Edited by Helaine E. Resnick, PhD, MPH
A Case for the Development of DPP-4 Inhibitors for Stroke Prevention in Diabetes?
The study by Darsalia et al. shows that linagliptin, a drug currently in use against type 2 diabetes, may also protect against stroke. In this issue of Diabetes (p. 1289), the investigators studied the antistroke efficacy of linagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, in type 2 diabetic mice. Type 2 diabetes is a risk factor for stroke, and stroke patients with diabetes have a greater risk of stroke recurrence and mortality than nondiabetic stroke patients. Glucagon-like peptide-1 (GLP-1) receptor (GLP-1R) agonists are in clinical use against type 2 diabetes. GLP-1R activation can also occur by prolonging the half-life of endogenous GLP-1 via inhibition of DPP-4. In a first set of experiments, 25-week-old obese diabetic mice were treated with either 10 mg/kg/body weight linagliptin per day or 2 mg/kg/body weight sulfonylurea glimepiride per day or vehicle for 7 weeks. Glimepiride was chosen because it does not affect the incretin system. At 4 weeks into the treatment, stroke was induced by transient middle cerebral artery occlusion. In a second set of experiments, 10-week-old control mice were treated with linagliptin, glimepiride, or vehicle for 7 weeks, similar to the first experiment. Again, all mice were subjected to stroke at 4 weeks. Linagliptin was efficacious against stroke in both type 2 diabetic mice and control mice. However, glimepiride showed antistroke efficacy only in control mice. The investigators propose that glimepiride’s neuroprotective effects are mediated by increased insulin secretion, whereas the efficacy of linagliptin is glucose independent and likely involves GLP-1. These results suggest that the development of DPP-4 inhibitors for stroke prevention and treatment in patients with diabetes, as well as in nondiabetic high-risk patients, may be warranted. — Laura Gehl, PhD
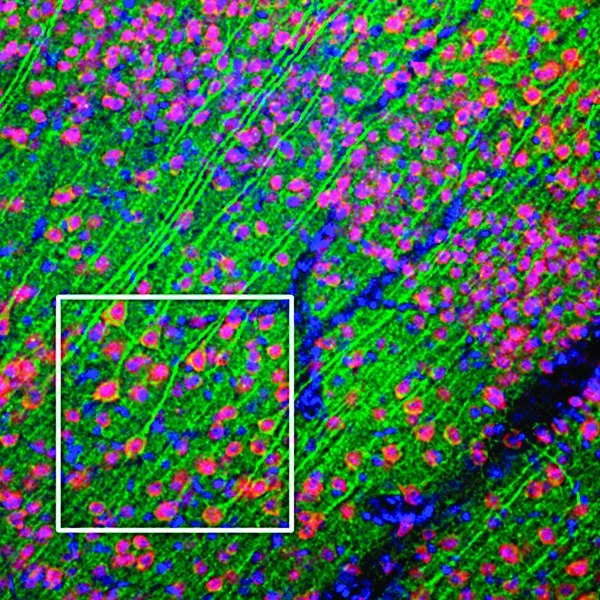
GLP-1R expression in the mouse cerebral cortex
Questioning the Use of Resveratrol for Managing Obesity-Related Metabolic Complications
Data in this issue of Diabetes (p. 1186) raise questions about the utility of resveratrol as a nutritional supplement in metabolic disorders. The study by Poulsen et al. explored the use of resveratrol in obese human subjects. The polyphenolic compound resveratrol, which is prevalent in nature, has been seen for years as a potential tool in managing obesity and obesity-related complications. In rodent models, resveratrol has been shown to protect against diet-induced metabolic abnormalities, and resveratrol is currently sold as an over-the-counter human nutritional supplement. Yet very few data are available to support the use of resveratrol in humans. Poulsen et al. conducted a randomized, placebo-controlled, double-blinded, parallel-group trial, in which 24 obese men with no other health problems were randomly assigned to resveratrol or placebo treatment for 4 weeks. Subjects in the resveratrol group received 1,500 mg trans-resveratrol daily for the 4-week period. Insulin sensitivity was assessed by hyperinsulinemic-euglycemic clamp. Subjects were clamped at a blood glucose level of ∼5 mmol/L with an insulin infusion of 0.5 mU/kg/min. Insulin levels increased from ∼75 pmol/L in the basal period to ∼315 pmol/L in the clamp period, and no significant differences were observed between the two groups for either period. Similarly, resveratrol failed to have a significant effect on fasting glucose, fasting insulin, blood pressure, resting energy expenditure, or inflammatory and metabolic biomarkers. Although the high incidence of obesity and type 2 diabetes calls for novel approaches to obesity management, these results do not support the use of resveratrol as a nutritional supplement for obese humans without a defined metabolic defect. — Laura Gehl, PhD
Blocking AβImproves Insulin Sensitivity in AD Mouse Model
In this issue of Diabetes (p. 1159), Zhang et al. found that not only does amyloid-β (Aβ) induce hepatic insulin resistance in vivo but that neutralizing Aβ also improves insulin sensitivity in an Alzheimer disease (AD) mouse model. In previous research, this group reported that in vitro Aβ induces insulin resistance via JAK/STAT3/SOCS-1 signaling. The new series of experiments confirmed that those results hold in vivo. Treatment with Aβ42 in C57BL/6J mice showed both increased fasting blood glucose levels and markedly reduced insulin sensitivity, without significant change in body weight. Further demonstrating the effects of Aβ, the researchers treated APPswe/PSEN1dE9 (APP/PS1) mice with control IgG or 6C8 or 1H3 anti-Aβ neutralizing antibodies. After 4 months of intraperitoneal injections, mice treated with either 6C8 or 1H3 anti-Aβ antibodies showed significantly decreased fasting blood glucose levels. After 9 months, mice treated with 6C8 anti-Aβ antibody showed both decreased fasting blood glucose levels and improved insulin sensitivity. Further experiments in APP/PS1 mice explored the mechanism of insulin resistance and showed that treatment with Aβ42 caused upregulation of SOCS-1 and activation of JAK2/STAT3 signaling in the liver. Neutralization of Aβ with 1H3 anti-Aβ antibody treatment inhibited the JAK2/STAT3/SOCS-1 signaling pathway. Expanding on this, the researchers used RNAi to knockdown JAK2 in APP/PS1 mice; consequently, JAK2/STAT3/SOCS1 signaling was inhibited, and both insulin sensitivity and glucose metabolism improved. This new report adds to the body of knowledge about the relationship between type 2 diabetes and AD and suggests that reducing Aβ levels or inhibiting JAK2 activity has potential as therapeutic interventions for insulin resistance and type 2 diabetes. — Deborah Elbaum, MD
Rac1 Regulates Contraction-Induced Glucose Uptake in Skeletal Muscle
In this issue of Diabetes (p. 1139), Sylow et al. establish that Ras-related C3 botulinum toxin substrate 1 (Rac1) is not only activated by muscle contraction but that it is also necessary for contraction-induced skeletal muscle glucose uptake. By quantifying the GTP-bound Rac1 immediately after exercise, the investigators demonstrated that Rac1 is activated by exercise in both mice and humans. Confirmatory experiments in mice showed Rac1 to be essential in contraction-induced glucose uptake. Soleus and extensor digitorum longus (EDL) muscles were incubated with one of two Rac1 inhibitors, NSC23766 or Rac1 inhibitor II. Treatment with either inhibitor decreased contraction-induced glucose uptake in the muscle. Treatment with NSC23766 reduced glucose uptake by ∼55% in both soleus and EDL muscle, and the corresponding decreases after treatment with Rac1 inhibitor II were 58% in soleus muscle and 22% in EDL. Transgenic mice with a muscle-specific inducible Rac1 knockout also showed decreased contraction-induced glucose uptake. Further experiments showed Rac1 activation to be independent of AMPK; neither targeted AMPK kinase activity deletion nor AICAR-induced AMPK activation altered normal Rac1 activation. This established that Rac1 is not activated downstream of AMPK. Rac1 is already known to be important for insulin-induced GLUT4 translocation; this series of experiments introduces and establishes its importance in exercise-induced skeletal muscle glucose uptake. As Rac1 activation appears to be either independent or upstream of AMPK activity (or both), further studies are needed to help elucidate this signal transduction pathway. In the future, pharmacological activators of Rac1 might prove to be novel therapies for the control of blood glucose levels. — Deborah Elbaum, MD


