Abstract
We designed an experiment to examine the effect of bile acid sequestration with Colesevelam on fasting and postprandial glucose metabolism in type 2 diabetes. To do so, we tested the hypothesis that Colesevelam increases the disposition index (DI), and this increase is associated with increased glucagon-like peptide-1 (GLP-1) concentrations. Thirty-eight subjects on metformin monotherapy were studied using a double-blind, placebo-controlled, parallel-group design. Subjects were studied before and after 12 weeks of Colesevelam or placebo using a labeled triple-tracer mixed meal to measure the rate of meal appearance (Meal Ra), endogenous glucose production (EGP), and glucose disappearance (Rd). Insulin sensitivity and β-cell responsivity indices were estimated using the oral minimal model and then used to calculate DI. Therapy with Colesevelam was associated with a decrease in fasting (7.0 ± 0.2 vs. 6.6 ± 0.2 mmol/L; P = 0.004) and postprandial glucose concentrations (3,145 ± 138 vs. 2,896 ± 127 mmol/6 h; P = 0.01) in the absence of a change in insulin concentrations. Minimal model–derived indices of insulin secretion and action were unchanged. Postprandial GLP-1 concentrations were not altered by Colesevelam. Although EGP and Rd were unchanged, integrated Meal Ra was decreased by Colesevelam (5,191 ± 204 vs. 5,817 ± 204 μmol/kg/6 h; P = 0.04), suggesting increased splanchnic sequestration of meal-derived glucose.
The bile acid sequestrants used therapeutically (for the treatment of hypercholesterolemia) such as cholestyramine, Colesevelam, and colestipol, increase the fecal excretion of bile acids by interrupting their enterohepatic circulation (1). This diminishes their ability to solubilize dietary lipids. The resulting contraction in the bile acid pool diverts hepatic cholesterol to the synthesis of bile acids with accompanying upregulation of hepatic LDL-receptor expression, increasing cholesterol clearance. This in turn results in lowering of LDL-cholesterol concentrations (2). Intriguingly, the use of such compounds in people with type 2 diabetes has been associated with decreases in HbA1c and fasting glucose concentrations (3,4).
These decreases have been observed in randomized, controlled clinical studies with treatment duration ranging from 6 to 26 weeks, in which an absolute decrease of ∼0.2–0.3% in HbA1c was observed (3,5,6). These data have resulted in Colesevelam being approved as a treatment for type 2 diabetes (4). However, the mechanism(s) by which bile acid sequestrants lower glucose concentrations remain uncertain. In vitro, bile acids alter the expression of genes via their interaction with the farnesoid X receptor (FXR), a nuclear receptor that acts as a ligand-activated transcription factor. Bile acids are endogenous ligands of this receptor, and binding results in downregulation of cholesterol-derived synthesis of bile acids as part of the negative-feedback regulation of bile acid synthesis (7). There is some uncertainty as to whether bile acids alter the expression of PEPCK, a rate-limiting step of gluconeogenesis through FXR-dependent mechanisms. However, FXR agonists also alter the expression of hepatocyte nuclear factor-4α, another important regulator of glucose metabolism (8–10). In rodents, bile acid sequestration improves insulin action (11,12), but this has not been readily apparent in humans (13,14).
Alternatively, bile acids may increase insulin secretion by release of glucagon-like peptide-1 (GLP-1) from enteroendocrine cells by signaling through the G-protein–coupled bile acid receptor (formerly TGR5) (15,16). Changes in the amount of fat delivery to the distal ileum will also directly affect enteroendocrine cell secretion (17). Bile acid sequestrants such as Colesevelam increase GLP-1 concentrations in rodents (11), although these effects are apparent in some (13) but not all (14,18) human studies. GLP-1 is a potent insulin secretagogue, and although it may affect insulin action in animals, to date there is no evidence that it significantly alters this parameter in humans (19).
The present experiment sought to determine the mechanism whereby Colesevelam lowers fasting and postprandial glucose concentrations. To do so, fasting and postprandial glucose metabolism were measured using the isotope dilution method. Insulin secretion and action were measured using the oral minimal model in individuals with type 2 diabetes. Subjects were studied at baseline and then following randomization to 12 weeks of Colesevelam or placebo. We report that Colesevelam lowers fasting and postprandial glucose concentrations without detectable alterations in insulin secretion, insulin action, or GLP-1 concentrations. A lowering of meal appearance rate (Meal Ra) suggests increased splanchnic sequestration of meal-derived glucose.
RESEARCH DESIGN AND METHODS
Subjects.
After approval from the Mayo Institutional Review Board, 39 subjects with type 2 diabetes on monotherapy with metformin gave written informed consent to participate in the study. All subjects were in good health, at stable weight, and did not engage in regular vigorous exercise. All subjects were instructed to follow a weight maintenance diet (∼55% carbohydrate, 30% fat, and 15% protein) for the period of study. Body composition was measured using dual-energy X-ray absorptiometry (DPX scanner; Lunar, Madison, WI).
Experimental design.
We used a randomized, double-blind, placebo-controlled parallel group design. After a baseline meal study, subjects received either Colesevelam hydrochloride (three 625-mg tablets twice daily, total dose 3.75 g daily) or identical placebo taken before breakfast and before the evening meal over a 12-week treatment period. Randomization using a randomized allocation sequence to assign sequentially labeled containers of medication was undertaken in the research pharmacy. Participants were examined in the Clinical Research Unit 6 weeks after the baseline study when compliance was assessed by counting remaining medication. Subsequently, subjects underwent a second meal study at the end of the 12-week treatment period. The study staff was unblinded after completion of all studies and compilation of all data. The trial was registered at www.clinicaltrials.gov (identifier: NCT00951899).
Subjects were admitted to the Clinical Research Unit at 1700 h on the evening prior to all of the meal studies. Subsequently, they consumed a standard 10 cal/kg meal (55% carbohydrate, 30% fat, and 15% protein), after which they fasted overnight. At 0630 (−180 min), a forearm vein was cannulated with an 18-gauge needle to allow infusions to be performed. An 18-gauge cannula was inserted retrogradely into a vein of the dorsum of the contralateral hand. This was placed in a heated Plexiglas box maintained at 55°C to allow sampling of arterialized venous blood. A primed (12 mg/kg) continuous (0.12 mg/kg/min) infusion of [6,6-2H2] glucose was initiated. Study medication was administered at 0900 (−30 min) on the second study day. At time 0, subjects consumed a meal consisting of three scrambled eggs, 55 g of Canadian bacon, 18 g of butter, 240 mL of water, and Jell-O containing 70 g of glucose labeled with [1-13C] glucose (4% enrichment). The meal provided ∼560 Kcal (43% carbohydrate, 18% protein, and 40% fat). An infusion of [6-3H] glucose was started at this time, and the infusion rate varied to mimic the anticipated appearance of meal [1-13C] glucose. The rate of infusion of [6,6-2H2] glucose was altered to approximate the anticipated fall in endogenous glucose production (EGP), thereby minimizing changes in specific activity (20,21).
Analytical techniques.
Plasma samples were placed on ice, centrifuged at 4°C, separated, and stored at −20°C until assayed. Glucose concentrations were measured using a glucose oxidase method (Yellow Springs Instruments, Yellow Springs, OH). Plasma insulin was measured using a chemiluminescence assay (Access Assay; Beckman Coulter, Chaska, MN). Plasma glucagon and C-peptide were measured by radioimmunoassay (Linco Research, St. Louis, MO). Collection tubes for GLP-1 had 100 μmol/L of dipeptidyl peptidase-4 inhibitor (Linco Research) added. Total GLP-1 concentrations were measured using a COOH-terminal assay (Linco Research). Plasma [6,6-2H2] glucose and [1-13C] glucose enrichments were measured using gas chromatographic mass spectrometry (Thermoquest, San Jose, CA) to simultaneously monitor the C-1, C-2, and C-3 to C-6 fragments, as described by Beylot et al. (22). In addition, [6-3H] glucose specific activity was measured by liquid scintillation counting following deproteinization and passage over anion and cation exchange columns (21).
Calculations.
The systemic rates of meal appearance (Rameal), EGP, and glucose disappearance (Rd) were calculated using Steele’s model (23). Rameal was calculated by multiplying rate of appearance of [1-13C] glucose (obtained from the infusion rate of [6-3H] glucose and the clamped plasma ratio of [6-3H] glucose and [1-13C] glucose) by the meal enrichment. EGP was calculated from the infusion rate of [6,62H2] glucose and the ratio of [6,62H2] glucose to endogenous glucose concentration. Rd was calculated by subtracting the change in glucose mass from the overall rate of glucose appearance (i.e., Rameal + EGP). Values from −30 to 0 min were averaged and considered as basal. Area above basal (AAB) or area under the curve (AUC) was calculated using the trapezoidal rule.
Net insulin action (SI) was measured using the oral minimal model (24). β-Cell responsivity indices were estimated using the oral C-peptide minimal model (25), incorporating age-associated changes in C-peptide kinetics (26). The model assumes that insulin secretion comprises a static and a dynamic component. The parameter ϕdynamic defines the dynamic responsivity index and is proportional to the rate of increase of glucose concentrations. ϕstatic represents the provision of new insulin to the releasable pool. An index of total β-cell responsivity to glucose (ϕtotal) is derived from the two indices (27). Disposition indices (DIs) were subsequently calculated by multiplying ϕtotal by SI. The effect of insulin on glucose disposal (SI*) was also measured using the previously described labeled oral minimal model (28).
Statistical analysis.
Data in the text are presented as (observed) means ± SEM. The primary analyses compared treatment groups at the post–12-week treatment visit using ANCOVA models incorporating the corresponding baseline study value as a covariate (e.g., fasting or peak concentration, AUC, or AAB). Similarly, treatment effects on SI, ϕtotal, and the DIs were assessed using ANCOVA models after first transforming to log scale to mitigate the skewed distribution of these responses. The corresponding least squares means adjusted for corresponding baseline study values are displayed in the Supplementary Data. In addition, changes in fasting, peak, and integrated hormone concentrations or glucose flux (baseline vs. post–12-week treatment) were assessed separately for each group using a paired t test, or signed-rank test, as warranted. A P value <0.05 was considered statistically significant.
RESULTS
Volunteer characteristics.
Thirty-nine subjects gave written, informed consent to participate in the study. One subject lost intravenous access during the baseline study, and consent was withdrawn. The remaining 38 were randomized to Colesevelam or placebo. Although recruitment was open to subjects treated with dietary and lifestyle modification alone or in combination with metformin, in practice, all subjects were receiving metformin at the time of participation and maintained this therapy throughout the study. There were 19 subjects randomly assigned to each arm (6 males and 13 females in both instances). No important differences were observed in baseline characteristics between the groups (Supplementary Table 1). The mean age was 62.6 ± 1.3 vs. 60.2 ± 1.5 years (Colesevelam vs. placebo). In addition, HbA1c (6.7 ± 0.2 vs. 6.8 ± 0.1%), fasting glucose (7.0 ± 0.3 vs. 7.4 ± 0.4 mmol/L), weight (92 ± 3 vs. 93 ± 3 kg), and BMI (30.8 ± 1.0 vs. 30.4 ± 0.9 kg/m2) were similar at the time of the screening visit, as would be expected from randomization. Weight did not change over the course of the experiment in either arm.
Plasma glucose concentrations in subjects treated with placebo or Colesevelam.
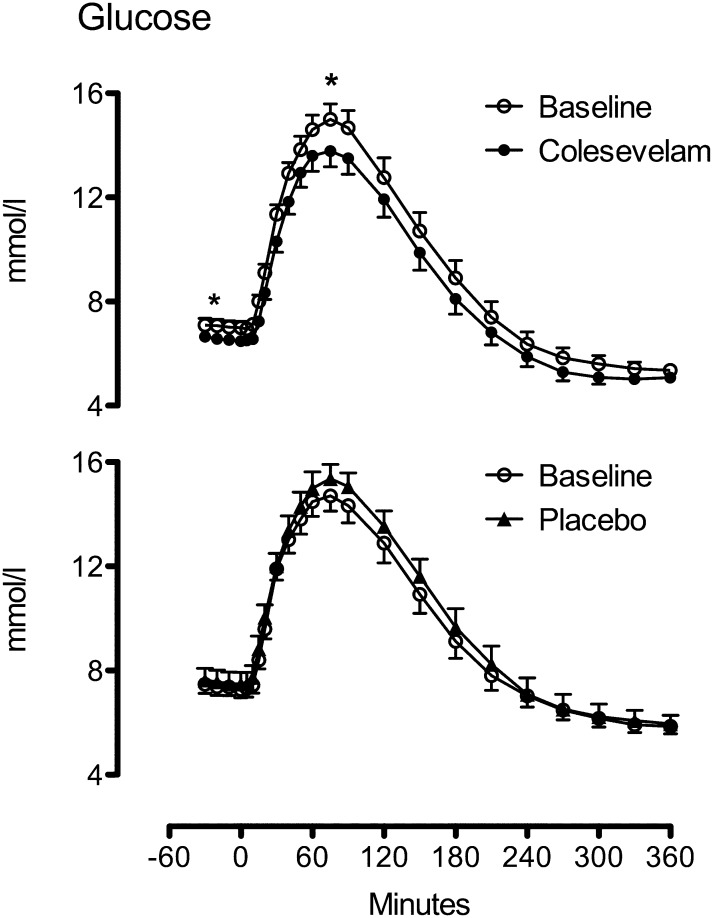
Compared with baseline, administration of Colesevelam resulted in lower fasting glucose (7.0 ± 0.2 vs. 6.6 ± 0.2 mmol/L; P = 0.004), a lower postmeal peak (15.4 ± 0.6 vs. 14.4 ± 0.6 mmol/L; P = 0.011), and AUC (3,286 ± 142 vs. 3,028 ± 130 mmol/6 h; P = 0.003) during the study (Fig. 1, top). In contrast, compared with baseline, administration of placebo did not alter fasting (7.4 ± 0.3 vs. 7.5 ± 0.5 mmol/L; P = 0.34), peak postprandial glucose (15.4 ± 0.6 vs. 15.8 ± 0.6 mmol/L; P = 0.24,) and AUC (3,394 ± 140 vs. 3,523 ± 168 mmol/6 h; P = 0.10) over the study (Fig. 1, bottom). The ANCOVA model indicated treatment group differences at 12 weeks for fasting and peak as well as AUC glucose concentrations (Supplementary Fig. 1 and Supplementary Table 3, P < 0.001). HbA1c was lowered by Colesevelam (6.7 ± 0.1 vs. 6.5 ± 0.2%; P = 0.009) but not by placebo (6.8 ± 0.1 vs. 6.9 ± 0.2%; P = 0.13), and the ANCOVA model indicated group differences at 12 weeks (P = 0.004).
FIG. 1.
Glucose concentrations at baseline (white circles) and after treatment with Colesevelam (black circles, top) or placebo (black triangles, bottom). *P < 0.05.
Insulin, C-peptide, and glucagon in subjects treated with placebo or Colesevelam.
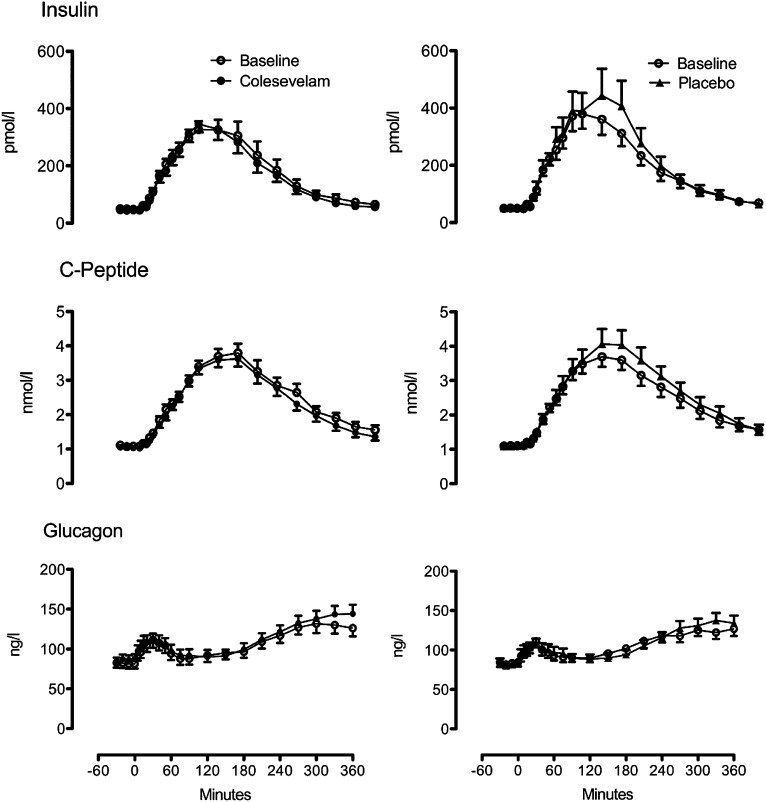
Fasting (48 ± 6 vs. 45 ± 5 pmol/L; P = 0.17) and postprandial insulin concentrations did not differ from baseline after 12 weeks of Colesevelam (Fig. 2, top left). In the placebo arm, fasting (50 ± 6 vs. 51 ± 6 pmol/L; P = 0.84) and peak postprandial insulin (450 ± 55 vs. 519 ± 95 pmol/L; P = 0.24) as well as insulin AAB (54.8 ± 6.8 vs. 63.5 ± 10.3 nmol per 6 h; P = 0.18) did not differ over 12 weeks of treatment (Fig. 2, top right). The ANCOVA comparing Colesevelam vs. placebo groups (week 12 response adjusted for corresponding baseline response) did not demonstrate lower AAB concentrations of insulin (P = 0.07) in the Colesevelam group compared with placebo (Supplementary Fig. 1 and Supplementary Table 3).
FIG. 2.
Insulin (top), C-peptide (middle), and glucagon (bottom) at baseline (white circles) and after treatment with Colesevelam (black circles) or placebo (black triangles).
Fasting C-peptide concentrations did not differ in the fasting state in the Colesevelam and placebo arm (Fig. 2, middle). Postprandial C-peptide concentrations were unchanged by treatment with Colesevelam (AAB 531 ± 37 vs. 504 ± 32 nmol/6 h; P = 0.18). In contrast, over the 12 weeks of placebo treatment, AAB C-peptide concentrations after meal ingestion increased (532 ± 50 vs. 599 ± 60 nmol/6 h; P = 0.03). The between-group differences in AAB concentrations (by ANCOVA) were significant (P < 0.01), with lower concentrations observed in the Colesevelam group compared with placebo (Supplementary Fig. 1 and Supplementary Table 3).
Fasting glucagon concentrations did not differ over the 12 weeks of study in either arm. Peak and integrated glucagon concentrations did not differ from baseline in either arm (Fig. 2, bottom). The ANCOVA results comparing week 12 response adjusted for corresponding baseline responses likewise did not show between-group differences (Supplementary Fig. 1 and Supplementary Table 3).
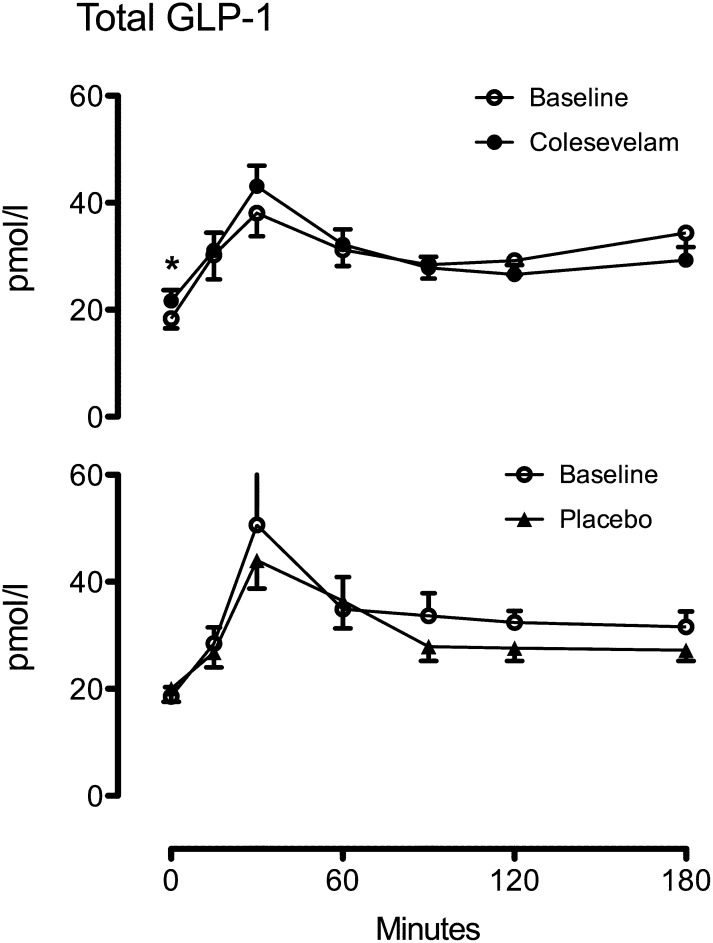
Total GLP-1 concentrations in subjects treated with placebo or Colesevelam.
Fasting total GLP-1 concentrations were increased slightly, but significantly, from baseline (18.3 ± 1.8 vs. 21.9 ± 2.1 pmol/L; P = 0.006) by 12 weeks of treatment with Colesevelam (Fig. 3, top). However, peak (44.0 ± 4.6 vs. 45.5 ± 3.6 pmol/L; P = 0.55) and AUC (5.2 ± 0.4 vs. 5.1 ± 0.3 nmol/3 h; P = 0.86) were unchanged over the period of measurement. In contrast, treatment with placebo did not alter fasting (18.6 ± 1.7 vs. 19.3 ± 2.4 pmol/L; P = 0.30), peak (55.1 ± 9.0 vs. 46.4 ± 4.9 pmol/L; P = 0.16), or AUC total GLP-1 concentrations (5.8 ± 0.6 vs. 5.2 ± 0.4 nmol/3 h; P = 0.23; Fig. 3, bottom). However, the ANCOVA results comparing week 12 response adjusted for corresponding baseline responses did not show between-group differences for fasting concentrations (P = 0.06) or for AUC concentrations of GLP-1 (P = 0.46; Supplementary Fig. 2 and Supplementary Table 3).
FIG. 3.
Total GLP-1 concentrations at baseline (white circles) and after treatment with Colesevelam (black circles, top) or placebo (black triangles, bottom). *P < 0.05.
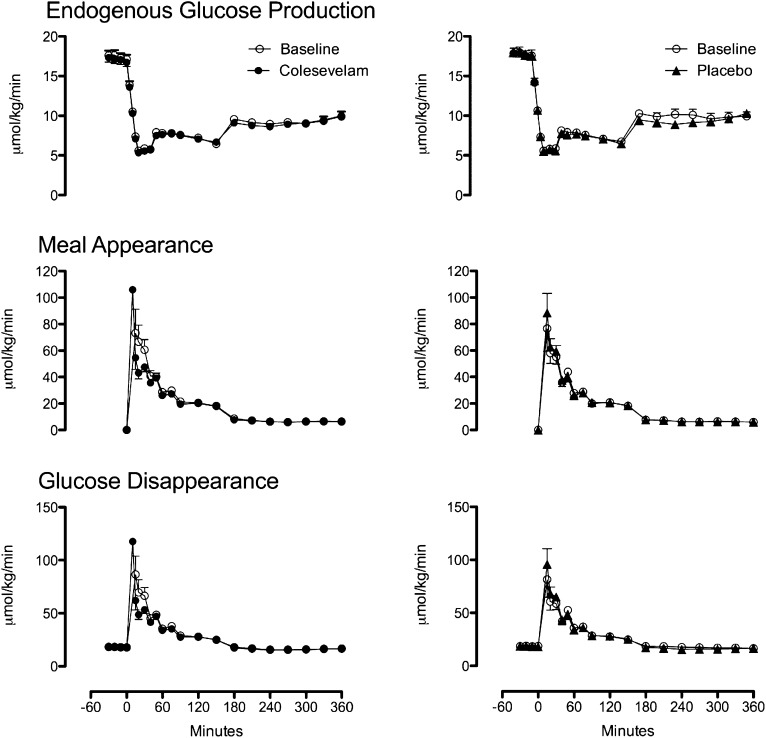
EGP, Meal Ra, and Rd in subjects treated with placebo or Colesevelam.
Fasting EGP (Fig. 4, top) was unchanged by treatment with Colesevelam (17.6 ± 0.6 vs. 17.2 ± 0.6 μmol/kg/min; P = 0.14) and placebo (17.7 ± 0.7 vs. 17.6 ± 0.7 μmol/kg/min; P = 0.81). Similarly, suppression of EGP was unchanged by Colesevelam or placebo. These were confirmed by ANCOVA analysis comparing week 12 response adjusted for corresponding baseline values (Supplementary Fig. 3 and Supplementary Table 3).
FIG. 4.
EGP (top), meal appearance (middle), and glucose disappearance (bottom) at baseline (white circles) and after treatment with Colesevelam (black circles) or placebo (black triangles).
Peak Meal Ra (Fig. 4, middle) did not differ from baseline after treatment with Colesevelam (81 ± 15 vs. 60 ± 6 μmol/kg/min; P = 0.15), or placebo (84 ± 12 vs. 88 ± 12 μmol/kg/min; P = 0.87). Similarly, AUC Meal Ra did not differ from baseline after treatment with Colesevelam (5,941 ± 402 vs. 5,413 ± 289 μmol/6 h; P = 0.20) or placebo (5,504 ± 354 vs. 5,613 ± 354 μmol/6 h; P = 0.48). However, ANCOVA comparing both peak and AUC values for Meal Ra at week 12 adjusted for baseline values demonstrated treatment group differences with lower peak (P = 0.01) and AUC (P = 0.04) values for Colesevelam (Supplementary Fig. 3).
Peak and AAB Rd (Fig. 4, bottom) did not differ from baseline after treatment with Colesevelam or placebo. However, ANCOVA for values at week 12 demonstrated lower peak Rd for Colesevelam (P = 0.01). AAB did not differ (P = 0.30) between groups (Supplementary Fig. 3 and Supplementary Table 3).
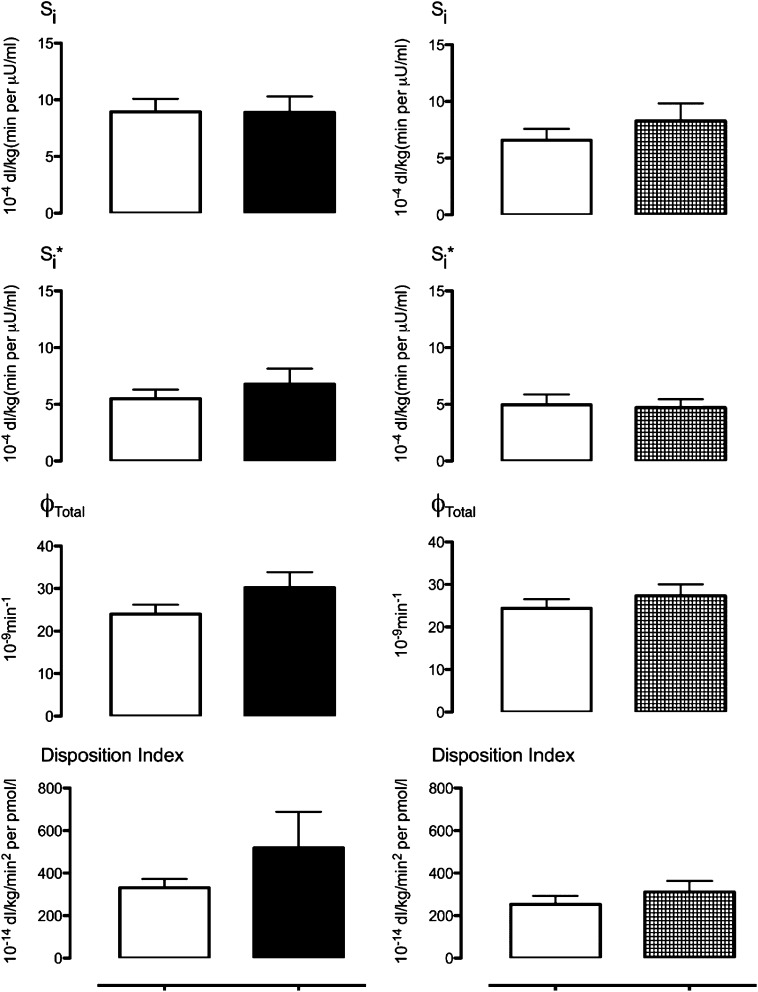
Insulin action, insulin-mediated glucose disposal, β-cell responsivity, and DI.
Insulin action (Fig. 5, top), insulin-mediated glucose disposal (Si*, Fig. 5, top middle), β-cell responsivity (ϕTotal, Fig. 5, bottom middle), and DI (Fig. 5, bottom) did not differ significantly from baseline after 12 weeks of therapy with Colesevelam or placebo. The ANCOVA results comparing each parameter at week 12 response adjusted for the corresponding baseline value did not show between-group differences (Supplementary Fig. 4).
FIG. 5.
Insulin action (Si), hepatic insulin action (Si*), β-cell responsivity (ϕTotal), and DI at baseline (white squares) and after treatment with Colesevelam (left) or placebo (right).
DISCUSSION
Administration of Colesevelam to individuals with type 2 diabetes results in lowering of glucose and, consequently, HbA1c concentrations. This was borne out by the current study. HbA1c, fasting, and postprandial glucose concentrations are lowered in absolute terms to a degree similar to previous observations, despite a lower baseline HbA1c (6.7 ± 0.2%) than that in prior studies. Indeed, the 5.7 and 6.5% decrease in fasting and peak postprandial glucose concentrations that we observed compares favorably with absolute reductions of 3 and 5% in subjects with a baseline HbA1c of 7.9% (3). Insulin concentrations in the fasting state were unchanged from basal values despite lower glucose concentrations after treatment with Colesevelam. Although these data suggest an effect on β-cell responsivity and possibly insulin action, these indices did not change during the study. Fasting total GLP-1 concentrations were slightly increased over 12 weeks of treatment by Colesevelam. However, when adjusted for baseline values, this change did not differ significantly from that observed with placebo treatment.
In the fasting state, glucose appearance is determined by the rate of EGP. Glucose concentrations increase when glucose appearance exceeds Rd and continues to increase until these rates are equal. Regulation of glucose concentrations after meal ingestion is more complex, with glucose entering the systemic circulation from two sources (EGP and the systemic appearance of ingested glucose); however, postprandial glucose concentrations again represent the net balance between the rate of glucose entry and the rate at which glucose leaves the systemic circulation (Rd). Therefore, differences in postprandial glucose concentrations ultimately arise due to differences, alone or in combination, of rates of meal glucose appearance, suppression of EGP, or stimulation of glucose uptake (29,30).
To measure these fluxes, we used the validated triple-tracer approach to minimize changes in both meal and endogenous plasma tracer-to-tracee ratios, thereby allowing simultaneous measurement of meal appearance and EGP (31,32). This approach is virtually model-independent (33). In the current experiment, there was no measurable effect on tracer-based measurement of EGP. However, when accounting for the variance in the systemic appearance of meal-derived glucose, peak and integrated meal appearance was decreased by Colesevelam. Meal appearance is a function of both gastric emptying and splanchnic meal extraction.
Marina et al. (14) previously suggested the possibility of an enteric mechanism to explain the effect of Colesevelam on glucose metabolism after observing that the compound has no effect on intravenous as opposed to oral glucose tolerance. Intriguingly, Colesevelam treatment was associated with cholecystokinin elevation after an oral challenge, leading to speculation that cholecystokinin improved glucose tolerance by delaying gastric emptying or altering EGP (14). Although gastric emptying was not measured in this study, when directly measured, 2 weeks of treatment with Colesevelam delayed gastric emptying slightly, but not significantly, 4 h after meal ingestion (34). Moreover, the time taken to empty 50% of stomach contents did not differ, making gastric emptying less likely to explain the effect on Meal Ra, given that differences were most apparent during the first 2 h of the study.
Another potential explanation is decreased gut absorption of ingested glucose. Since bile acid sequestration decreases endogenous ligand for FXR, and mice lacking FXR exhibit delayed intestinal absorption of ingested glucose, resulting in decreased peak glucose concentrations after an oral challenge (35), this could be one feasible explanation for our findings.
Hyperglycemia and hyperinsulinemia both stimulate splanchnic glucose uptake, and compounds that alter insulin secretion or action will alter this parameter. Although Colesevelam did not alter the measured indices of insulin secretion and action, fasting and postprandial glucose concentrations were lower in the presence of the compound, despite unchanged insulin concentrations. FXR−/− mice exhibit decreased insulin and glucose concentrations during feeding as well as suppression of gluconeogenic genes, again suggesting beneficial effects on insulin action, although there was no effect on hepatic insulin signaling (36).
Direct measurement of insulin action in subjects with type 2 diabetes with a hyperinsulinemic-euglycemic clamp has failed to demonstrate an effect of Colesevelam (37,38). It is possible that these experimental designs missed a small effect on insulin action because the insulin dose used resulted in maximal suppression of hepatic EGP. Therefore, these experiments could not exclude a small effect on hepatic insulin action. In contrast, in this and other studies using model-based indices of insulin action, no effect (on insulin action) was detected (13,14,37,38). Beysen et al. (13) also suggested an effect of bile acid sequestration on glycogenolysis, but the differences observed between treatment and placebo arms were due to an increase in gluconeogenesis during placebo treatment, not necessarily due to an effect of Colesevelam in decreasing glycogenolysis. No effect on EGP (the sum of gluconeogenesis and glycogenolysis) was observed in our experiment.
Bile acid sequestration is associated with increased hepatic lipogenesis and increased hepatic fat content (39,40). Given that such intervention, as well as loss of FXR (36) is associated with increased triglycerides [see Supplementary Data and (3)], it is possible that with Colesevelam treatment, some ingested glucose is used in lipogenesis.
An increase in portal insulin concentrations in response to Colesevelam could affect splanchnic extraction of the meal and therefore Meal Ra. Modulation of FXR, which is important to the mechanism of action of Colesevelam on glucose metabolism (41), may also affect insulin secretion (42). Orally active FXR agonists directly increase insulin concentrations while lowering glucose concentrations (therefore implying an effect on insulin secretion) in vivo. However, indices of β-cell function failed to detect a significant effect of Colesevelam on ϕTotal and DI. This may have been due to an experiment inadequately powered to detect the (small) effect of the compound on these indices. Indeed, given the observed variation in DI, to have 80% power to detect a 25% difference between groups would have required ∼125 subjects per group.
Enteroendocrine cell secretion is altered by bile acids and by bile acid sequestrants either acting directly on L-cells (15,16,43) or via alteration of intraluminal contents (17). A link between bile acid concentrations and (fasting) GLP-1 secretion has been suggested (11). However, it is far from certain that these findings can explain the glucose lowering of Colesevelam. Reliable measurement of fasting incretin concentrations is problematic, as these concentrations are typically close to the limit of assay detectability. GLP-1 concentrations have been measured in other experiments seeking to determine the mechanism of Colesevelam-mediated glucose lowering. Garg et al. (18) studied subjects with type 1 diabetes using a liquid meal and concluded that GLP-1 concentrations were unchanged by Colesevelam. Beysen et al. (13) demonstrated an effect of Colesevelam on both total GLP-1 concentrations in response to a mixed meal. However, the GLP-1 concentrations observed with Colesevelam did not differ from those observed after treatment with placebo. A significant increase was observed only because GLP-1 prior to treatment was lower in the group randomized to Colesevelam (compared with placebo). Since metformin may also increase GLP-1 concentrations (44,45), it is unclear if these differences might be explained by between-arm differences in oral antidiabetes treatment at baseline. In our experiment, although Colesevelam slightly increased fasting GLP-1, the change observed did not differ significantly from that with placebo, making it less likely that the actions of Colesevelam are GLP-1 mediated.
In this experiment, we demonstrate an effect of Colesevelam on both fasting and postprandial glucose concentrations. Measurement of insulin secretion and action using the oral labeled and unlabeled minimal model failed to show a significant effect on insulin secretion and action. Moreover, there was no evidence that glucose lowering is produced by alterations in GLP-1 concentrations. The use of the model-independent triple-tracer technique [which avoids the pitfalls of dual-tracer methodology (31,46)] allowed accurate measurement of ingested meal appearance. This enabled detection of a small effect of Colesevelam on Meal Ra. The decrease in meal appearance could be explained by decreased intestinal absorption or increased hepatic uptake of ingested glucose. Both of these scenarios are supported by rodent models, and further human studies will be required to determine the mechanism by which bile-acid binding resins decrease meal appearance.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the Mayo Clinic General Clinical Research Center. A.V. and C.C. are supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases grants DK-78646 and DK-82396.
A.V. received research grants from Merck and Daiichi-Sankyo and has consulted for sanofi-aventis, Novartis, and Bristol-Myers Squibb. No other potential conflicts of interest relevant to this article were reported.
G.S. researched data and ran the studies. M.S. conducted some of the meal studies and researched data. F.P. and C.D.M. undertook mathematical modeling of insulin secretion and action. J.H.L. researched data. C.C. reviewed and edited the manuscript. A.R.Z. undertook statistical analysis. R.A.R. contributed to discussion and reviewed and edited the manuscript. A.V. researched data and wrote the manuscript. A.V. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Daiichi-Sankyo for providing grant support for this investigator-initiated study.
Footnotes
Clinical trial reg. no. NCT00951899, clinicaltrials.gov.
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-0923/-/DC1.
REFERENCES
- 1.Charlton-Menys V, Durrington PN. Human cholesterol metabolism and therapeutic molecules. Exp Physiol 2008;93:27–42 [DOI] [PubMed] [Google Scholar]
- 2.Staels B, Kuipers F. Bile acid sequestrants and the treatment of type 2 diabetes mellitus. Drugs 2007;67:1383–1392 [DOI] [PubMed] [Google Scholar]
- 3.Zieve FJ, Kalin MF, Schwartz SL, Jones MR, Bailey WL. Results of the glucose-lowering effect of WelChol study (GLOWS): a randomized, double-blind, placebo-controlled pilot study evaluating the effect of colesevelam hydrochloride on glycemic control in subjects with type 2 diabetes. Clin Ther 2007;29:74–83 [DOI] [PubMed] [Google Scholar]
- 4.Staels B, Fonseca VA. Bile acids and metabolic regulation: mechanisms and clinical responses to bile acid sequestration. Diabetes Care 2009;32(Suppl. 2):S237–S245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fonseca VA, Rosenstock J, Wang AC, Truitt KE, Jones MR. Colesevelam HCl improves glycemic control and reduces LDL cholesterol in patients with inadequately controlled type 2 diabetes on sulfonylurea-based therapy. Diabetes Care 2008;31:1479–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg RB, Fonseca VA, Truitt KE, Jones MR. Efficacy and safety of colesevelam in patients with type 2 diabetes mellitus and inadequate glycemic control receiving insulin-based therapy. Arch Intern Med 2008;168:1531–1540 [DOI] [PubMed] [Google Scholar]
- 7.Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev 2009;89:147–191 [DOI] [PubMed] [Google Scholar]
- 8.Stayrook KR, Bramlett KS, Savkur RS, et al. Regulation of carbohydrate metabolism by the farnesoid X receptor. Endocrinology 2005;146:984–991 [DOI] [PubMed] [Google Scholar]
- 9.Hirota K, Daitoku H, Matsuzaki H, et al. Hepatocyte nuclear factor-4 is a novel downstream target of insulin via FKHR as a signal-regulated transcriptional inhibitor. J Biol Chem 2003;278:13056–13060 [DOI] [PubMed] [Google Scholar]
- 10.Yamagata K, Daitoku H, Shimamoto Y, et al. Bile acids regulate gluconeogenic gene expression via small heterodimer partner-mediated repression of hepatocyte nuclear factor 4 and Foxo1. J Biol Chem 2004;279:23158–23165 [DOI] [PubMed] [Google Scholar]
- 11.Shang Q, Saumoy M, Holst JJ, Salen G, Xu G. Colesevelam improves insulin resistance in a diet-induced obesity (F-DIO) rat model by increasing the release of GLP-1. Am J Physiol Gastrointest Liver Physiol 2010;298:G419–G424 [DOI] [PubMed] [Google Scholar]
- 12.Chen L, McNulty J, Anderson D, et al. Cholestyramine reverses hyperglycemia and enhances glucose-stimulated glucagon-like peptide 1 release in Zucker diabetic fatty rats. J Pharmacol Exp Ther 2010;334:164–170 [DOI] [PubMed] [Google Scholar]
- 13.Beysen C, Murphy EJ, Deines K, et al. Effect of bile acid sequestrants on glucose metabolism, hepatic de novo lipogenesis, and cholesterol and bile acid kinetics in type 2 diabetes: a randomised controlled study. Diabetologia 2012;55:432–442 [DOI] [PubMed] [Google Scholar]
- 14.Marina AL, Utzschneider KM, Wright LA, Montgomery BK, Marcovina SM, Kahn SE. Colesevelam improves oral but not intravenous glucose tolerance by a mechanism independent of insulin sensitivity and β-cell function. Diabetes Care 2012;35:1119–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirasawa A, Tsumaya K, Awaji T, et al. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med 2005;11:90–94 [DOI] [PubMed] [Google Scholar]
- 16.Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun 2005;329:386–390 [DOI] [PubMed] [Google Scholar]
- 17.Hofmann AF. Bile acid sequestrants improve glycemic control in type 2 diabetes: a proposed mechanism implicating glucagon-like peptide 1 release. Hepatology 2011;53:1784. [DOI] [PubMed] [Google Scholar]
- 18.Garg SK, Ritchie PJ, Moser EG, Snell-Bergeon JK, Freson BJ, Hazenfield RM. Effects of colesevelam on LDL-C, A1c and GLP-1 levels in patients with type 1 diabetes: a pilot randomized double-blind trial. Diabetes Obes Metab 2011;13:137–143 [DOI] [PubMed] [Google Scholar]
- 19.Vella A, Rizza RA. Extrapancreatic effects of GIP and GLP-1. Horm Metab Res 2004;36:830–836 [DOI] [PubMed] [Google Scholar]
- 20.Bock G, Dalla Man C, Micheletto F, et al. The effect of DPP-4 inhibition with sitagliptin on incretin secretion and on fasting and postprandial glucose turnover in subjects with impaired fasting glucose. Clin Endocrinol (Oxf) 2010;73:189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalla Man C, Bock G, Giesler PD, et al. Dipeptidyl peptidase-4 inhibition by vildagliptin and the effect on insulin secretion and action in response to meal ingestion in type 2 diabetes. Diabetes Care 2009;32:14–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beylot M, Previs SF, David F, Brunengraber H. Determination of the 13C-labeling pattern of glucose by gas chromatography-mass spectrometry. Anal Biochem 1993;212:526–531 [DOI] [PubMed] [Google Scholar]
- 23.Steele R, Bjerknes C, Rathgeb I, Altszuler N. Glucose uptake and production during the oral glucose tolerance test. Diabetes 1968;17:415–421 [DOI] [PubMed] [Google Scholar]
- 24.Dalla Man C, Caumo A, Basu R, Rizza R, Toffolo G, Cobelli C. Minimal model estimation of glucose absorption and insulin sensitivity from oral test: validation with a tracer method. Am J Physiol Endocrinol Metab 2004;287:E637–E643 [DOI] [PubMed] [Google Scholar]
- 25.Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C. Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes 2001;50:150–158 [DOI] [PubMed] [Google Scholar]
- 26.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 1992;41:368–377 [DOI] [PubMed] [Google Scholar]
- 27.Cobelli C, Toffolo GM, Dalla Man C, et al. Assessment of beta-cell function in humans, simultaneously with insulin sensitivity and hepatic extraction, from intravenous and oral glucose tests. Am J Physiol Endocrinol Metab 2007;293:E1–E15 [DOI] [PubMed] [Google Scholar]
- 28.Dalla Man C, Caumo A, Basu R, Rizza R, Toffolo G, Cobelli C. Measurement of selective effect of insulin on glucose disposal from labeled glucose oral test minimal model. Am J Physiol Endocrinol Metab 2005;289:E909–E914 [DOI] [PubMed] [Google Scholar]
- 29.Dinneen SF. Mechanism of postprandial hyperglycaemia in diabetes mellitus. Eur J Gastroenterol Hepatol 1995;7:724–729 [PubMed] [Google Scholar]
- 30.Dinneen S, Gerich J, Rizza R. Carbohydrate metabolism in non-insulin-dependent diabetes mellitus. N Engl J Med 1992;327:707–713 [DOI] [PubMed] [Google Scholar]
- 31.Vella A, Rizza RA. Application of isotopic techniques using constant specific activity or enrichment to the study of carbohydrate metabolism. Diabetes 2009;58:2168–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basu R, Di Camillo B, Toffolo G, et al. Use of a novel triple-tracer approach to assess postprandial glucose metabolism. Am J Physiol Endocrinol Metab 2003;284:E55–E69 [DOI] [PubMed] [Google Scholar]
- 33.Toffolo G, Basu R, Dalla Man C, Rizza R, Cobelli C. Assessment of postprandial glucose metabolism: conventional dual- vs. triple-tracer method. Am J Physiol Endocrinol Metab 2006;291:E800–E806 [DOI] [PubMed] [Google Scholar]
- 34.Odunsi-Shiyanbade ST, Camilleri M, McKinzie S, et al. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin Gastroenterol Hepatol 2010;8:159–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Dijk TH, Grefhorst A, Oosterveer MH, et al. An increased flux through the glucose 6-phosphate pool in enterocytes delays glucose absorption in Fxr-/- mice. J Biol Chem 2009;284:10315–10323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duran-Sandoval D, Cariou B, Percevault F, et al. The farnesoid X receptor modulates hepatic carbohydrate metabolism during the fasting-refeeding transition. J Biol Chem 2005;280:29971–29979 [DOI] [PubMed] [Google Scholar]
- 37.Henry RR, Aroda VR, Mudaliar S, Garvey WT, Chou HS, Jones MR. Effects of colesevelam on glucose absorption and hepatic/peripheral insulin sensitivity in patients with type 2 diabetes mellitus. Diabetes Obes Metab 2012;14:40–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartz SL, Lai Y-L, Xu J, et al. The effect of colesevelam hydrochloride on insulin sensitivity and secretion in patients with type 2 diabetes: a pilot study. Metab Syndr Relat Disord 2010;8:179–188 [DOI] [PubMed] [Google Scholar]
- 39.Herrema H, Meissner M, van Dijk TH, et al. Bile salt sequestration induces hepatic de novo lipogenesis through farnesoid X receptor- and liver X receptor alpha-controlled metabolic pathways in mice. Hepatology 2010;51:806–816 [DOI] [PubMed] [Google Scholar]
- 40.Le T-A, Chen J, Changchien C, et al. San Diego Integrated NAFLD Research Consortium (SINC) Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology 2012;56:922–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Renga B, Mencarelli A, Vavassori P, Brancaleone V, Fiorucci S. The bile acid sensor FXR regulates insulin transcription and secretion. Biochim Biophys Acta 2010;1802:363–372 [DOI] [PubMed] [Google Scholar]
- 42.Prawitt J, Abdelkarim M, Stroeve JHM, et al. Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity. Diabetes 2011;60:1861–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 2009;10:167–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mannucci E, Tesi F, Bardini G, et al. Effects of metformin on glucagon-like peptide-1 levels in obese patients with and without Type 2 diabetes. Diabetes Nutr Metab 2004;17:336–342 [PubMed] [Google Scholar]
- 45.Mulherin AJ, Oh AH, Kim H, Grieco A, Lauffer LM, Brubaker PL. Mechanisms underlying metformin-induced secretion of glucagon-like peptide-1 from the intestinal L cell. Endocrinology 2011;152:4610–4619 [DOI] [PubMed] [Google Scholar]
- 46.Livesey G, Wilson PD, Dainty JR, et al. Simultaneous time-varying systemic appearance of oral and hepatic glucose in adults monitored with stable isotopes. Am J Physiol 1998;275:E717–E728 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.