Abstract
Relative to diets enriched in palmitic acid (PA), diets rich in oleic acid (OA) are associated with reduced risk of type 2 diabetes. To gain insight into mechanisms underlying these observations, we applied comprehensive lipidomic profiling to specimens collected from healthy adults enrolled in a randomized, crossover trial comparing a high-PA diet to a low-PA/high-OA (HOA) diet. Effects on insulin sensitivity (SI) and disposition index (DI) were assessed by intravenous glucose tolerance testing. In women, but not men, SI and DI were higher during HOA. The effect of HOA on SI correlated positively with physical fitness upon enrollment. Principal components analysis of either fasted or fed-state metabolites identified one factor affected by diet and heavily weighted by the PA/OA ratio of serum and muscle lipids. In women, this factor correlated inversely with SI in the fasted and fed states. Medium-chain acylcarnitines emerged as strong negative correlates of SI, and the HOA diet was accompanied by lower serum and muscle ceramide concentrations and reductions in molecular biomarkers of inflammatory and oxidative stress. This study provides evidence that the dietary PA/OA ratio impacts diabetes risk in women.
Western-style diets that are high in fat content have been linked to increased risk of type 2 diabetes (1,2). The two most prevalent fatty acids (FAs) in this diet are palmitic acid (PA; C16:0) and oleic acid (OA; C18:1), each present in approximately equal amounts as a percentage of dietary energy. Although total dietary fat consumption is comparably high in Mediterranean countries, epidemiological studies show that these populations have a paradoxically low prevalence of type 2 diabetes and cardiovascular disease (1,2). Owing to liberal use of olive oil, the typical Mediterranean diet is rich in OA and low in PA (3–5). Numerous studies in cultured cells suggest that exposure to high PA disrupts insulin action and provokes proinflammatory signaling events, whereas OA mitigates these adverse responses (6–8). However, exposure of cells to high concentrations of PA may not reflect normal physiology, raising doubts about the clinical relevance of such experiments (9).
Progress toward a clearer understanding of the role of specific dietary FA in conferring cardioprotective and/or antidiabetic benefits requires carefully controlled dietary intervention studies. Although previous dietary trials have attempted to elucidate the distinct metabolic properties of PA and OA (10,11), most of these studies relied on prescribed diets and/or did not actually measure the impact of the experimental diets on the FA composition of circulating and cellular lipids. As a result, the current literature on this topic is conflicted and difficult to interpret. In this study, we present new findings testing the hypothesis that replacing dietary PA with OA would impact insulin sensitivity. Because a previous study found sex differences in lipid metabolism (12), we also sought to consider sex as a factor that might influence metabolic responses to a change in dietary FA composition.
RESEARCH DESIGN AND METHODS
Subjects, screening, and overall design.
This study was approved by institutional committees associated with the University of Vermont General Clinical Research Center (GCRC).
Healthy men (n = 9) and women (n = 9), aged 18–40 years, with a BMI >18 and <30 were recruited for this study. These 18 volunteers constituted the cohort for all results in this article, except for studies of muscle protein expression and muscle ceramide content performed in an additional 10 volunteers (5 women and 5 men), who also participated in the same protocol (see Supplementary Data).
Exclusion criteria included regular aerobic exercise training, dyslipidemia (13), and evidence of type 2 diabetes or insulin resistance (14). Women were enrolled if they did not receive hormonal forms of contraception and manifested normal ovulation based both on a urine luteinizing hormone test and serum concentrations of estradiol and progesterone.
Screening indicated a habitual intake of 37% kcal total fat, 14.5% saturated fat, and 12% monounsaturated fat, consistent with the usual American diet (15). After screening, all subjects ingested a low-fat/low-PA, baseline/control diet for 7 days (protein, 19.7% kcal; carbohydrate, 51.6% kcal; fat, 28.4% kcal; PA, 5.3% kcal; and OA, 15.9% kcal) (13). On the morning of day 8 of the baseline/control diet, fasting blood and muscle tissue were collected at 0700 h (16), and 3 h after a breakfast (one-third daily kcal), muscle biopsy and blood collection were repeated. Then, the subjects participated in a crossover study of 3-wk diet periods, consisting of a diet resembling the habitual diet and high in PA (HPA; fat, 40.4% kcal; PA, 16.0% kcal; and OA, 16.2% kcal) or a diet low in PA and high in OA (HOA; 40.1% kcal; 2.4% kcal; and 28.8% kcal, respectively) (Supplementary Table 1). These diets were separated by a 1-week period on the baseline/control diet. Repeat blood collection and muscle biopsy in the fasted and fed state were carried out on the 22nd day of each experimental diet (HPA and HOA). Further details concerning the diets were described previously (16) and in the Supplementary Data.
In women, postexperimental diet evaluations took place in the luteal phase of the cycle prior to menstruation. On the first day of the baseline diet and at the end of the HPA and HOA diets, body composition was assessed, including upper body (android), truncal, legs, and lower body (gynoid) regions (GE Lunar Prodigy Densitometer, Version 5.6; GE Healthcare) (17). On the 21st day of each experimental diet, after an overnight fast in the GCRC, we completed a frequently sampled intravenous glucose tolerance test (18). We used a modified version of the MINMOD program (18) to estimate the following parameters: glucose effectiveness (Sg), the capacity of glucose to mediate its own disposal; acute insulin response to glucose (AIRg); insulin sensitivity index (SI), the capacity of insulin to promote glucose disposal; and disposition index (DI): AIRg * SI (19). The usual range of SI is ∼0–15 × 10−4 (min−1/μU/mL), with the MINMOD program generating values for SI that have been multiplied by 104. While verifying the adequacy of the data from the intravenous glucose tolerance test, we noted that some women seemed to exhibit lower nadirs for blood glucose concentration during one of the diets. Since we knew that some of these women were more physically fit than others, we elected to explore the correlation of peak oxygen consumption with cycling exercise (VO2peak) with diet change in SI. VO2peak was measured at screening (20). Physical activity was assessed daily using an ActiGraph Activity Monitor, worn at the waist (catalog number GT1M; ActiGraph, Pensacola, FL).
Metabolic measurements.
Glucose concentration was measured using a YSI 2300 Stat Plus glucose analyzer (YSI Inc., Yellow Springs, OH), and serum insulin concentration was measured by radioimmunoassay (Linco Research Inc., St. Charles, MO) (GCRC Core Laboratory). Standard radioimmunoassay kits and a Wallac Wizard 1470-010 automatic γ counter (PerkinElmer) were used for assays of leptin and adiponectin (Linco RIA kits; Linco Research Inc.). β-Hydroxybutyrate was measured by a standard method (Wako, Richmond, VA). Ceramides were extracted and analyzed based on the methods of Merrill et al. (21) using flow-injection tandem mass spectrometry. Nonesterified FA (NEFA) and total FA (free plus esterified) in serum were assessed by capillary gas chromatography/mass spectrometry (22). Fasting and fed, muscle, and serum concentrations of acylcarnitines (AC) and amino acids were measured by direct-injection electrospray tandem mass spectrometry (23). Muscle organic acids were quantified as previously described (24).
FA composition and concentration of skeletal muscle diacylglycerol, triacylglycerol, and phospholipids.
The FA composition of diacylglycerol (DAG) and triacylglycerol (TAG) as well as serum and muscle phospholipids was analyzed by gas chromatography using recently described methods (16).
Bio-Plex analysis of signaling cascades active in muscle.
Bio-Rad Bio-Plex phosphoprotein assays (Bio-Rad, Hercules, CA) and Bio-Plex total target assays (Luminex xMAP technology; Luminex) were used to detect the activity (phosphorylation) of inhibitor of κBα (IκBα), nuclear factor-κBp65, c-Jun N-terminal kinase (JNK), Akt, and insulin-receptor substrate-1 (IRS-1) in lysates derived from 5–10 mg of muscle biopsy samples.
Serum concentration (pg/mL) of interleukin-6, interleukin-10, tumor necrosis factor-α, and ferritin (all measured on the n = 18 cohort).
Serum cytokine concentrations were measured after the HPA and HOA diets in the fasting and fed states. Custom Bio-Plex (Bio-Rad, Hercules, CA) 3-plex kits were designed containing coupled beads and antibodies recognizing human interleukin-6 (IL-6), IL-10, and tumor necrosis factor-α (TNF-α). All assays were performed in duplicate according to the manufacturer’s instructions. Because of the interrelationships of physical fitness, iron status, insulin sensitivity, and oxidant stress (25–28), serum ferritin was measured and related to diet change (Fletcher Allen Health Center, direct chemiluminescence assay; Siemens ADVIA Centaur Ferritin; Siemens Healthcare Global).
Statistics.
All data are expressed as mean ± SEM. Analyses were performed with SAS, version 9.2 (SAS Institute). This study used a two-treatment, two-period, two-sequence crossover design. Diet effects were analyzed using a repeated-measures ANOVA, including sequence and treatment effects, with the baseline value as a covariate, when available. Because sex-specific responses to the diets were anticipated (12), men and women were analyzed both as a group and separately. For some analyses, we used the same model using ranks. All correlations reported are Spearman rank correlations.
Principal components analysis (PCA) was used to reduce the dimensionality of both the fasting and fed data and to aid in explaining the highest variance within the overall dataset. Orthogonal rotation was used to aid in the interpretation of the components. Diet differences in component scores were examined using the repeated-measures ANOVA methods described above. In addition, select components were included as time-varying covariates in the analysis to examine the relationship between dependent variables of interest and the component scores. Additional details are provided in the Supplementary Data.
RESULTS
Body composition, physical fitness, and physical activity.
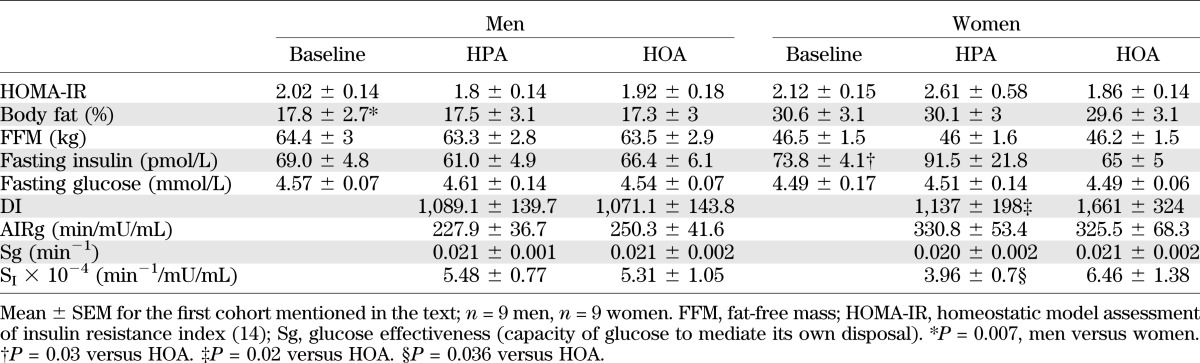
The diets did not affect body weight, BMI, or whole-body composition (Table 1). In men and women combined, the HPA diet period was associated with greater android adiposity (percent fat) (P = 0.037) and a trend for higher truncal adiposity (P = 0.068). A similar trend (P = 0.060) for increased android adiposity was observed when women (but not men) were analyzed separately. However, there was no diet effect on total or regional fat mass in men and women together or separately (Supplementary Table 2). Compared with women, men exhibited higher VO2peak (mL/kg/min) at screening (49.11 ± 3.71 vs. 36.22 ± 4.32) and lower percent body fat after the baseline diet (Table 1). In both men and women, physical activity correlated positively with VO2peak, regardless of the diet (r = 0.66–0.87; P < 0.02).
TABLE 1.
Demographic and metabolic characteristics
Comprehensive analyses of circulating and cellular lipids.
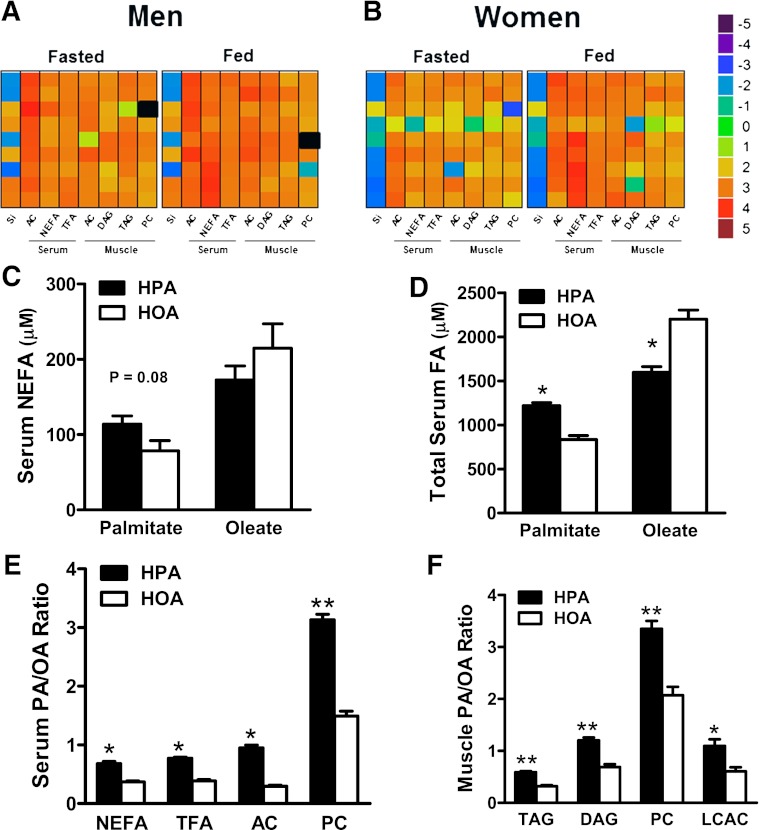
A major goal of this study was to compare the effects of the two diets on the quantity and quality of circulating and intracellular lipid pools. Because lipid metabolism fluctuates dramatically in response to acute changes in nutrient and/or hormonal status, biological specimens were collected in both the fasted and postprandial states. The heat maps in Fig. 1A and B provide compelling evidence that even a short-term change in dietary fats can have broad-ranging effects on circulating as well as muscle lipids. Thus, when comparing the HOA to the HPA diet, the PA/OA ratio was increased in nearly every lipid pool analyzed including muscle TAG, DAG, and phosphatidylcholine (PC) (Fig. 1A and B). The impact of the diets on the FA composition of lipids was universally evident in every subject, and in general, this effect was more robust in the fed than the fasted state. Notably, the diet also affected the PA/OA ratio measured in circulating NEFAs, which are derived principally from lipolysis of adipose tissue TAG (Fig. 1C). Likewise, the pronounced diet-induced shift in the PA/OA ratio of mitochondrial-derived AC metabolites, both in the circulation and in muscle (Fig. 1E and F), indicates that the diets had a strong influence on the types of FA undergoing β-oxidation, not only in the fed state but also during fasting. Data in Fig. 1C–F are shown for men and women combined, but for most variables, similar results were found for men and women separately (see Supplementary Data).
FIG. 1.
The FA composition of the diet is reflected in a broad range of circulating and intramuscular lipids (see text for abbreviations, except where indicated). A and B: Heat maps depicting diet-induced changes in the PA/OA ratio of blood and muscle lipids according to the scale on the right (total FA [TFA]). Change scores were calculated from absolute values of log base 5 transformed PA/OA data in the fasted or fed state on the HPA versus HOA diet (HPA/HOA). Each square represents an individual subject, and black indicates a missing value. SI reflects insulin sensitivity measured in the fasted state. Results in men and women were combined to show diet effects on serum concentrations (μmol/L) of nonesterified PA and OA (C); serum concentration (μmol/L) of total PA and OA (D); the PA/OA ratio in serum NEFA, TFA, AC, and PC (E); and the PA/OA ratio in skeletal muscle lipid metabolites (F): TAG, DAG, PC, and LCAC. *P ≤ 0.001, **P ≤ 0.01 denote diet effect.
Diet effects on insulin secretion and SI.
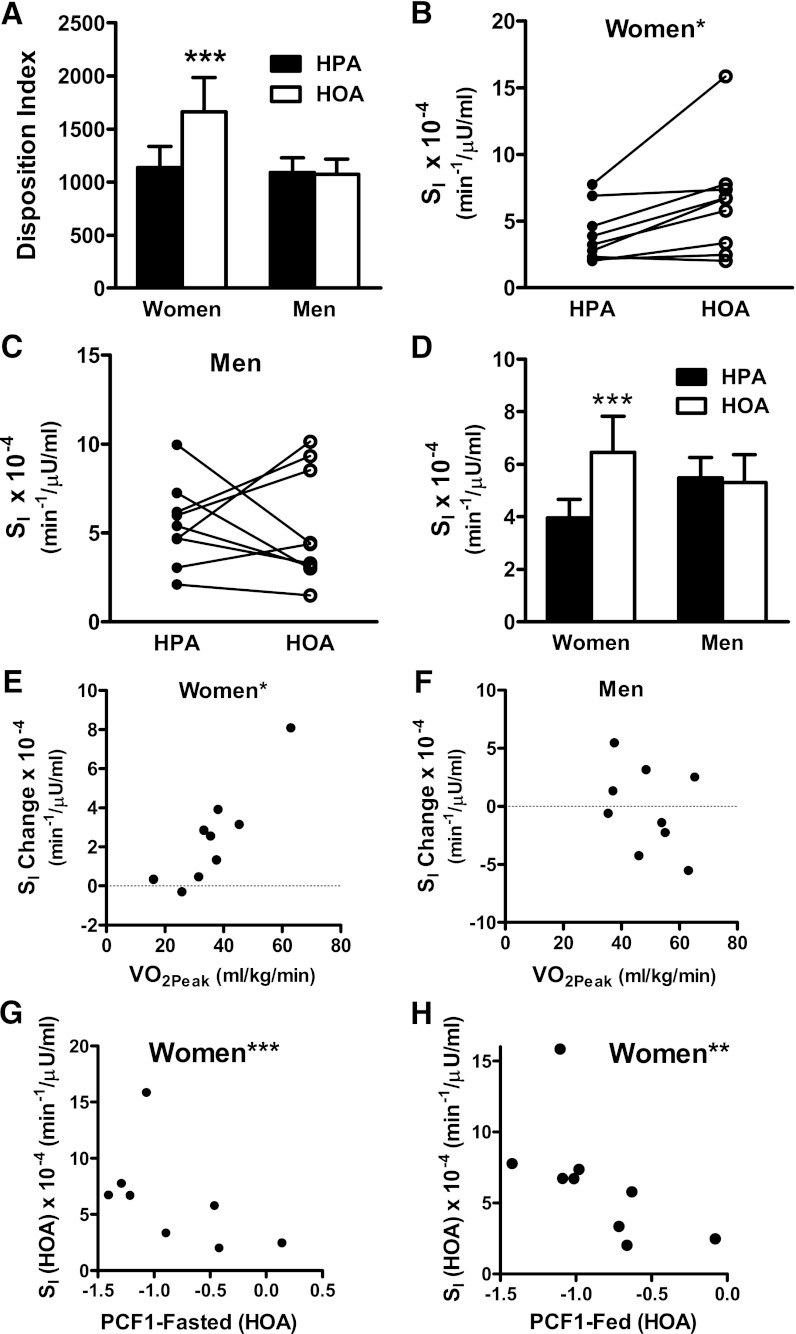
Because sex-specific responses to the diets were anticipated a priori (12), men and women were analyzed both as a group and separately. Both the DI and SI were similar between men and women regardless of the diet condition. When the diet effect on DI was analyzed by repeated-measures ANOVA, the diet group by sex interaction approached statistical significance (P = 0.06). In women only, the DI was 46% higher during HOA (P = 0.02) (Table 1 and Fig. 2A). The diet group by sex interaction for SI was not significant using normal distribution statistics (P = 0.109), but trended toward significance when calculated with rank-transformed data (P = 0.079). Analysis of men and women separately showed that eight of nine female subjects manifested higher SI when consuming the HOA diet (Fig. 2B; P = 0.03), whereas in men, only four of nine showed a higher SI on the same diet (Fig. 2C and Supplementary Table 3). In women, the average increase in SI during the HOA diet was 63% (P = 0.036, NS, men) (Fig. 2D and Table 1). In women, but not men, we identified a strong rank correlation between VO2peak and the diet-induced change in SI (Spearman r = 0.90; P = 0.001) (Fig. 2E and F). Thus, the most impressive gains in SI during the HOA diet occurred in women who were most physically fit at the inception of the study. Importantly, the diet effect on SI and the relationship between SI and VO2peak were maintained (P = 0.027 and P = 0.007, respectively) even after exclusion of one female subject who demonstrated the most robust change in SI. Additional details pertaining to the relationship between the SI response to the diets and baseline characteristics of male and female subjects are provided in the Supplementary Data.
FIG. 2.
The HOA diet improved insulin secretion and sensitivity in women. A: DI. B and C: Insulin sensitivity index (SI) in individual women and men measured during the HOA and HPA diets. D: SI. Relationship between diet-induced change in SI (HOA − HPA) (SI Change) and VO2peak in women (E) and men (F) (*Spearman r = 0.90; P ≤ 0.001). In women, during the HOA diet, SI correlated inversely with PCF1-Fasted (G) (***r = −0.786, P = 0.021) and PCF1-Fed (H) (**r = −0.850, P = 0.004). ***P ≤ 0.05 denotes a diet effect.
PCA.
The dimension reduction strategy of PCA was used to reduce all metabolites measured, including those in serum/plasma, muscle, and urine, into a smaller number of orthogonal variables. This analysis was performed separately for fasted and fed conditions. PCA identified 31 factors among 329 total variables measured in the fasted state. Only one of these factors, Principal Components Factor1-Fasted (PCF1-Fasted) was affected by diet in both men and women (P < 0.0001) and almost uniformly reflected the PA/OA ratio of serum and muscle lipids (Supplementary Table 3). PCA identified 31 factors among 277 variables measured in the fed state. PCF1-Fed was affected by diet in both men and women (P < 0.0001) and again reflected the PA/OA ratio of lipids (Supplementary Table 4). PA contributed a positive loading score and OA a negative score; thus, F1 was higher during the HPA compared with the HOA diet (P < 0.0001). These computer-generated factors provided an unbiased, composite index of systemic FA composition that was then used to evaluate the relationship between the PA/OA content of biological lipids and glucose homeostasis. In women but not men, both PCF1-Fasted and PCF1-Fed correlated inversely with SI assessed during the HOA diet (r = −0.786, P = 0.021; and r = −0.850, P = 0.004, respectively) (Fig. 2F and G). Inclusion of PCF1-Fed in an ANCOVA model abrogated the diet effect on SI in women, whereas this relationship was maintained after adjusting for PCF1-Fasted. In aggregate, these findings suggest that the diet effects on SI in women were related to and possibly mediated by the PA/OA ratio of serum and muscle lipids.
Diet-induced changes in candidate mediators of insulin resistance.
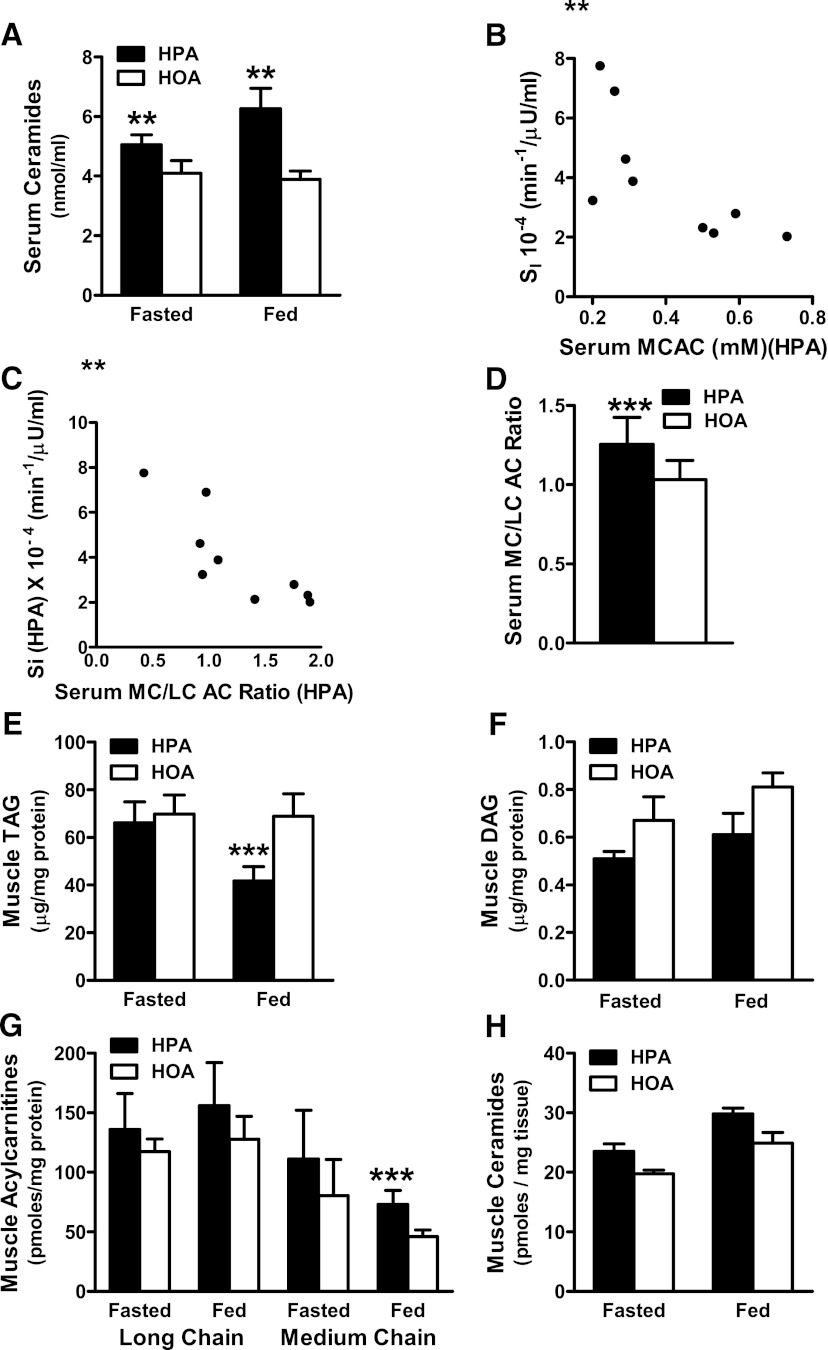
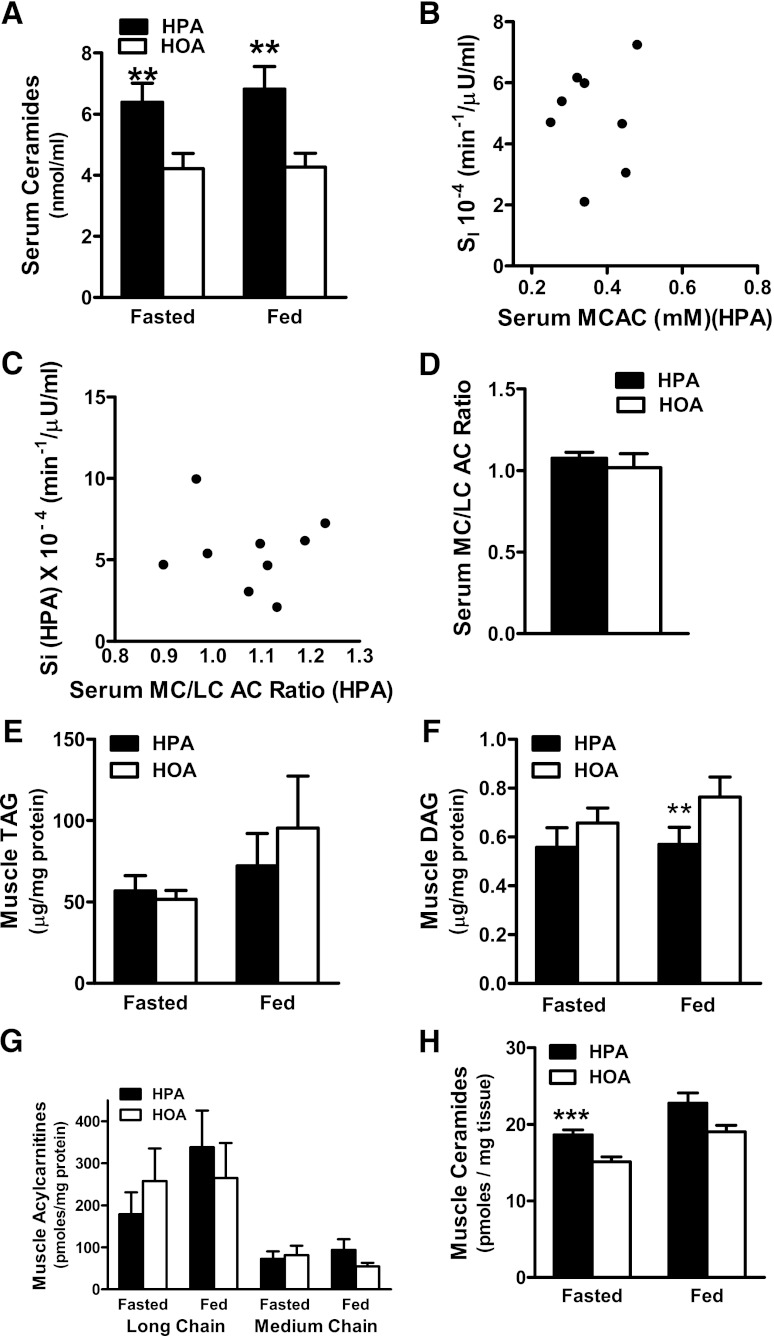
To gain mechanistic insights into the insulin-sensitizing effect of the HOA diet in women, we initially focused our analysis on systemic lipid metabolites that have been linked to insulin resistance, including ceramides and AC. In women (Fig. 3A) and men (Fig. 4A), total ceramide concentrations in serum were higher during the HPA diet in both the fasting and fed states; and nearly every ceramide species measured in serum increased in response to the HPA diet (Supplementary Table 5). However, we did not detect an inverse correlation between circulating ceramides and SI. By contrast, SI in women measured during the HPA diet correlated inversely with serum concentration of medium chain AC (MCAC) (Fig. 3B) and with the serum medium-chain to long-chain AC ratio (MCAC/LCAC) (Fig. 3C). In women (Fig. 3D), the serum MCAC/LCAC ratio was higher after the HPA diet compared with HOA. These diet effects were not evident in men (Fig. 4).
FIG. 3.
Dietary FA composition affected lipid biomarkers of insulin resistance in women. A: Serum ceramide concentrations measured in the fasting and fed states. B: Relationship between SI and MCAC measured in the fed state during the HPA diet (**r = −0.783, P = 0.013). C: Relationship between SI and the serum MCAC/LCAC measured in the fed state during the HPA diet (**r = −0.867, P = 0.002). D: Serum MCAC/LCAC ratio in the fed state. Muscle biopsy specimens harvested in the fasted and fed states were used to quantify intramuscular concentrations of TAG (E), DAG (F), and LCAC and MCAC (G) and total ceramides (H). **P ≤ 0.01, ***P ≤ 0.05 denote a diet effect.
FIG. 4.
Dietary FA composition affected lipid biomarkers of insulin resistance in men. A: Serum ceramide concentrations measured in the fasting and fed states. B: Relationship between SI and MCAC measured in the fed state during the HPA diet (r = −0.33, P = 0.38). C: Relationship between SI and the serum MCAC/LCAC measured in the fed state during the HPA diet (r = 0.05, P = 0.90). D: Serum MCAC/LCAC ratio in the fed state. Muscle biopsy specimens harvested in the fasted and fed states were used to quantify intramuscular concentrations of TAG (E), DAG (F), and LCAC and MCAC (G) and total ceramides (H). **P ≤ 0.01, ***P ≤ 0.05 denote a diet effect.
We next examined concentrations in muscle of specific metabolites that are known markers and/or suspected mediators of insulin resistance, including TAG, DAG, AC, and ceramides (29,30). In women, muscle TAG measured in the fed state was higher during the HOA diet (P = 0.05; Fig. 3E), whereas muscle DAG content was unaffected by diet (Fig. 3F). Notably, the diet-induced change in the OA content of intramuscular TAG correlated positively with VO2peak (r = 0.667; P = 0.05), again suggesting an interaction between diet and physical fitness in women. Muscle MCAC levels in the fed state were 58% higher during the HPA compared with the HOA diet in women (Fig. 3G) but not in men (Fig. 4G).
Muscle ceramides were measured in a separate cohort of five men and five women. In men, fasting levels of total muscle ceramides were 23% higher during HPA compared with HOA diet (P = 0.023) (Fig. 4H), whereas in women, similar fractional increases in total muscle ceramides (19 to 20%) did not reach statistical significance (Fig. 3H). In this second cohort, SI increased during the HOA diet in all five women (P = 0.043).
Diet-induced changes in molecular markers of insulin resistance and inflammation.
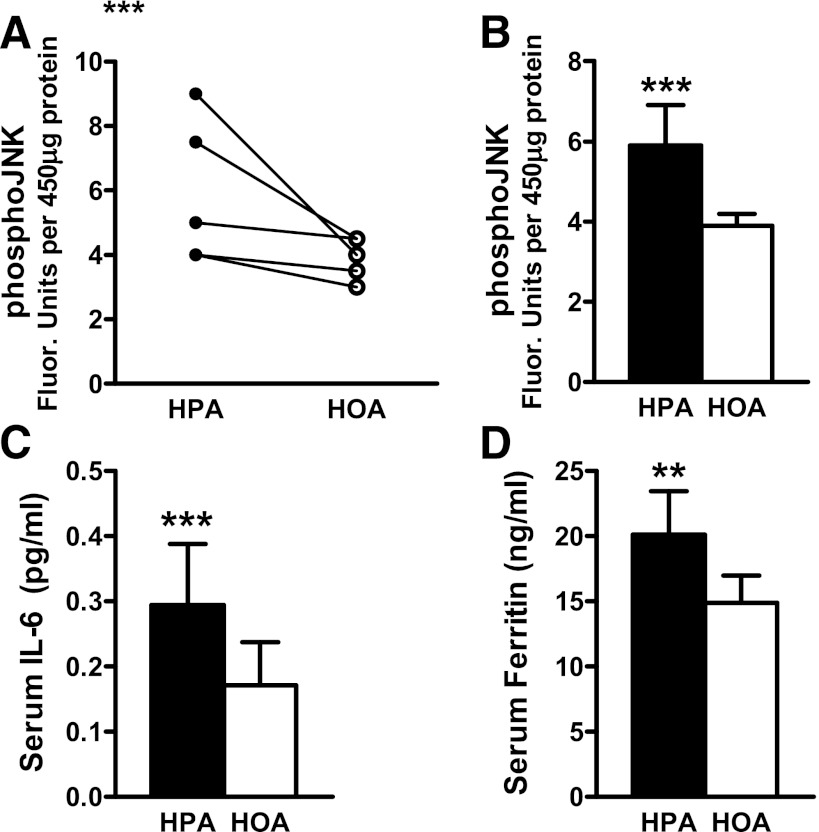
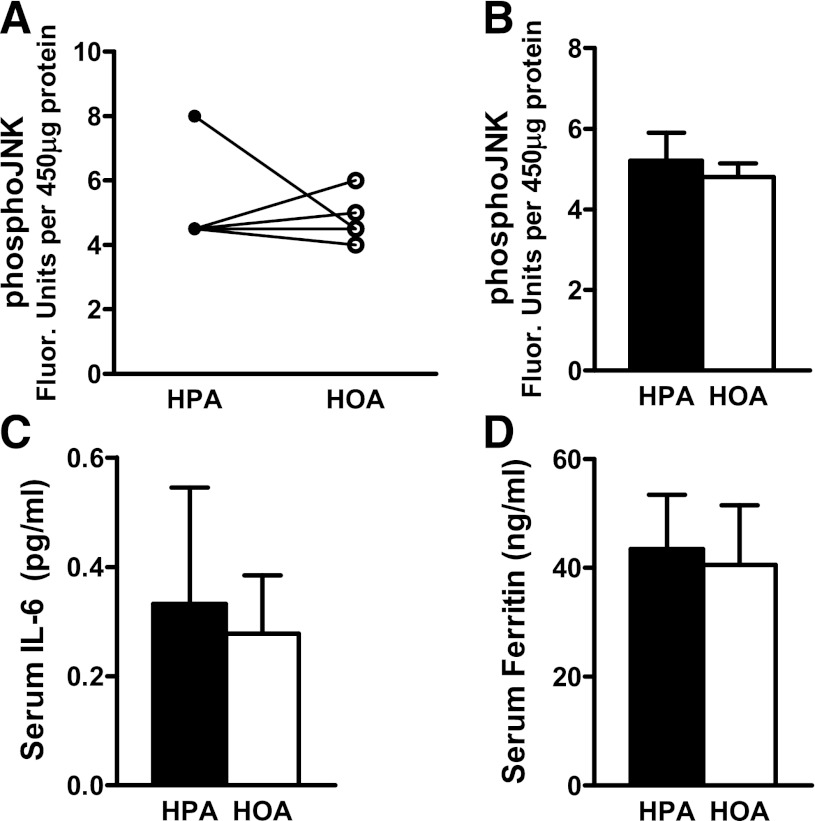
In female subjects, we found no evidence of a diet effect on phosphorylated Akt (pAkt) relative to total Akt or serine phosphorylation of IRS-1 (pIRS-1 [Ser636/Ser639]) relative to total IRS-1. However, in men, both pAkt (Ser473) and the pAkt/total Akt ratio measured in the fed state were higher during the HOA diet compared with the HPA diet (both P ≤ 0.01), and these values increased in all five men. It is noteworthy that these measures were made in specimens collected 3 h after feeding, which might not reflect muscle signaling changes occurring during the acute-phase insulin response. In women, muscle levels of phosphorylated c-Jun N-terminal kinase (pJNK) (Thr183/Tyr185) were lower after the HOA diet compared with the HPA diet during the fasted state (P = 0.02, using ranks) (Fig. 5A and B), but we observed no diet effect on pJNK in men (Fig. 6A and B).
FIG. 5.
Dietary FA composition affected molecular markers of insulin resistance and oxidant stress in women. Skeletal muscle biopsies harvested in the fasted state were used to assess pJNK using the Bio-Plex phosphoprotein assay (Bio-Rad); results are shown for individual women (A) and group averages measured after each diet (B). Blood samples harvested in the fasted state were used to measure serum IL-6 (C) and serum ferritin (D). **P ≤ 0.01, ***P ≤ 0.05 denote a diet effect.
FIG. 6.
Dietary FA composition did not affect molecular markers of insulin resistance and oxidant stress in men. Skeletal muscle biopsies harvested in the fasted state were used to assess pJNK using the Bio-Plex phosphoprotein assay; results are shown for individual men (A) and group averages measured after each diet (B). Blood samples harvested in the fasted state were used to measure serum IL-6 (C) and serum ferritin (D).
Assessment of systemic inflammatory tone.
In both women and men, serum concentrations of IL-10 and TNF-α were unaffected by the diets. Notably, however, six of the nine women had a higher fasting IL-6 concentration during the HPA diet (P = 0.05, using ranks) (Fig. 5C). Additionally, serum ferritin concentration was higher during the HPA diet in all nine women (P = 0.014; P = 0.007 using ranks), and the mean concentration was 35% higher on the HPA compared with the HOA diet (P = 0.014; Fig. 5D). In men, IL-6 and ferritin were unaffected by diet (Fig. 6).
DISCUSSION
This study provides direct evidence that replacing dietary palmitate with oleate can benefit clinically relevant measures of metabolic wellness in healthy individuals. In women, a diet-induced shift favoring less saturation and more monounsaturation of cellular lipids (lower PA/OA ratio) was associated with improvements in both DI and SI. All women were studied in the postluteal phase of the menstrual cycle, verified by measurements of estrogen and progesterone. Considering that estrogen can alter expression of genes linked to regulation of lipid oxidation (31,32), these results may not necessarily be generalizable to nonovulating women. Whereas a diet effect on SI was evident in women, results in men were variable, implying that sex influences the interplay between dietary FA composition and clinical outcomes. However, due to insufficient power, we are unable to form firm conclusions regarding gender specificity.
Both candidate and unbiased lipidomic profiling approaches were used to gain a comprehensive view of how the experimental diets impacted systemic lipid composition. Remarkably, we found that a relatively short-term change in the quality and quantity of specific dietary FA affected the FA composition of nearly every class of biological lipids evaluated in blood and muscle biopsy specimens. We also targeted several prominent lipid metabolites previously implicated as mediators and/or strong markers of insulin resistance. Notably, the HPA diet increased circulating levels and muscle content of ceramides, thus resembling abnormal ceramide metabolism observed in subjects with insulin resistance and/or type 2 diabetes (33). These exciting results provide the first demonstration that a shift in dietary FA composition can actually alter systemic and cellular ceramide metabolism in humans. Surprisingly, however, our analysis failed to detect a convincing association between ceramides and SI, suggesting that short-term changes in these lipid molecules do not necessarily influence insulin action.
Intriguingly, in women, the HPA diet also increased muscle and blood concentrations of MCAC measured in the fed state. Moreover, the circulating concentration of MCAC in the fed state emerged as a strong negative correlate of SI when women were consuming the HPA diet. Earlier studies likewise identified a link between insulin resistance and MCAC assessed in human muscle and primary human skeletal myocytes (34). Most MCAC are generated in the mitochondrial matrix and originate from medium-chain acyl-CoA intermediates of the β-oxidation pathway. Accordingly, we measured muscle mRNA abundance of medium-chain acyl-CoA dehydrogenase but did not find a diet effect on expression of this gene (not shown), pointing to other explanations for the HPA diet-induced rise in MCAC content.
Yet unclear is whether the AC can act as signaling molecules or if these metabolites are strictly reporting on changes in mitochondrial substrate flux and/or load. Although convincing evidence that AC play a direct role in mediating insulin resistance is lacking, support for this possibility comes from a recent study showing that exposure of RAW264.7 cells to low micromolar concentrations of MCAC stimulated activity of the stress-sensitive transcription factor nuclear factor-κB (35). Alternatively, these metabolites might serve as markers of mitochondrial stress and/or cellular events that are known to influence insulin action (29). For example, accumulation of lipid-derived medium chain acyl-CoAs might reflect a mitochondrial environment that is conducive to the production of reactive oxygen species (ROS) (36), perturbations in cellular redox balance (30), and/or inhibition of pyruvate dehydrogenase (37,38).
Fitting with potential shifts in cellular stress, we speculate that the HOA diet lowered oxidant and inflammatory stress in women but not men. In support of this possibility, we found that serum concentrations of IL-6 and ferritin were lower in women during the HOA diet. Circulating levels of IL-6 are elevated in patients with obesity and/or type 2 diabetes, although the role of this cytokine as a direct mediator of insulin resistance remains controversial (39,40). Ferritin, best known as a major iron storage protein, is also recognized as an acute-phase protein (41). Its expression is upregulated by cytokines and ROS during conditions of infection and ramped inflammation (28). Under these conditions, the robust increase in circulating ferritin serves to reduce iron bioavailability, thereby mitigating ROS production and oxidant damage (28). Because iron intake was identical during the two diets and the treatment order was randomized, we surmise that increased ferritin levels in women consuming the HPA diet might reflect heightened oxidative and/or inflammatory stress. It is also important to consider that the distinct antioxidant properties of the vegetable oils used to formulate the HOA and HPA diets might have contributed to the overall effects of the two experimental regimens (42). Notably, however, with the exception of the virgin olive oil added to the HPA diet, the oils used in this study were highly purified (see Supplementary Data).
Although the molecular mechanisms linking lipid dysregulation, inflammation, and/or oxidative stress to insulin resistance are still unfolding, strong evidence implicates a role for JNK1, a member of the mitogen-activated family of serine kinases that serves as a major hub for several discrete signaling pathways involved in monitoring metabolic stress (30,43). In both cultured cells and animal models, JNK1 is activated in response to IL-6, TNF-α, ROS, endoplasmic reticulum stress, and exposure to surplus lipids, resulting in serine phosphorylation and consequent inhibition of IRS-1 (30,43). Most compelling, prolonged high-fat feeding increases JNK phosphorylation in rodents, and genetic ablation of JNK1 protects against obesity-induced insulin resistance (43). Likewise, in the current study, the insulin-sensitizing properties of the HOA diet in women were accompanied by a reduction in muscle levels of pJNK. Our findings suggest that a shift in the FA composition of the diet was sufficient to modulate JNK activity, which in turn contributed to corresponding changes in insulin action. At this stage, we are uncertain as to whether JNK was responding to fluctuations in ROS production, inflammatory tone, endoplasmic reticulum stress (44), and/or other lipid-sensitive signaling molecules. Perhaps subtle perturbations in multiple pathways converged at the JNK nexus.
Interestingly, in women but not men, physical fitness was identified as a strong, positive modifier of changes in cellular lipid composition as well as SI. These results suggest that the extent to which dietary FAs penetrate cellular lipids and affect glucose homeostasis in women depends on physical activity. Because exercise promotes metabolic wellness, a common assumption is that physically inactive individuals have the most to gain by improving dietary habits. Instead, we found that the most active, physically fit women gained the greatest benefit from replacing PA with OA, suggesting that exercise and dietary OA acted synergistically on a common molecular target. Whereas a previous study found that OA increases the energy cost of exercise (20), perhaps by promoting mitochondrial uncoupling (45), investigations to delineate the distinct uncoupling properties of specific FAs have produced conflicting results (46,47). Exercise also decreases the saturation index of muscle lipids and promotes synthesis, storage, and turnover of intramuscular TAG (48,49). Thus, the strong interaction between diet and physical activity might relate to adaptations in muscle lipid droplet metabolism (50). Hinting at this possibility, we found that diet-induced changes in the fractional OA content of muscle TAG correlated positively with VO2peak in women.
In summary, this investigation supports the notion that palmitate imposes a heavy metabolic burden on subcellular machinery, including muscle mitochondria, which was most evident in women during the period after consumption of an HPA meal. In simple terms, when women were consuming the HPA meals, we found less FA safely sequestered into muscle TAG and more FA routed toward the production of AC and ceramides. In women, the metabolic effects of the HPA diet were dependent on physical fitness and associated with decreased insulin sensitivity and insulin secretion. Still uncertain is whether the results of this study point toward sexually dimorphic responses to the experimental diets or, alternatively, if larger cohorts, longer exposures, and/or interventions in populations at risk for diabetes might reveal comparable effects in men and women. These are important questions that highlight the need for additional dietary trials to better establish the efficacy of replacing palmitate with oleate as a nutritional strategy to combat chronic metabolic disease.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health Grants R01-DK-073284 and R01-DK-082803, and these studies were conducted at The University of Vermont GCRC, funded by grant RR-00109 from the National Center for Research Resources, National Institutes of Health, U.S. Public Health Service.
No potential conflicts of interest relevant to this article were reported.
C.L.K., J.Y.B., and D.M.M. researched the data, contributed to discussion, and wrote the manuscript. M.E.P. and T.R.K. researched the data and contributed to discussion. R.S., J.B., O.I., and K.I.C. researched the data. N.K.F. and C.M.C. contributed to discussion. C.L.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Data on the effects of these diets on insulin sensitivity were presented in abstract form at The Obesity Society’s 28th Annual Scientific Meeting, San Diego, California, 8–12 October 2010. In addition, at the same meeting, we presented a limited amount of data, described in this article, on the PA/OA ratio of muscle lipids as well as data showing the LDL-lowering effect of the HOA diet (not presented in this article) (Obesity 2010;18:S103). Finally, some of the data in this study were published in abstract form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
The authors thank the staff of the University of Vermont GCRC for dietary, nursing, body composition, and exercise services, administration, and informatics support. The authors also thank the many subjects for patience and hard work in enduring the rigorous protocol; Dr. Julia Johnson, University of Massachusetts Medical School, for assistance with testing of ovulation status; and Julie Smith, MS, RD, The University of Vermont, for help with diet development under the overall supervision of C.L.K. and Emily Tarleton, MS, RD, LD, Bionutrition Manager at The University of Vermont GCRC, in consultation with C.M.C., Pennington Biomedical Research Center.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-0363/-/DC1.
REFERENCES
- 1.Kien CL. Dietary interventions for metabolic syndrome: role of modifying dietary fats. Curr Diab Rep 2009;9:43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Astrup A, Dyerberg J, Elwood P, et al. The role of reducing intakes of saturated fat in the prevention of cardiovascular disease: where does the evidence stand in 2010? Am J Clin Nutr 2011;93:684–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esposito K, Maiorino MI, Ceriello A, Giugliano D. Prevention and control of type 2 diabetes by Mediterranean diet: a systematic review. Diabetes Res Clin Pract 2010;89:97–102 [DOI] [PubMed] [Google Scholar]
- 4.Gillingham LG, Harris-Janz S, Jones PJ. Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors. Lipids 2011;46:209–228 [DOI] [PubMed] [Google Scholar]
- 5.Hu FB, van Dam RM, Liu S. Diet and risk of type II diabetes: the role of types of fat and carbohydrate. Diabetologia 2001;44:805–817 [DOI] [PubMed] [Google Scholar]
- 6.Coll T, Eyre E, Rodríguez-Calvo R, et al. Oleate reverses palmitate-induced insulin resistance and inflammation in skeletal muscle cells. J Biol Chem 2008;283:11107–11116 [DOI] [PubMed] [Google Scholar]
- 7.Yuzefovych L, Wilson G, Rachek L. Different effects of oleate vs. palmitate on mitochondrial function, apoptosis, and insulin signaling in L6 skeletal muscle cells: role of oxidative stress. Am J Physiol Endocrinol Metab 2010;299:E1096–E1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wen H, Gris D, Lei Y, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol 2011;12:408–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li LO, Klett EL, Coleman RA. Acyl-CoA synthesis, lipid metabolism and lipotoxicity. Biochim Biophys Acta 2010;1801:246–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vessby B, Uusitupa M, Hermansen K, et al. KANWU Study Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: The KANWU Study. Diabetologia 2001;44:312–319 [DOI] [PubMed] [Google Scholar]
- 11.Tierney AC, McMonagle J, Shaw DI, et al. Effects of dietary fat modification on insulin sensitivity and on other risk factors of the metabolic syndrome—LIPGENE: a European randomized dietary intervention study. Int J Obes (Lond) 2011; 35:800–809 [DOI] [PubMed] [Google Scholar]
- 12.Kien CL, Bunn JY. Gender alters the effects of palmitate and oleate on fat oxidation and energy expenditure. Obesity (Silver Spring) 2008;16:29–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285:2486–2497 [DOI] [PubMed] [Google Scholar]
- 14.Stern SE, Williams K, Ferrannini E, DeFronzo RA, Bogardus C, Stern MP. Identification of individuals with insulin resistance using routine clinical measurements. Diabetes 2005;54:333–339 [DOI] [PubMed] [Google Scholar]
- 15.Roger VL, Go AS, Lloyd-Jones DM, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 2011;123:e18–e209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kien CL, Everingham KI, D Stevens R, Fukagawa NK, Muoio DM. Short-term effects of dietary fatty acids on muscle lipid composition and serum acylcarnitine profile in human subjects. Obesity (Silver Spring) 2011;19:305–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heymsfield SB, Smith R, Aulet M, et al. Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nutr 1990;52:214–218 [DOI] [PubMed] [Google Scholar]
- 18.Saad MF, Anderson RL, Laws A, et al. A comparison between the minimal model and the glucose clamp in the assessment of insulin sensitivity across the spectrum of glucose tolerance. Insulin Resistance Atherosclerosis Study. Diabetes 1994;43:1114–1121 [DOI] [PubMed] [Google Scholar]
- 19.Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 1993;42:1663–1672 [DOI] [PubMed] [Google Scholar]
- 20.Børsheim E, Kien CL, Pearl WM. Differential effects of dietary intake of palmitic acid and oleic acid on oxygen consumption during and after exercise. Metabolism 2006;55:1215–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merrill AH, Jr, Sullards MC, Allegood JC, Kelly S, Wang E. Sphingolipidomics: high-throughput, structure-specific, and quantitative analysis of sphingolipids by liquid chromatography tandem mass spectrometry. Methods 2005;36:207–224 [DOI] [PubMed] [Google Scholar]
- 22.Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009;9:311–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koves TR, Li P, An J, et al. PPARgamma coactivator-1alpha -mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem 2005;280:33588–33598 [DOI] [PubMed] [Google Scholar]
- 24.Jensen MV, Joseph JW, Ilkayeva O, et al. Compensatory responses to pyruvate carboxylase suppression in islet beta-cells. Preservation of glucose-stimulated insulin secretion. J Biol Chem 2006;281:22342–22351 [DOI] [PubMed] [Google Scholar]
- 25.Brown RT, McIntosh SM, Seabolt VR, Daniel WA., Jr Iron status of adolescent female athletes. J Adolesc Health Care 1985;6:349–352 [DOI] [PubMed] [Google Scholar]
- 26.Brownlie T, 4th, Utermohlen V, Hinton PS, Haas JD. Tissue iron deficiency without anemia impairs adaptation in endurance capacity after aerobic training in previously untrained women. Am J Clin Nutr 2004;79:437–443 [DOI] [PubMed] [Google Scholar]
- 27.Rajpathak SN, Wylie-Rosett J, Gunter MJ, et al. Diabetes Prevention Program (DPP) Research Group Biomarkers of body iron stores and risk of developing type 2 diabetes. Diabetes Obes Metab 2009;11:472–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arosio P, Levi S. Cytosolic and mitochondrial ferritins in the regulation of cellular iron homeostasis and oxidative damage. Biochim Biophys Acta 2010;1800:783–792 [DOI] [PubMed] [Google Scholar]
- 29.Muoio DM. Intramuscular triacylglycerol and insulin resistance: guilty as charged or wrongly accused? Biochim Biophys Acta 2010;1801:281–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muoio DM, Newgard CB. Obesity-related derangements in metabolic regulation. Annu Rev Biochem 2006;75:367–401 [DOI] [PubMed] [Google Scholar]
- 31.D’Eon TM, Souza SC, Aronovitz M, Obin MS, Fried SK, Greenberg AS. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem 2005;280:35983–35991 [DOI] [PubMed] [Google Scholar]
- 32.Kamei Y, Suzuki M, Miyazaki H, et al. Ovariectomy in mice decreases lipid metabolism-related gene expression in adipose tissue and skeletal muscle with increased body fat. J Nutr Sci Vitaminol (Tokyo) 2005;51:110–117 [DOI] [PubMed] [Google Scholar]
- 33.Haus JM, Kashyap SR, Kasumov T, et al. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 2009;58:337–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kovalik JP, Slentz D, Stevens RD, et al. Metabolic remodeling of human skeletal myocytes by cocultured adipocytes depends on the lipolytic state of the system. Diabetes 2011;60:1882–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adams SH, Hoppel CL, Lok KH, et al. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr 2009;139:1073–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson EJ, Lustig ME, Boyle KE, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 2009;119:573–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugden MC, Holness MJ. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am J Physiol Endocrinol Metab 2003;284:E855–E862 [DOI] [PubMed] [Google Scholar]
- 38.Muoio DM, Noland RC, Kovalik JP, et al. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metab 2012;15:764–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jové M, Planavila A, Laguna JC, Vázquez-Carrera M. Palmitate-induced interleukin 6 production is mediated by protein kinase C and nuclear-factor kappaB activation and leads to glucose transporter 4 down-regulation in skeletal muscle cells. Endocrinology 2005;146:3087–3095 [DOI] [PubMed] [Google Scholar]
- 40.Al-Khalili L, Bouzakri K, Glund S, Lönnqvist F, Koistinen HA, Krook A. Signaling specificity of interleukin-6 action on glucose and lipid metabolism in skeletal muscle. Mol Endocrinol 2006;20:3364–3375 [DOI] [PubMed] [Google Scholar]
- 41.Van Campenhout A, Van Campenhout C, Lagrou AR, et al. Impact of diabetes mellitus on the relationships between iron-, inflammatory- and oxidative stress status. Diabetes Metab Res Rev 2006;22:444–454 [DOI] [PubMed] [Google Scholar]
- 42.Hatipoğlu A, Kanbağli O, Balkan J, et al. Hazelnut oil administration reduces aortic cholesterol accumulation and lipid peroxides in the plasma, liver, and aorta of rabbits fed a high-cholesterol diet. Biosci Biotechnol Biochem 2004;68:2050–2057 [DOI] [PubMed] [Google Scholar]
- 43.Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature 2002;420:333–336 [DOI] [PubMed] [Google Scholar]
- 44.Fu S, Yang L, Li P, et al. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature 2011;473:528–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tonkonogi M, Krook A, Walsh B, Sahlin K. Endurance training increases stimulation of uncoupling of skeletal muscle mitochondria in humans by non-esterified fatty acids: an uncoupling-protein-mediated effect? Biochem J 2000;351:805–810 [PMC free article] [PubMed] [Google Scholar]
- 46.Borst P, Loos JA, Christ EJ, Slater EC. Uncoupling activity of long-chain fatty acids. Biochim Biophys Acta 1962;62:509–518 [DOI] [PubMed] [Google Scholar]
- 47.Esteves TC, Parker N, Brand MD. Synergy of fatty acid and reactive alkenal activation of proton conductance through uncoupling protein 1 in mitochondria. Biochem J 2006;395:619–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dobrzyn P, Pyrkowska A, Jazurek M, Szymanski K, Langfort J, Dobrzyn A. Endurance training-induced accumulation of muscle triglycerides is coupled to upregulation of stearoyl-CoA desaturase 1. J Appl Physiol 2010;109:1653–1661 [DOI] [PubMed] [Google Scholar]
- 49.Chabowski A, Zendzian-Piotrowska M, Nawrocki A, Gorski J. Not only accumulation, but also saturation status of intramuscular lipids is significantly affected by PPARgamma activation. Acta Physiol (Oxf) 2012;205:145–158 [DOI] [PubMed] [Google Scholar]
- 50.Amati F, Dubé JJ, Alvarez-Carnero E, et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes 2011;60:2588–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.