Abstract
Glucagon and glucagon-like peptide (GLP)-1 are the primary products of proglucagon processing from the pancreas and gut, respectively. Giving dual agonists with glucagon and GLP-1 activity to diabetic, obese mice causes enhanced weight loss and improves glucose tolerance by reduction of food intake and by increase in energy expenditure (EE). We aimed to observe the effect of a combination of glucagon and GLP-1 on resting EE and glycemia in healthy human volunteers. In a randomized, double-blinded crossover study, 10 overweight or obese volunteers without diabetes received placebo infusion, GLP-1 alone, glucagon alone, and GLP-1 plus glucagon simultaneously. Resting EE—measured using indirect calorimetry—was not affected by GLP-1 infusion but rose significantly with glucagon alone and to a similar degree with glucagon and GLP-1 together. Glucagon infusion was accompanied by a rise in plasma glucose levels, but addition of GLP-1 to glucagon rapidly reduced this excursion, due to a synergistic insulinotropic effect. The data indicate that drugs with glucagon and GLP-1 agonist activity may represent a useful treatment for type 2 diabetes and obesity. Long-term studies are required to demonstrate that this combination will reduce weight and improve glycemia in patients.
Glucagon and glucagon-like peptide (GLP)-1 are, respectively, pancreatic and intestinal hormones derived from the same proglucagon peptide but with divergent roles in metabolism. Glucagon has primarily been characterized as a counterregulatory hormone that responds to hypoglycemia and fasting by stimulating glycogenolysis and gluconeogenesis, as well as hepatic fatty acid β-oxidation and ketogenesis (1). Glucagon is an integral part of the body’s neurohormonal response to stress together with cortisol and catecholamines (2). On the other hand, GLP-1 is released postprandially and has primary roles in enhancing the β-cell insulin response to eating, enhancing β-cell survival, inhibiting gastric emptying, and suppressing appetite (3). A third proglucagon derivative, oxyntomodulin, possesses both glucagon receptor (GcgR) and GLP-1 receptor (GLP-1R) agonist activity (4,5) and is also released from the gut postprandially.
GLP-1 and its analogs are used for their insulinotropic actions as therapies for type 2 diabetes. As GLP-1 suppresses appetite (6), patients generally experience weight loss with GLP-1 analog therapy (3). However, the magnitude of weight loss is restricted by dose-limiting nausea and vomiting (7), as well as by the fact that GLP-1 tends to reduce energy expenditure (EE) (8). In this connection, glucagon has emerged as a suitable therapeutic partner for GLP-1. Glucagon is also known to reduce appetite (9) but in addition increases EE (10). The combination of GLP-1 and glucagon therefore makes an attractive proposition for obesity therapy. Consistent with this hypothesis, the weak GcgR/GLP-1R coagonist oxyntomodulin is known to suppress appetite (11) and to increase EE, causing considerable weight loss in obese volunteers (12,13).
The hyperglycemic effect of glucagon has deterred investigation of its potential as an obesity treatment. However, oxyntomodulin and other GcgR/GLP-1R coagonists have been shown to have neutral or beneficial glycemic effects in rodents with diet-induced obesity (14–16). This has been hypothesized by others to be due to one or more mechanisms: 1) intrinsic GLP-1R agonism having an effect opposing and neutralizing that mediated by GcgR stimulation, 2) the metabolic benefits of body weight loss outweighing any diabetogenic effect of GcgR stimulation, and 3) an unexpectedly beneficial metabolic effect of sustained GcgR stimulation (15).
These observations suggest that combined administration of GcgR and GLP-1R agonists, or of a single coagonist, could be useful to treat type 2 diabetes and obesity. However, the separate and combined effects of GLP-1 and glucagon on EE and glycemia have not previously been demonstrated in humans. We therefore decided to investigate the effects of the GLP-1 and glucagon combination in healthy human volunteers. Specifically, we wished to confirm that glucagon increases resting EE and that this effect is retained when GLP-1 is combined with glucagon. Secondly, we wished to confirm that GLP-1 is able to ameliorate the hyperglycemia induced by glucagon.
RESEARCH DESIGN AND METHODS
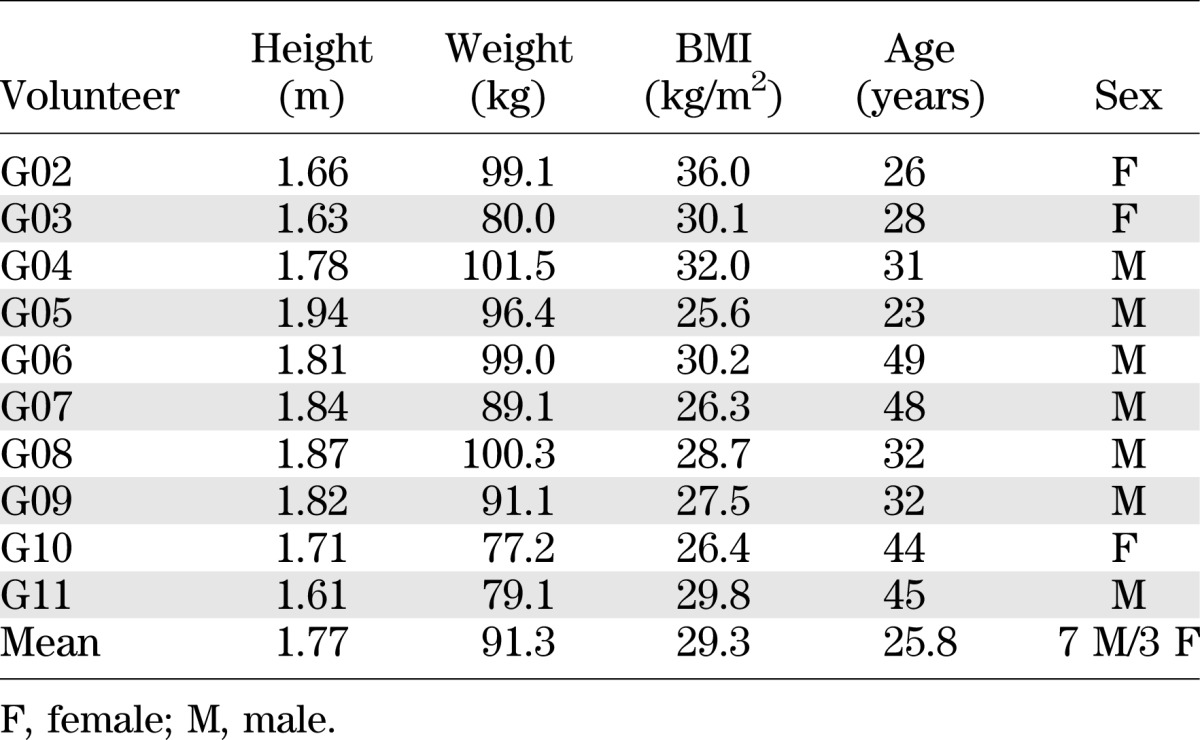
The study was approved by the Hammersmith and Queen Charlotte’s Research Ethics Committee (09/H0707/76) and carried out according to the principles of the Declaration of Helsinki. Ten overweight/obese volunteers were recruited by advertisement (Table 1). Potential participants were screened and determined to be healthy by medical history, physical examination, hematological and biochemical testing, and 12-lead electrocardiogram. All female participants were premenopausal and had regular menstrual cycles. None were taking the combined oral contraceptive pill, but one volunteer was taking a progestogen contraceptive pill that was deemed acceptable, as progestogen-based contraception has previously been found not to alter EE (17). Volunteers were placed briefly under a calorimeter canopy to confirm that they would be comfortable in it during study visits.
TABLE 1.
Demographic details of volunteers in the study
Volunteers attended for five consecutive study visits spaced ≥2 days apart. The initial visit was an unblinded “dummy” visit where gelofusine (B. Braun, Crawley, U.K.) was infused to acclimatize volunteers to study procedures. For avoidance of liver glycogen depletion, volunteers were asked to avoid alcohol and strenuous exercise for 24 h prior to each study visit and were given a high-energy snack (each containing 3.8 g fat, 20.4 g carbohydrate, and 1.9 g protein; Alpen, Weetabix, U.K.) to consume at 2200 h the night before the study. They arrived at the clinical research facility at 0830 h having eaten a small, low-fat breakfast of two McVitie’s Rich Tea biscuits (each containing 1.3 g fat, 5.9 g carbohydrate, and 0.6 g protein; United Biscuits) at 0700 h. Upon arrival, volunteers were asked to empty their bladder; urine was collected at the end of each study visit to provide an estimate of urinary nitrogen excretion.
After dual cannulation to provide a blood sampling point and to allow intravenous infusion of hormones, volunteers were asked to swallow a core temperature measurement capsule (Equivital; Hidalgo, Swavesey, U.K.). They were placed under the indirect calorimeter canopy (Gas Exchange Monitor; GEM Nutrition, Daresbury, U.K.) at 0 min (0900 h). Before each measurement, the calorimeter was calibrated with “zero” (0.00% O2 and 0.00% CO2) and “span” (20.00% O2, 1.00% CO2) gases (BOC Gases, Surrey, U.K.). Volunteers lay semirecumbent on a bed and were allowed to watch television or listen to music. Calorimeter measurements were allowed to stabilize during the first 30 min. Resting EE, respiratory quotient (RQ), and carbohydrate and fat oxidation rates were estimated from measurements of Vo2 and Vco2 recorded each minute, with adjustment for urinary nitrogen excretion. Protein oxidation rate over the entire study visit was estimated from urinary nitrogen excretion (18,19). Baseline resting EE was defined as the mean of measurements taken during the final 15 min of this 45-min baseline phase.
At 45 min, a hormone infusion was started, which lasted for 45 min. Volunteers received intravenous infusions of placebo (gelofusine), glucagon (50 ng/kg/min; Novo Nordisk, Crawley, U.K.), GLP-17–36amide (0.8 pmol/kg/min; Clinalfa Basic, Bachem, Switzerland), or combined GLP-1 and glucagon at the above-mentioned doses, with infusion allocation determined in a four-way, randomized design. Gelofusine was used as the vehicle for hormone infusions in order to minimize absorption of peptides to infusion lines and syringes (20). The infusion was “ramped” to establish stable plasma hormone levels rapidly, with an infusion rate four times the nominal rate for the initial 5 min reduced to twice the nominal rate for the next 5 min and then to the nominal rate for the remaining 35 min. The dose of glucagon was selected after a dose-finding study to determine a well-tolerated dose of glucagon with consistent effects on EE.
Calorimetry continued during the 45-min infusion. As with the baseline phase, calorimetry measurements were allowed to stabilize for 30 min before measurements from the final 15 min were used to calculate the infusion-phase resting EE, RQ, and substrate oxidation rates. At 90 min, the infusion and calorimetry were stopped. Each study visit was terminated at 105 min.
Blood samples were collected at 30, 45, 60, 75, 90, and 105 min and assayed for insulin, glucose, nonesterified fatty acids (NEFAs), thyroid hormones, cortisol, GLP-1, glucagon, and total/acyl ghrelin levels. Samples for analysis of plasma GLP-1 and glucagon were collected in lithium heparin tubes with 100 µL aprotinin (1,000 kallikrein inhibitor units) and were measured using established radioimmunoassays (21,22). NEFAs were measured by the Department of Chemical Pathology, Great Ormond Street Hospital National Health Service Trust. Ghrelin was measured using total ghrelin and active (acyl) ghrelin ELISA assays (Millipore, Watford, U.K.). Other analytes were measured by the Department of Chemical Pathology, Imperial College Healthcare National Health Service Trust.
Statistical analysis was carried out, as noted in the text and Figure captions, using GraphPad Prism 5.0d (GraphPad Software, San Diego, CA) and STATA 12 (StataCorp, College Station, TX). The change from baseline in the calorimetric data was modeled using a linear mixed model with random intercepts to model patient heterogeneity. This model allowed the assessment of the presence of a period effect or a period-by-treatment interaction. The within-subject comparison was based on the treatment effect as long as there was not a significant period-by-treatment interaction. In addition, the baseline calorimetric data and patient weight were included in the model to estimate the adjusted treatment effect. Two-way repeated-measures ANOVA with Bonferroni post hoc test was used to compare differences in glucose, insulin, and ghrelin levels. Area under the curve was calculated using the trapezoidal rule, and differences were compared using one-way repeated-measures ANOVA with Tukey post hoc test. Plasma NEFA concentrations were analyzed with one-way repeated-measures ANOVA and Bonferroni post hoc test. Substrate oxidation rates were analyzed using one-way repeated-measures ANOVA. Unless otherwise noted, results are presented as means ± SEM. Statistical significance was defined as P < 0.05.
RESULTS
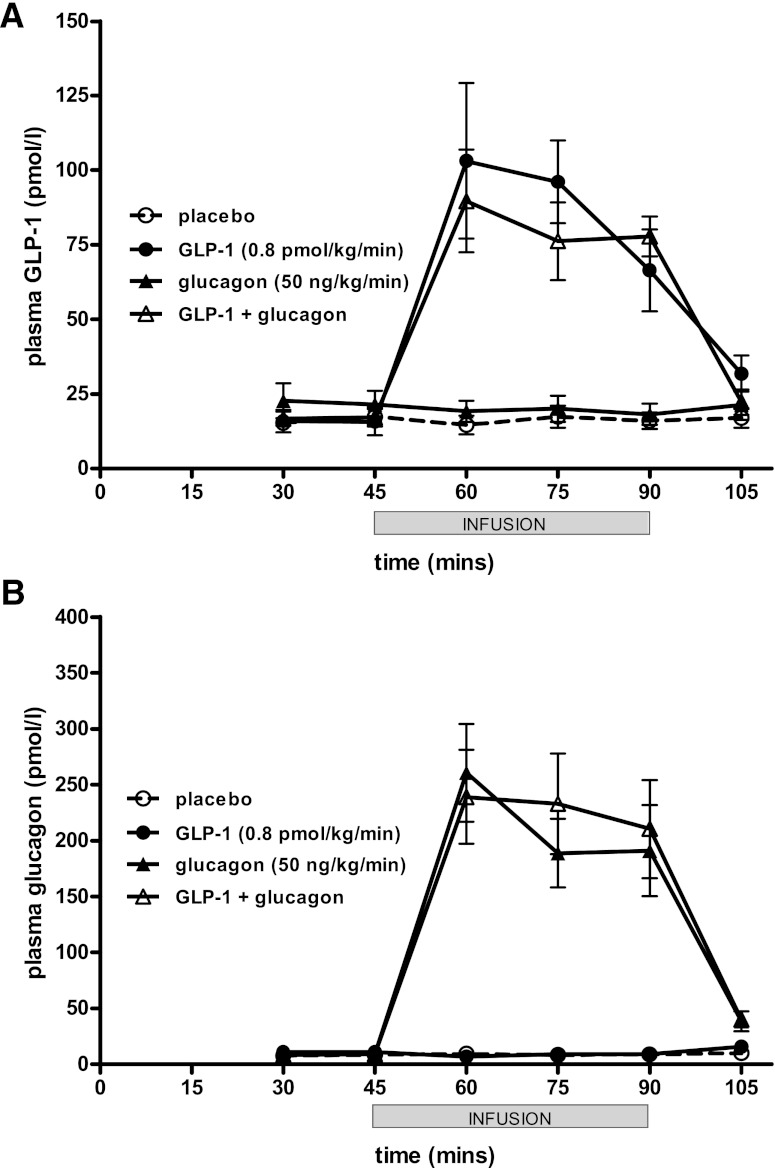
Mean plasma levels of GLP-1 at 30 min before the start of the infusion were 15–23 pmol/L. In those arms receiving the infusion of GLP-1 at 0.8 pmol/kg/min, GLP-1 levels rose to 90–103 pmol/L 60 min after initiation (Fig. 1A). Similarly, glucagon levels at 30 min before starting were 8–11 pmol/L. In those arms receiving the infusion of glucagon at 50 ng/kg/min, glucagon levels rose to 239–260 pmol/L (Fig. 1B). After the infusion was stopped at 90 min, glucagon and GLP-1 levels also rapidly fell to baseline. No adverse events were recorded with the hormone infusions.
FIG. 1.
Plasma GLP-1 (A) and glucagon (B) levels after hormone infusion. Mean ± SEM plasma venous levels plotted. Duration of infusion from 45 to 90 min denoted by gray bar.
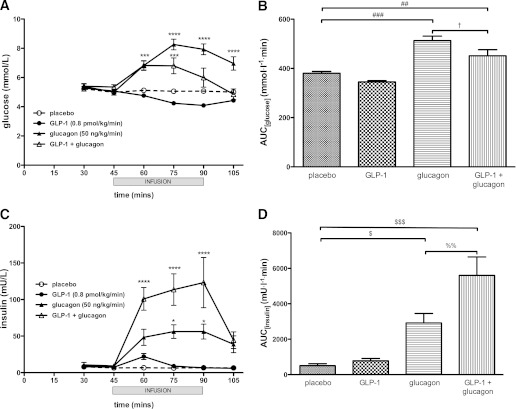
GLP-1 infusion was accompanied by a slight decline in plasma glucose levels from 5.3 ± 0.1 to 4.1 ± 0.1 mmol/L as expected (Fig. 2A). Glucagon infusion, conversely, induced a rise in plasma glucose to a peak of 8.3 ± 0.4 mmol/L at 75 min. This rise was blunted when GLP-1 and glucagon were combined, with a peak of 7.1 ± 0.5 mmol/L at 75 min, declining to 6.2 ± 0.7 mmol/L at the end of the infusion at 90 min (Fig. 2A).
FIG. 2.
Effects of glucagon, GLP-1, and GLP-1 plus glucagon infusion on glucose (A and B) and insulin (C and D) levels. A and C: Mean ± SEM venous plasma glucose and serum insulin levels plotted. Duration of infusion from 45 to 90 min denoted by gray bar. *P < 0.05, ***P < 0.001, ****P < 0.0001 compared with placebo. B: Area under the glucose curve ±SEM plotted. Significantly different area under the curve values are indicated as follows: ##P < 0.01, ###P < 0.001 compared with placebo; †P < 0.05 compared with glucagon. D: Area under the insulin curve ±SEM plotted. Significantly different area under the curve values are indicated as follows: $P < 0.05, $$$P < 0.001 compared with placebo; %%P < 0.01 compared with glucagon.
Consistent with its glucose-dependent insulinotropic effect, GLP-1 induced an early, small rise in insulin levels from 8.1 ± 2.2 to 22.0 ± 4.1 mU/L, which fell back to baseline at later time points (Fig. 2C). Glucagon infusion caused a more marked rise in insulin to a peak of 56.2 ± 10.2 mU/L at 90 min. The combination of GLP-1 and glucagon caused a synergistic rise in insulin levels to a peak of 135.6 ± 35.7 mU/L—a sixfold rise compared with GLP-1 alone and a 2.4-fold rise compared with glucagon alone (Fig. 2C).
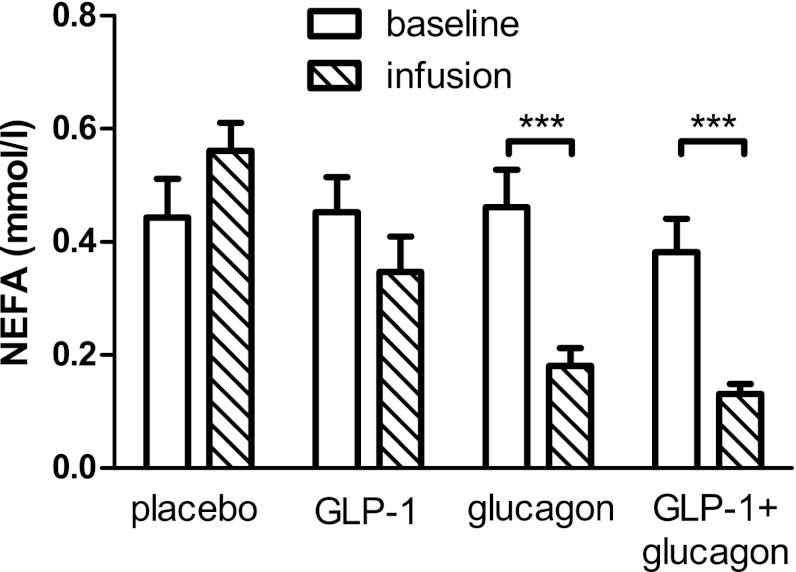
Infusion of glucagon caused a sharp drop in NEFA levels from 0.46 ± 0.07 to 0.18 ± 0.03 mmol/L (Fig. 3). Similarly, the GLP-1 plus glucagon combination caused a fall in NEFA levels from 0.38 ± 0.06 to 0.13 ± 0.18 mmol/L. No significant change in NEFA levels was seen in the placebo or GLP-1 arms.
FIG. 3.
Effects of glucagon, GLP-1, and GLP-1 plus glucagon infusion on NEFA levels. Means ± SEM plotted for NEFA levels during the baseline phase (30 min) and at the end of the infusion phase (90 min). ***P < 0.001 for difference between baseline and infusion phase values.
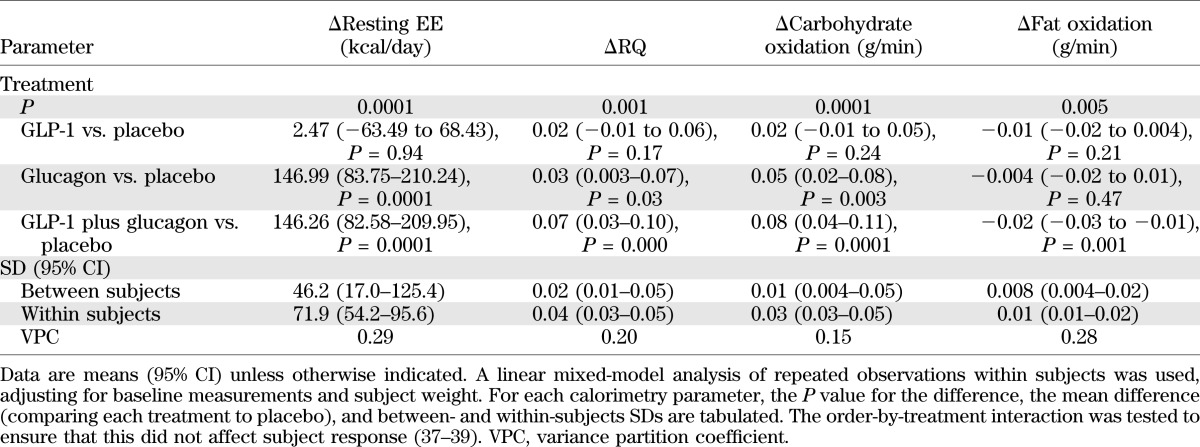
Baseline resting EE was not significantly different between infusion arms. A linear mixed model analysis of repeated measurements, taking into account subject weight, baseline measurements, and the order of infusion (period by treatment), was used to analyze the differences in resting EE induced by the different infusions: no significant interactions were found with these three possible confounding factors (Fig. 4A and Table 2). There was no significant change in resting EE during GLP-1 infusion. However, resting EE increased significantly during glucagon infusion by a mean of 146.99 kcal/day. This effect was retained when GLP-1 and glucagon were infused in combination, with a mean increase in resting EE of 146.26 kcal/day.
FIG. 4.
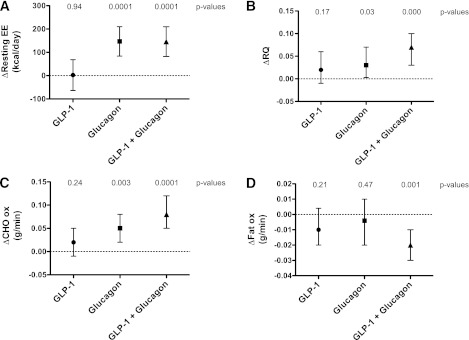
Effects of infusions on EE. The mean changes in each parameter (±95% CI) between baseline and infusion phases are plotted. Baseline resting EEs were as follows: 1,487 ± 70.9 kcal/day (placebo), 1,487 ± 65.9 kcal/day (GLP-1), 1,469 ± 66.0 kcal/day (glucagon), and 1,485 ± 67.4 kcal/day (GLP-1 plus glucagon); they were not significantly different (P = 0.7253, one-way repeated-measures ANOVA). Baseline RQ values for all arms were 0.822 ± 0.018 (placebo), 0.857 ± 0.018 (GLP-1), 0.851 ± 0.020 (glucagon), and 0.834 ± 0.022 (GLP-1 plus glucagon), and these were not significantly different (P = 0.5138, one-way repeated-measures ANOVA). A linear mixed-model analysis of repeated observations within subjects was used to compare these differences for each arm. P values for the observed differences in each parameter are indicated at the top of each graph. A: ΔResting EE (expressed as kilocalories per day). B: ΔRQ (Vco2/Vo2). C: ΔCarbohydrate (CHO) oxidation (ox) rates (grams per minute). D: ΔFat oxidation rates (grams per minute).
TABLE 2.
Statistical analysis of calorimetry data
Baseline RQ values were again not significantly different between infusion arms. The changes in RQ upon infusion were analyzed using the linear mixed model (Fig. 4B and Table 2). There was no significant change in RQ for the GLP-1 infusion arm. There was a significant increase in RQ in the glucagon arm as well as in the GLP-1 plus glucagon arm.
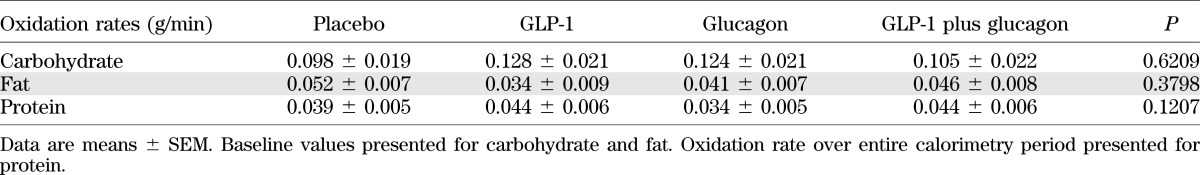
Substrate oxidation rates are summarized in Table 3, which shows that there were no significant differences comparing all arms of the study at baseline. Carbohydrate and fat oxidation rates were analyzed using the linear mixed model (Fig. 4C and D and Table 2). There was no significant change in carbohydrate oxidation rates with GLP-1 infusion. There was a significantly increased carbohydrate oxidation rate with glucagon infusion and a similar increase with GLP-1 plus glucagon infusion. There was no significant change in fat oxidation rate either with GLP-1 infusion or with glucagon infusion, whereas the combined GLP-1 plus glucagon infusion significantly decreased fat oxidation rates.
TABLE 3.
Substrate oxidation rates
There was no significant variation in thyroid hormone levels between the baseline phase and the infusion phase apart from a small, statistically significant reduction in thyrotropin levels when volunteers were given glucagon, which was not seen in the GLP-1 plus glucagon arm and which is therefore of uncertain biological significance (data not shown). There was no significant difference in core temperature (data not shown). There were no significant changes in systolic and diastolic blood pressure, although there was a trend toward a higher pulse rate in the glucagon and GLP-1 plus glucagon infusion arms compared with the placebo and GLP-1 infusion arms (data not shown).
Ghrelin is a key appetite-regulatory hormone that increases food intake (23). Acyl ghrelin, incorporating an octanoyl group linked to the Ser3 residue, is the active form of ghrelin that activates its growth hormone secretagogue receptor 1a (24). As glucagon and GLP-1 have been known to suppress appetite (6,25) and ghrelin secretion (26,27), the plasma levels of ghrelin during infusion were examined (Fig. 5). Infusion of GLP-1 alone did not influence circulating total and acyl ghrelin levels. Glucagon infusion alone reduced total and acyl ghrelin but not to a statistically significant degree. By contrast, the combination of GLP-1 and glucagon significantly reduced total and acyl ghrelin.
FIG. 5.
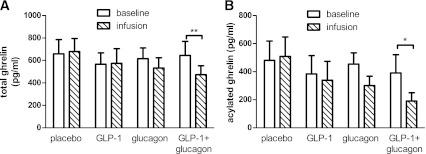
Effects of infusions on total (A) and acylated (B) ghrelin levels (picograms per milliliter). Means ± SEM plotted for the baseline phase and at the end of the infusion phase. *P < 0.05, **P < 0.01.
DISCUSSION
In this clinical study, we have verified for the first time that combined infusion of GLP-1 and glucagon increases resting EE acutely, by the equivalent of ~150 kcal/day, in a manner similar to that of glucagon infusion alone. We speculate that this phenomenon could be mediated by increased thermogenesis in brown adipose tissue (28) and by futile substrate cycling (29). These effects may be triggered directly by glucagon acting on tissue GcgR (e.g., in brown adipose tissue) or indirectly via an increase in catecholamines (2). No consistent effects of glucagon or GLP-1 plus glucagon were seen on the thyroid hormone axis or on cortisol secretion (data not shown). One caveat is that our observations on EE relate to the acute effects of the GLP-1 plus glucagon combination; a longer-term study will be required to confirm that chronic GLP-1R/GcgR agonism in humans leads to a sustained elevation of EE, as was seen in diet-induced obesity rodent models (15). If such an elevation of EE were to be sustained, it would represent ∼7.5% of the typical 2,000 kcal/day resting EE of a 40-year-old male of 100 kg in weight and 1.75 m in height.
At the dose used, glucagon provokes an acute rise in plasma glucose levels, probably via glycogen breakdown. Hyperglycemia is an expected effect of glucagon. Indeed, glucagon antagonists have been mooted as treatments for diabetes on the basis that sustained hyperglucagonemia contributes to the pathogenesis of diabetes (1), although there are major concerns that this strategy may promote hepatic steatosis and liver injury (30). We found that coinfusion of GLP-1 blunted the hyperglycemic effect of glucagon via a synergistic insulinotropic effect, although it did not completely neutralize the acute rise in glucose. It remains possible that the acute hyperglycemic effect would resolve with continued combination treatment, due to increased insulin release and exhaustion of liver glycogen stores. Indeed, the initial hyperglycemia did resolve toward the end of the 45-min infusion of GLP-1 plus glucagon—unlike with glucagon alone (Fig. 2A). It is also noteworthy that oxyntomodulin, administered for 4 weeks to human volunteers, did not result in hyperglycemia (13).
Our study used overweight-obese healthy volunteers without diabetes. Would the synergistic insulinotropic effect of the GLP-1 and glucagon combination be seen in patients with type 2 diabetes and decreased β-cell reserve? Despite the fact that such patients exhibit decreased sensitivity to incretins at physiological levels, it is notable that GLP-1 at pharmacological levels is still able to stimulate a significant insulin response (31). Early indications from clinical trials also suggest that long-term GLP-1R agonist therapy carries positive benefits for insulin secretion and β-cell function (32). Moreover, if chronic treatment induces enhanced weight loss, this may improve glycemia in the long run by enhancing insulin sensitivity. Further studies are therefore required to understand the effect of the GLP-1 and glucagon combination when given for extended time periods and to verify that there is an overall beneficial effect on glycemia in patients with type 2 diabetes.
We observed that plasma NEFA concentrations fell with glucagon and with combined GLP-1 plus glucagon infusions, triggered by the insulinotropic effect of these infusions. We did not observe an increase in fat oxidation rates but, rather, observed a drop with the GLP-1 plus glucagon combination. Although it is traditionally taught that glucagon stimulates lipolysis (33), at the doses used combined glucagon and GLP-1 infusions did not appear to have any direct effect on lipolysis by adipose tissue. Indeed, it has been shown that glucagon’s insulinotropic effect causes a fall in NEFA levels, probably mediated by inhibition of hormone-sensitive lipase (34,35). This finding contrasts with results of studies by Day et al. (15), where chronic treatment with a GLP-1R/GcgR coagonist in diet-induced obese mice was shown to cause phosphorylation and activation of hormone-sensitive lipase. It remains to be seen, therefore, whether chronic GLP-1R/GcgR stimulation may induce a lipolytic state in humans, and this should be addressed in long-term studies of the combination.
Lastly, we show that an intravenous infusion of GLP-1 and glucagon is able to suppress the secretion of ghrelin and that that there was a trend toward suppression of ghrelin levels with intravenous glucagon alone. The suppression of ghrelin secretion by glucagon appears to be mediated by central mechanisms involving the hypothalamus and pituitary and not via insulin-mediated suppression of ghrelin secretion (26,36). We did not observe an effect of GLP-1 alone on ghrelin secretion in our study, unlike Hagemann et al. (27), who found that GLP-1 suppressed ghrelin secretion. The differences may be explained by the fact that their study used a 50% higher dose of GLP-1, and it is notable that the suppression of ghrelin levels was observed after a longer infusion (210–360 min), whereas our study was curtailed after a 45-min infusion. Our data suggest that the combination of GLP-1 and glucagon may indirectly influence appetite by reducing the levels of the orexigenic hormone ghrelin in addition to their respective direct effects on appetite.
In conclusion, our observations provide initial evidence that GLP-1R/GcgR coagonists, such as oxyntomodulin and related hormone analogs, may be suitable to treat type 2 diabetes and obesity. However, further studies are required to investigate whether sustained weight loss and beneficial effects on glycemia persist during chronic treatment.
ACKNOWLEDGMENTS
This study was supported by the Imperial College Healthcare Charity (7006/R50U). Investigative Medicine is funded by the Medical Research Council (MRC), the Biotechnology and Biological Sciences Research Council (BBSRC), the National Institute for Health Research (NIHR), an Integrative Mammalian Biology Capacity Building award, an FP7-HEALTH-2009-241592 EuroCHIP grant, and by funding from the NIHR Imperial Biomedical Research Centre within the Academic Health Sciences Centre. S.R.B. is supported by an NIHR Senior Investigator Award and the MRC. G.S.F. is supported by an NIHR Senior Investigator award. T.M.T. is supported by grants from the MRC. K.A.M. and A.D.S. are recipients of Wellcome Trust Clinical Research Training fellowships. R.C.T., V.S., and B.C.T.F. are recipients of MRC Clinical Research Training fellowships, and B.C.T.F. received an NIHR Clinical Lectureship. A.A. is an NIHR Academic Foundation Year 2 trainee. E.S.C. is supported by a BBSRC Diet and Health Research Industry Club grant. A.V. is the recipient of a National Health and MRC overseas-based Clinical Research fellowship.
No potential conflicts of interest relevant to this article were reported.
T.M.T. designed the study, conducted the study, wrote the manuscript, and reviewed and commented on the manuscript. B.C.T.F. designed the study, conducted the study, and reviewed and commented on the manuscript. K.A.M., R.C.T., E.S.C., and V.S. conducted the study and reviewed and commented on the manuscript. J.G.M. performed the statistical analysis and reviewed and commented on the manuscript. K.C.R.B., A.D.S., A.V., and A.A. conducted the study and reviewed and commented on the manuscript. G.S.F. advised on the calorimetric measurements and data analysis and reviewed and commented on the manuscript. M.A.G. advised on the hormone immunoassays and reviewed and commented on the manuscript. S.R.B. designed the study and reviewed and commented on the manuscript. S.R.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors are grateful to P.R. Bech and A. Hogben, Section of Investigative Medicine, for assistance with assays and to L. Huson, McMichael Wellcome Trust Clinical Research Facility, for statistical advice. The authors also acknowledge N. Germain-Zito, Section of Investigative Medicine, and the staff of the National Institute for Health Research/Wellcome Trust Clinical Research Facility at Imperial College Healthcare National Health Service Trust, without whom this project would not have been possible.
REFERENCES
- 1.Cryer PE. Minireview: glucagon in the pathogenesis of hypoglycemia and hyperglycemia in diabetes. Endocrinology 2012;153:1039–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones BJ, Tan T, Bloom SR. Minireview: glucagon in stress and energy homeostasis. Endocrinology 2012;153:1049–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest 2007;117:24–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bataille D, Gespach C, Tatemoto K, et al. Bioactive enteroglucagon (oxyntomodulin): present knowledge on its chemical structure and its biological activities. Peptides 1981;2(Suppl. 2):41–44 [DOI] [PubMed] [Google Scholar]
- 5.Dakin CL, Gunn I, Small CJ, et al. Oxyntomodulin inhibits food intake in the rat. Endocrinology 2001;142:4244–4250 [DOI] [PubMed] [Google Scholar]
- 6.Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest 1998;101:515–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Astrup A, Rössner S, Van Gaal L, et al. NN8022-1807 Study Group Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 2009;374:1606–1616 [DOI] [PubMed] [Google Scholar]
- 8.Flint A, Raben A, Rehfeld JF, Holst JJ, Astrup A. The effect of glucagon-like peptide-1 on energy expenditure and substrate metabolism in humans. Int J Obes Relat Metab Disord 2000;24:288–298. [DOI] [PubMed]
- 9.Schulman JL, Carleton JL, Whitney G, Whitehorn JC. Effect of glucagon on food intake and body weight in man. J Appl Physiol 1957;11:419–421 [DOI] [PubMed] [Google Scholar]
- 10.Nair KS. Hyperglucagonemia increases resting metabolic rate in man during insulin deficiency. J Clin Endocrinol Metab 1987;64:896–901 [DOI] [PubMed] [Google Scholar]
- 11.Cohen MA, Ellis SM, Le Roux CW, et al. Oxyntomodulin suppresses appetite and reduces food intake in humans. J Clin Endocrinol Metab 2003;88:4696–4701 [DOI] [PubMed] [Google Scholar]
- 12.Wynne K, Park AJ, Small CJ, et al. Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: a randomised controlled trial. Int J Obes (Lond) 2006;30:1729–1736 [DOI] [PubMed] [Google Scholar]
- 13.Wynne K, Park AJ, Small CJ, et al. Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double-blind, randomized, controlled trial. Diabetes 2005;54:2390–2395 [DOI] [PubMed] [Google Scholar]
- 14.Maida A, Lovshin JA, Baggio LL, Drucker DJ. The glucagon-like peptide-1 receptor agonist oxyntomodulin enhances beta-cell function but does not inhibit gastric emptying in mice. Endocrinology 2008;149:5670–5678 [DOI] [PubMed] [Google Scholar]
- 15.Day JW, Ottaway N, Patterson JT, et al. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat Chem Biol 2009;5:749–757 [DOI] [PubMed] [Google Scholar]
- 16.Pocai A, Carrington PE, Adams JR, et al. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes 2009;58:2258–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pelkman CL, Chow M, Heinbach RA, Rolls BJ. Short-term effects of a progestational contraceptive drug on food intake, resting energy expenditure, and body weight in young women. Am J Clin Nutr 2001;73:19–26 [DOI] [PubMed] [Google Scholar]
- 18.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949;109:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 1983;55:628–634 [DOI] [PubMed] [Google Scholar]
- 20.Kraegen EW, Lazarus L, Meler H, Campbell L, Chia YO. Carrier solutions for low-level intravenous insulin infusion. BMJ 1975;3:464–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet 1987;2:1300–1304 [DOI] [PubMed] [Google Scholar]
- 22.Ghatei MA, Uttenthal LO, Bryant MG, Christofides ND, Moody AJ, Bloom SR. Molecular forms of glucagon-like immunoreactivity in porcine intestine and pancreas. Endocrinology 1983;112:917–923 [DOI] [PubMed] [Google Scholar]
- 23.Wren AM, Small CJ, Ward HL, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 2000;141:4325–4328 [DOI] [PubMed] [Google Scholar]
- 24.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999;402:656–660 [DOI] [PubMed] [Google Scholar]
- 25.Geary N, Kissileff HR, Pi-Sunyer FX, Hinton V. Individual, but not simultaneous, glucagon and cholecystokinin infusions inhibit feeding in men. Am J Physiol 1992;262:R975–R980 [DOI] [PubMed] [Google Scholar]
- 26.Arafat MA, Otto B, Rochlitz H, et al. Glucagon inhibits ghrelin secretion in humans. Eur J Endocrinol 2005;153:397–402. [DOI] [PubMed]
- 27.Hagemann D, Holst JJ, Gethmann A, Banasch M, Schmidt WE, Meier JJ. Glucagon-like peptide 1 (GLP-1) suppresses ghrelin levels in humans via increased insulin secretion. Regul Pept 2007;143:64–68 [DOI] [PubMed] [Google Scholar]
- 28.Billington CJ, Briggs JE, Link JG, Levine AS. Glucagon in physiological concentrations stimulates brown fat thermogenesis in vivo. Am J Physiol 1991;261:R501–R507 [DOI] [PubMed] [Google Scholar]
- 29.Miyoshi H, Shulman GI, Peters EJ, Wolfe MH, Elahi D, Wolfe RR. Hormonal control of substrate cycling in humans. J Clin Invest 1988;81:1545–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ali S, Drucker DJ. Benefits and limitations of reducing glucagon action for the treatment of type 2 diabetes. Am J Physiol Endocrinol Metab 2009;296:E415–E421 [DOI] [PubMed] [Google Scholar]
- 31.Holst JJ, Knop FK, Vilsbøll T, Krarup T, Madsbad S. Loss of incretin effect is a specific, important, and early characteristic of type 2 diabetes. Diabetes Care 2011;34(Suppl. 2):S251–S257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garber AJ. Incretin effects on β-cell function, replication, and mass: the human perspective. Diabetes Care 2011;34(Suppl 2):S258–S263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bertin E, Arner P, Bolinder J, Hagström-Toft E. Action of glucagon and glucagon-like peptide-1-(7-36) amide on lipolysis in human subcutaneous adipose tissue and skeletal muscle in vivo. J Clin Endocrinol Metab 2001;86:1229–1234 [DOI] [PubMed] [Google Scholar]
- 34.Samols E, Marri G, Marks V. Interrelationship of glucagon, insulin and glucose. The insulinogenic effect of glucagon. Diabetes 1966;15:855–866 [DOI] [PubMed] [Google Scholar]
- 35.Ranganath L, Schaper F, Gama R, Morgan L. Does glucagon have a lipolytic effect? Clin Endocrinol (Oxf) 2001;54:125–126 [DOI] [PubMed] [Google Scholar]
- 36.Arafat AM, Perschel FH, Otto B, et al. Glucagon suppression of ghrelin secretion is exerted at hypothalamus-pituitary level. J Clin Endocrinol Metab 2006;91:3528–3533 [DOI] [PubMed] [Google Scholar]
- 37.Altman DG. Practical Statistics for Medical Research. London, Chapman and Hall, 1991 [Google Scholar]
- 38.Bland M. An Introduction to Medical Statistics. Oxford, U.K., Oxford University Press, 1995 [Google Scholar]
- 39.Goldstein H, Browne W, Rasbash J. Partitioning variation in multilevel models. Underst Stat 2002;1:223–231 [Google Scholar]