AIF decreases mitochondrial oxidative phosphorylation and protects from obesity and diabetes. Cell 2007;131:476–491 [DOI] [PubMed] [Google Scholar]
- 31.Zechner C, Lai L, Zechner JF, et al. Total skeletal muscle PGC-1 deficiency uncouples mitochondrial derangements from fiber type determination and insulin sensitivity. Cell Metab 2010;12:633–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han D-H, Nolte LA, Ju J-S, Coleman T, Holloszy JO, Semenkovich CF. UCP-mediated energy depletion in skeletal muscle increases glucose transport despite lipid accumulation and mitochondrial dysfunction. Am J Physiol Endocrinol Metab 2004;286:E347–E353 [DOI] [PubMed] [Google Scholar]
- 33.Ritov VB, Menshikova EV, Azuma K, et al. Deficiency of electron transport chain in human skeletal muscle mitochondria in type 2 diabetes mellitus and obesity. Am J Physiol Endocrinol Metab 2010;298:E49–E58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han D-H, Hancock CR, Jung SR, Higashida K, Kim SH, Holloszy JO. Deficiency of the mitochondrial electron transport chain in muscle does not cause insulin resistance. PLoS ONE 2011;6:e19739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cartier LJ, Ohira Y, Chen M, Cuddihee RW, Holloszy JO. Perturbation of mitochondrial composition in muscle by iron deficiency. Implications regarding mitochondrial biogenesis. J Biol Chem 1986;261:13827–13832 [PubMed] [Google Scholar]
- 36.Kelley DE, Simoneau JA. Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest 1994;94:2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodpaster BH, Wolfe RR, Kelley DE. Effects of obesity on substrate utilization during exercise. Obes Res 2002;10:575–584 [DOI] [PubMed] [Google Scholar]
- 38.Colberg SR, Hagberg JM, McCole SD, Zmuda JM, Thompson PD, Kelley DE. Utilization of glycogen but not plasma glucose is reduced in individuals with NIDDM during mild-intensity exercise. J Appl Physiol 1996;81:2027–2033 [DOI] [PubMed] [Google Scholar]
- 39.Blaak EE, Wagenmakers AJM, Glatz JFC, et al. Plasma FFA utilization and fatty acid-binding protein content are diminished in type 2 diabetic muscle. Am J Physiol Endocrinol Metab 2000;279:E146–E154 [DOI] [PubMed] [Google Scholar]
- 40.Thyfault JP, Kraus RM, Hickner RC, Howell AW, Wolfe RR, Dohm GL. Impaired plasma fatty acid oxidation in extremely obese women. Am J Physiol Endocrinol Metab 2004;287:E1076–E1081 [DOI] [PubMed] [Google Scholar]
- 41.Felber JP, Ferrannini E, Golay A, et al. Role of lipid oxidation in pathogenesis of insulin resistance of obesity and type II diabetes. Diabetes 1987;36:1341–1350 [DOI] [PubMed] [Google Scholar]
- 42.Groop LC, Saloranta C, Shank M, Bonadonna RC, Ferrannini E, DeFronzo RA. The role of free fatty acid metabolism in the pathogenesis of insulin resistance in obesity and noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1991;72:96–107 [DOI] [PubMed] [Google Scholar]
- 43.Tappy L, Felber JP, Jequier E: Energy and substrate metabolism in obesity and postobese state. Diabetes Care 1991:14:1180–1188 [DOI] [PubMed] [Google Scholar]
- 44.Braun B, Sharoff C, Chipkin SR, Beaudoin F. Effects of insulin resistance on substrate utilization during exercise in overweight women. J Appl Physiol 2004;97:991–997 [DOI] [PubMed] [Google Scholar]
- 45.Boon H, Blaak EE, Saris WHM, Keizer HA, Wagenmakers AJM, van Loon LJC. Substrate source utilisation in long-term diagnosed type 2 diabetes patients at rest, and during exercise and subsequent recovery. Diabetologia 2007;50:103–112 [DOI] [PubMed] [Google Scholar]
- 46.Horowitz JF, Klein S. Oxidation of nonplasma fatty acids during exercise is increased in women with abdominal obesity. J Appl Physiol 2000;89:2276–2282 [DOI] [PubMed] [Google Scholar]
- 47.Golay A, Felber JP, Meyer HU, Curchod B, Maeder E, Jéquier E. Study on lipid metabolism in obesity diabetes. Metabolism 1984;33:111–116 [DOI] [PubMed] [Google Scholar]
- 48.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963;1:785–789 [DOI] [PubMed] [Google Scholar]