Abstract
The metabolism of lactate to pyruvate in the mediobasal hypothalamus (MBH) regulates hepatic glucose production. Because astrocytes and neurons are functionally linked by metabolic coupling through lactate transfer via the astrocyte-neuron lactate shuttle (ANLS), we reasoned that astrocytes might be involved in the hypothalamic regulation of glucose metabolism. To examine this possibility, we used the gluconeogenic amino acid proline, which is metabolized to pyruvate in astrocytes. Our results showed that increasing the availability of proline in rats either centrally (MBH) or systemically acutely lowered blood glucose. Pancreatic clamp studies revealed that this hypoglycemic effect was due to a decrease of hepatic glucose production secondary to an inhibition of glycogenolysis, gluconeogenesis, and glucose-6-phosphatase flux. The effect of proline was mimicked by glutamate, an intermediary of proline metabolism. Interestingly, proline’s action was markedly blunted by pharmacological inhibition of hypothalamic lactate dehydrogenase (LDH) suggesting that metabolic flux through LDH was required. Furthermore, short hairpin RNA–mediated knockdown of hypothalamic LDH-A, an astrocytic component of the ANLS, also blunted the glucoregulatory action of proline. Thus our studies suggest not only a new role for proline in the regulation of hepatic glucose production but also indicate that hypothalamic astrocytes are involved in the regulatory mechanism as well.
Astrocytes have long been considered as support cells for neurons in the central nervous system (CNS). Studies in recent years, however, have suggested that astrocytes are involved in a number of active roles, including information processing, signal transduction, regulation of neural plasticity (1–3), and, more recently, memory consolidation (4). Astrocytes and neurons are functionally linked through metabolic coupling driven by neuronal activity (5,6). Astrocytes produce lactate from various substrates, and lactate is subsequently released from astrocytes, taken up by neurons, and converted back to pyruvate. The latter is next oxidized for energy production in neurons. Lactate transfer from astrocytes to neurons is possible thanks to the activity of the astrocyte-neuron lactate shuttle (ANLS) (7,8). Directional lactate transfer through the ANLS is based on the differential distribution of monocarboxylate transporters (MCTs) and lactate dehydrogenase (LDH) isoenzymes (7,9). Astrocytes express MCT4 and LDH-A, whereas neurons are enriched in MCT2 and LDH-B (7,9). The conversion of pyruvate to lactate in astrocytes requires the action of LDH-A. Lactate is then exported through the MCT4, taken up by neurons through MCT2, and converted back to pyruvate by the action of LDH-B. Because glucose and pyruvate are both known LDH-dependent hypothalamic modulators of glucose metabolism (10), we reasoned that lactate transfer from astrocytes to neurons might be involved. To test this concept, we chose the gluconeogenic amino acid proline as a probe. Astrocytes but not neurons metabolize gluconeogenic amino acids into pyruvate (11), in contrast to glucose and pyruvate, which can be metabolized by both astrocytes and neurons. Amino acids gain access to the CNS through a facilitative transport system (12). Proline is first converted to glutamate (11) and subsequently metabolized to α-ketoglutarate, which in turn enters the tricarboxylic acid cycle to form pyruvate (Fig. 1A). Next, pyruvate is metabolized to acetyl-CoA and subsequently carboxylated to malonyl-CoA, a key molecule in the central regulation of hepatic glucose metabolism by hypothalamic nutrient sensing (13). We therefore sought to investigate whether the metabolism of the gluconeogenic amino acid proline into pyruvate in the MBH regulates hepatic glucose production by a mechanism requiring the functionality of the ANLS.
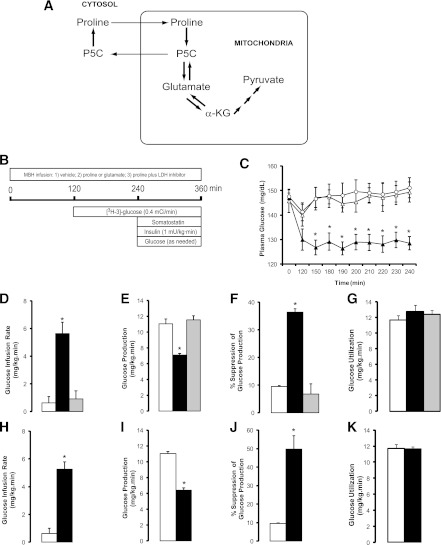
FIG. 1.
Increased availability of the gluconeogenic amino acids proline and glutamate in the MBH lowers plasma glucose by inhibiting endogenous glucose production through a carbohydrate sensing pathway. A, Schematic of proline and glutamate metabolism. B, Schematic of pancreatic clamp procedure in rats receiving central infusions of gluconeogenic amino acids. C, Effect of the central administration of vehicle (n = 6, ○), proline (n = 6, ▲), and proline plus LDH inhibitor oxamate (n = 5, △) on plasma glucose levels. D–G, Effect of the central administration of vehicle (n = 6, white bars), proline (n = 6, black bars), and proline plus LDH inhibitor oxamate (n = 5, gray bars) on glucose infusion rate (D), glucose production (E), suppression of glucose production (F), and glucose utilization (G) during pancreatic clamps. H–K, Effect of the central administration of vehicle (n = 6, white bars) or glutamate (n = 6, black bars) on glucose infusion rate (H), glucose production (I), suppression of glucose production (J), and glucose utilization (K) during pancreatic clamps. *P < 0.05 vs. vehicle. All values are mean ± SEM. α-KG, α-ketoglutarate; P5C, Δ'-pyrroline-5-carboxylic acid.
RESEARCH DESIGN AND METHODS
Animal preparation.
The animal studies were approved by the Institutional Animal Care and Use Committee of the Albert Einstein College of Medicine. Ten-week-old male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) underwent stereotactic surgery for implantation of double cannulas in the MBH, as previously described (14). On recovery, we placed indwelling vascular catheters for infusion and sampling (14). Postoperative recovery was monitored by measuring daily food intake and body weight. Proper placement of the cannulas was confirmed histologically in brain slices prepared postmortem.
Central and systemic infusions.
The in vivo experiments lasted a total of 360 min and were carried out in conscious rats that were restricted to 60 kcal of food the night before to ensure comparable nutritional status. Separate groups of rats received infusion into the MBH for 6 h as follows: 1) vehicle (artificial cerebrospinal fluid), 2) 12 nmol proline, 3) 12 nmol proline plus 8 nmol LDH inhibitor (oxamate) (10), or 4) 4 nmol glutamate. To assess the effect of acute elevations of circulating proline on glucose kinetics, we performed systemic infusions of either vehicle or proline (90 µmol/kg · min) in animals that simultaneously received either vehicle or LDH inhibitor into the MBH. Similar studies were also performed in animals without brain cannulas.
Measurements of in vivo glucose kinetics.
During the intrahypothalamic or systemic infusion, plasma metabolite levels were monitored under basal (0–240 min) and pancreatic clamp (240–360 min) conditions. A primed continuous infusion of 3-[3H]glucose (40-µCi bolus followed by a 0.4 µCi/min infusion) (Perkin Elmer, San Jose, CA) was initiated at 120 min and maintained throughout the study to assess glucose kinetics by tracer dilution methodology, as described previously (15). At the end of the experiments, the rats were anesthetized, and tissue samples were freeze-clamped in situ and stored at −80°C for subsequent analysis.
Analytical procedures and calculations.
Plasma glucose was measured with an Analox instrument (Analox Instruments USA Inc., Lunenburg, MA). Plasma levels of hormones were determined by either ELISA (insulin, adiponectin) or radioimmunoassay (glucagon). The rates of phosphoenolpyruvate gluconeogenesis, glycogenolysis, and flux through glucose-6-phosphatase (Glc-6-Pase) were calculated as previously described (15).
Hypothalamic injections of short hairpin RNA lentiviral vectors.
A 2-μL dose (108 particles/μL) of prevalidated recombinant lentiviral vectors (Thermo Fisher Scientific, Waltham, MA) carrying either rat LDH-A short hairpin RNA (shRNA) (mature target sequence 5′-CTCAATTTGGTCCAGCGAA-3′ based on accessions NM_017025 XM_001080828) or a proprietary control nonsilencing shRNA (RHS4348) were injected into the MBH through chronically implanted cannulas. Specificity of the mature target sequence was confirmed with the Basic Local Alignment Search Tool (BLAST, at http://blast.ncbi.nlm.nih.gov/Blast.cgi). Ten days after the injections, the animals were catheterized for clamp studies.
Western blot analyses.
MBH samples from rats injected with shRNA vectors were homogenized in radioimmunoprecipitation assay SDS buffer and analyzed by Western blot as described previously (14) with primary antibodies to LDH-A (Abcam, Cambridge, MA) and β-tubulin (Covance, Princeton, NJ).
G6pc and Pck1 gene expression in liver.
G6pc and Pck1 gene expression in the liver was measured using quantitative real-time PCR, as previously described (14).
Statistical analyses.
Statistical comparisons were assessed by unpaired Student t test or ANOVA as appropriate by means of GraphPad Prism 5.04 software. Data are presented as means ± SEM.
RESULTS
To determine whether enhancing proline metabolism in the MBH modulates glucose metabolism, we first examined whether a primary increase in hypothalamic proline per se was sufficient to modulate hepatic glucose production. To increase the central availability of proline selectively, we infused this amino acid or vehicle into the MBH of conscious rats (Fig. 1B). The dose of proline selected in these studies was based on the circulating levels of all gluconeogenic amino acids (16). Central proline decreased plasma glucose and insulin levels (Fig. 1C and Supplementary Table 1). To investigate the mechanism by which proline decreased glucose levels, we next assessed its effects during pancreatic basal insulin clamps designed to control for the circulating levels of glucoregulatory hormones (Fig. 1B). In the presence of basal circulating insulin levels, the rate of glucose infusion required to maintain glucose at its basal levels was marginal in rats infused with vehicle in the MBH (Fig. 1D). In contrast, after central infusion of proline, glucose had to be infused at a significantly higher rate to prevent hypoglycemia (Fig. 1D). Glucose kinetics analysis showed that a marked inhibition of endogenous glucose production (Fig. 1E and F) completely accounted for the effect of central proline on whole-body glucose metabolism. In fact, the rate of glucose utilization did not change (Fig. 1G). Before its conversion to pyruvate, proline is metabolized to glutamate (Fig. 1A). We reasoned that glutamate in the MBH should be able to reproduce the effect of proline. To test this possibility, we infused glutamate into the MBH and measured glucose kinetics under basal conditions and during pancreatic clamps. The dose of glutamate used in these studies was well below those known to produce neuroexcitotoxic effects in vivo (17–19). As predicted, glutamate in the MBH completely recapitulated the effect of proline on basal circulating glucose (vehicle 159 ± 3 vs. glutamate 136 ± 2 mg/dL) and endogenous glucose production during pancreatic clamps (Fig. 1H–K).
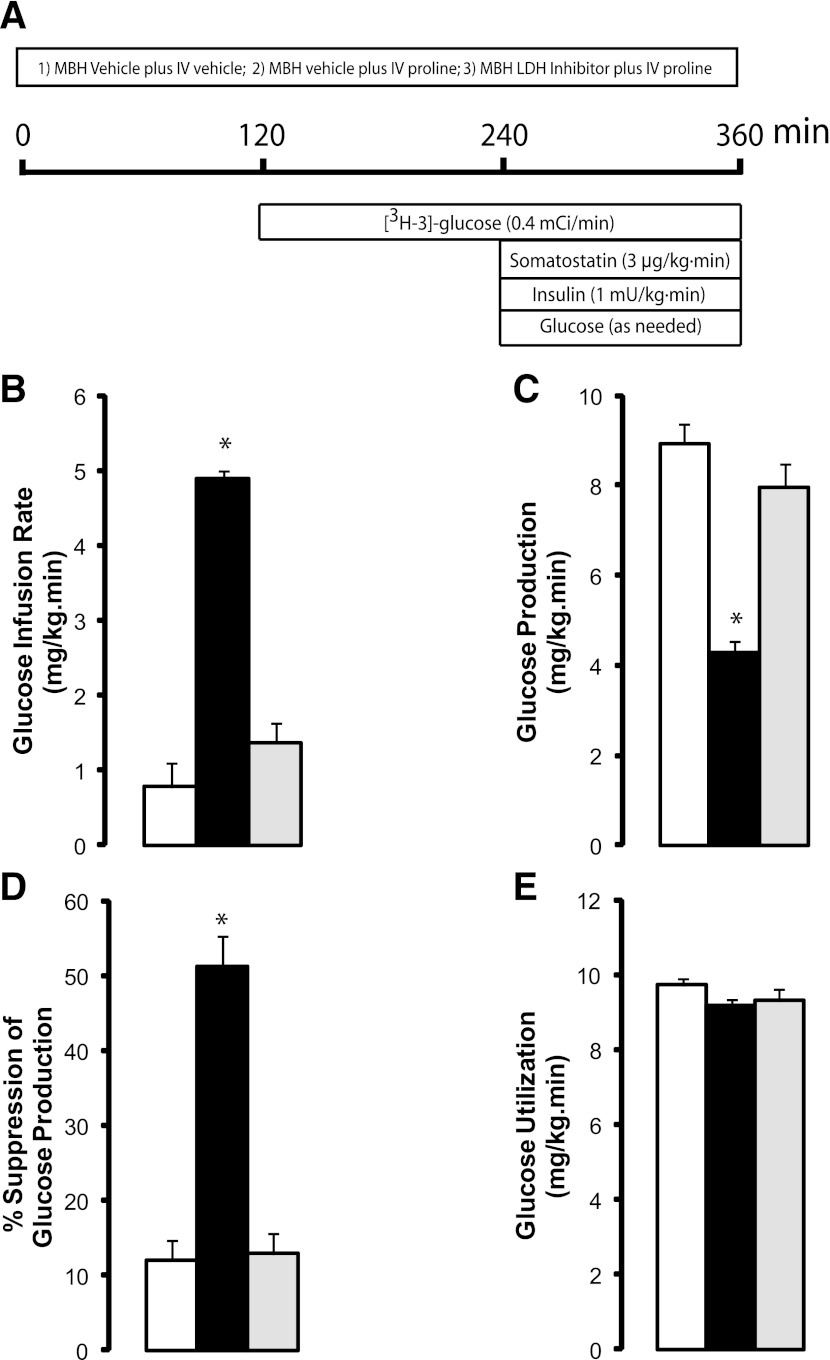
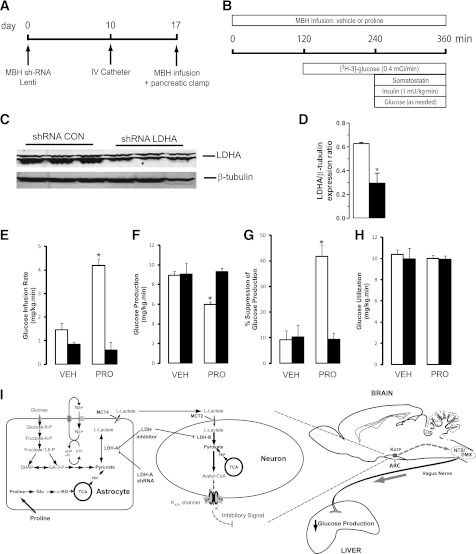
To delineate the mechanisms by which central proline metabolism modulates liver glucose homeostasis, we estimated the in vivo flux through Glc-6-Pase and the relative contributions of glycogenolysis and gluconeogenesis to liver glucose production. Central proline markedly decreased the flux through Glc-6-Pase in the liver (Fig. 2A). Inhibition of hepatic glycogenolysis largely accounted for the decrease in glucose production, although a decrease in gluconeogenesis was also observed (Fig. 2B and C). The hepatic expression of both G6pc and Pck1 was decreased (Fig. 2D and E), whereas the levels of glucoregulatory hormones were unchanged (Supplementary Table 1). Because proline can be metabolized to pyruvate, and the latter regulates liver glucose fluxes on its conversion to lactate (10), we examined the possibility that the metabolism of proline to pyruvate in hypothalamic astrocytes modulates glucose homeostasis. To probe this hypothesis, we infused proline in conjunction with oxamate, a competitive inhibitor of LDH (10). Hypothalamic LDH inhibition negated the glucose-lowering effect of central proline (Fig. 1C). During pancreatic clamps, LDH inhibition also negated the effects of central proline on glucose production (Fig. 1D–F), liver glucose fluxes (Fig. 2A–C), and hepatic G6pc and Pck1 mRNA expression (Fig. 2D–E). Importantly, we have previously shown that central LDH inhibition per se (oxamate alone) does not modify these parameters (10). We next asked whether a moderate increase in circulating proline was capable of regulating liver glucose production and whether these effects required LDH flux in the MBH. We therefore performed systemic infusions of proline in conscious rats while simultaneously infusing either vehicle or LDH inhibitor in the MBH (Fig. 3A and Supplementary Table 2). During pancreatic clamps, high levels of circulating proline completely reproduced the effect of central proline on liver glucose production (Fig. 3B–E). More importantly, under similar conditions the infusion of LDH inhibitor into the MBH markedly blunted the effect of systemic proline on glucose metabolism (Fig. 3B–D), indicating that the effect of circulating proline was centrally mediated and LDH dependent. To rule out the possibility that hypothalamic cannulas artificially disrupted the integrity of the MBH, we performed similar experiments in animals without cannulas. Under these conditions, systemic proline was equally capable of regulating liver glucose production (Supplementary Fig. 1). Next, to examine the consequences of perturbing the ANLS functionality on the gucoregulatory action of proline, we injected lentiviral particles carrying an LDH-A–specific or a nonsilencing (control) shRNA into the MBH of rats (Fig. 4A). Control studies in cultured cells demonstrated that silencing LDH-A does not affect the expression of LDH-B (Supplementary Fig. 2). LDH-A was decreased by 50% in animals injected with LDH-A shRNA compared with a control (nonsilencing) shRNA as determined in samples of MBH analyzed by western blot (Fig. 4C and D). More importantly, during pancreatic clamp studies (Fig. 4B and Supplementary Table 3) the infusion of proline into the MBH of these LDH-A deficient animals failed to inhibit glucose production (Fig. 4E–H), nor did the vectors themselves seem to have independent effects on glucose metabolism (Fig. 4E–H).
FIG. 2.
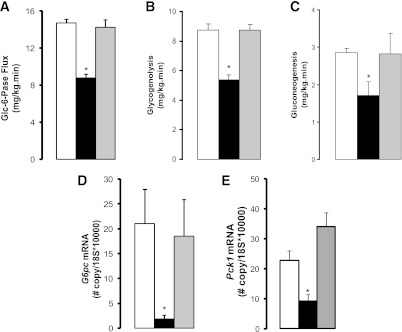
Central infusion of proline in the MBH modulates liver glucose homeostasis through its metabolism to lactate. Effects of the central administration of vehicle (n = 6, white bars), proline (n = 6, black bars), and proline plus LDH inhibitor oxamate (n = 5, gray bars) on the in vivo flux of Glc-6-Pase (A), the relative contributions of glycogenolysis (B) and gluconeogenesis (C), and the hepatic expression of G6pc (D) and Pck1 (E) genes. G6pc and Pck1 gene expression is normalized to that of the housekeeping gene 18s. *P < 0.05 vs. vehicle. All values are mean ± SEM.
FIG. 3.
Circulating proline regulates hepatic glucose production through a central LDH-dependent mechanism. A: Schematic of pancreatic clamp procedure in rats receiving systemic infusions of proline plus either vehicle or LDH inhibitor in the MBH. B–E: Effects of the systemic administration of vehicle (n = 6, white bars), proline with central vehicle (n = 6, black bars), and proline plus central LDH inhibitor (n = 6, gray bars) on glucose infusion rate (B), glucose production (C), suppression of glucose production (D), and glucose utilization (E). *P < 0.05 vs. vehicle. All values are mean ± SEM. IV, intravenous.
FIG. 4.
The glucoregulatory action of proline requires flux through astrocytic LDH-A in the MBH. A: Time line for studies on the effect of lentiviral shRNA-mediated LDH-A knockdown and pancreatic insulin clamps. B: Schematic of pancreatic clamp procedure in rats receiving central (MBH) infusions of proline. C: Representative Western blot of LDH-A knockdown in the MBH of rats. D: Quantification of LDH-A expression in the MBH of rats receiving intrahypothalamic injections of either a control shRNA lentivirus (n = 6, white bar) or LDH-A shRNA lentivirus (n = 6, black bar). E–H: Effect of central vehicle (VEH) and proline (PRO) in animals injected in the MBH with either control nonsilencing (n = 6, white bars) or LDH-A shRNA (n = 6, black bars) on glucose infusion rate (E), glucose production (F), suppression of glucose production (G), and glucose utilization (H). I: Schematic of the proposed mechanism for the regulation of liver glucose fluxes by proline by the ANLS in the hypothalamus. Astrocytes express MCT4 and LDH-A, whereas neurons are enriched in MCT2 and LDH-B. *P < 0.05 vs. control. All values are mean ± SEM. α-KG, α-ketoglutarate; ARC, arcuate nucleus of the hypothalamus; CON, control; DHAP, dihydroxyacetone phosphate; DMX, dorsal motor nucleus of the vagus; Glu, glutamate; KATP, ATP-sensitive potassium channel; NTS, nucleus of the solitary tract; PEP, phosphoenolpyruvate; SUR, sulfonylurea receptor; TCA, tricarboxylic acid cycle.
DISCUSSION
Our studies showed that enhancing the availability of the gluconeogenic amino acid proline within the MBH acutely lowers blood glucose through a robust inhibition of liver glucose production secondary to an inhibition of hepatic glycogenolysis and gluconeogenesis. To bring about this modulatory action, proline engages a biochemical network that requires its conversion to glutamate and carbon flux through LDH in the MBH. In fact, central glutamate completely recapitulated the action of proline. It could be argued that the action of glutamate results from its excitotoxic activity; this is unlikely, however, because the doses of glutamate necessary to bring about excitotoxic effects are much higher than the doses used in this study (17–19). Moreover, either pharmacological or genetic inhibition of hypothalamic LDH, which lies downstream of glutamate, completely attenuated the effect of proline. If proline-derived glutamate had independent effects, these would have persisted or perhaps even been enhanced under these latter conditions. Importantly, the physiological relevance of this regulatory mechanism is highlighted by our observation that moderate elevations of circulating proline acutely regulate liver glucose production, suggesting that postprandial increases of circulating proline can engage this central regulatory mechanism in a manner similar to leucine (14). A substrate-like effect of circulating proline in our studies is unlikely, because liver gluconeogenesis from amino acids contributes to the regulation of blood glucose levels only under conditions such as prolonged fasting (beyond 24 h), pregnancy, ketotic hypoglycemia, and prolonged exercise (20). Of interest, the central infusion of equimolar doses of the nonmetabolizable compound mannitol did not modify glucose metabolism (21), ruling out osmolarity effects. Interestingly, although the available evidence suggests that the signal that is relayed to the liver to adjust glucose fluxes arises in neurons (13), the ability of LDH-A to robustly attenuate the glucoregulatory action of proline strongly suggests not only that proline metabolism to lactate in astrocytes is required but also a role for the ANLS. Our findings are relevant to the ongoing debate on the physiological roles of the ANLS, which has been shown to play a critical role in neuronal energetics (5,6) and more recently in long-term memory consolidation (4) in animal models. Of note, the extent of LDH-A knockdown required to attenuate central proline sensing is comparable to that required in other published studies for disruption of the function of various CNS protein targets (22–24). On the basis of these experimental results, we postulate that proline-derived pyruvate is converted to lactate in astrocytes, exported to neurons, and metabolized back to pyruvate to generate acetyl-CoA and malonyl-CoA (Fig. 4I), the putative key signal that initiates communication with the liver via vagal outflow (13). Previous studies on the modulation of glucose metabolism by macronutrients in the CNS have emphasized the role of hypothalamic neurons (13,14,25). To our knowledge, the rodent studies presented here are the first to suggest a role for proline and for the ANLS in the regulation of liver glucose metabolism.
In conclusion, our studies on the hypothalamic sensing of proline advance our understanding of the mechanisms by which amino acids modulate glucose homeostasis. The new network identified here may be relevant for the development of nutritional or pharmacological interventions for the control of hyperglycemia.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health grants DK45024 to R.G.-J. and DK20541 to the Diabetes Research and Training Center.
No potential conflicts of interest relevant to this article were reported.
I.A.-C., C.M.K., and T.K.T.L. researched and analyzed data and reviewed the manuscript. Y.S. researched data and reviewed the manuscript. R.G.-J. designed the experiments, analyzed data, and wrote the manuscript. R.G.-J. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the following people at the Albert Einstein College of Medicine for their expert technical assistance in these studies: Bing Liu (animal surgery) and Stanislaw Gaweda (biochemical analyses).
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-0228/-/DC1.
I.A.-C. is currently affiliated with the Department of Basic Science Research, National Institute of Geriatrics, Mexico City, Mexico. T.K.T.L. is currently affiliated with the Departments of Physiology and Medicine, University Health Network and University of Toronto, Toronto, Ontario, Canada.
REFERENCES
- 1.Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol 2010;72:335–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature 2010;463:232–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci 2009;32:421–431 [DOI] [PubMed] [Google Scholar]
- 4.Suzuki A, Stern SA, Bozdagi O, et al. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 2011;144:810–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsacopoulos M, Magistretti PJ. Metabolic coupling between glia and neurons. J Neurosci 1996;16:877–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pellerin L, Bouzier-Sore AK, Aubert A, et al. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia 2007;55:1251–1262 [DOI] [PubMed] [Google Scholar]
- 7.Pellerin L, Pellegri G, Bittar PG, et al. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev Neurosci 1998;20:291–299 [DOI] [PubMed] [Google Scholar]
- 8.Pellerin L, Magistretti PJ. Neuroenergetics: calling upon astrocytes to satisfy hungry neurons. Neuroscientist 2004;10:53–62 [DOI] [PubMed] [Google Scholar]
- 9.Pellerin L, Pellegri G, Martin JL, Magistretti PJ. Expression of monocarboxylate transporter mRNAs in mouse brain: support for a distinct role of lactate as an energy substrate for the neonatal vs. adult brain. Proc Natl Acad Sci USA 1998;95:3990–3995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lam TK, Gutierrez-Juarez R, Pocai A, Rossetti L. Regulation of blood glucose by hypothalamic pyruvate metabolism. Science 2005;309:943–947 [DOI] [PubMed] [Google Scholar]
- 11.Bakken IJ, White LR, Unsgård G, Aasly J, Sonnewald U. [U-13C]glutamate metabolism in astrocytes during hypoglycemia and hypoxia. J Neurosci Res 1998;51:636–645 [DOI] [PubMed] [Google Scholar]
- 12.Smith QR. Transport of glutamate and other amino acids at the blood-brain barrier. J Nutr 2000;130(4S Suppl):1016S–1022S [DOI] [PubMed] [Google Scholar]
- 13.Pocai A, Obici S, Schwartz GJ, Rossetti L. A brain-liver circuit regulates glucose homeostasis. Cell Metab 2005;1:53–61 [DOI] [PubMed] [Google Scholar]
- 14.Su Y, Lam TK, He W, et al. Hypothalamic leucine metabolism regulates liver glucose production. Diabetes 2012;61:85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutiérrez-Juárez R, Obici S, Rossetti L. Melanocortin-independent effects of leptin on hepatic glucose fluxes. J Biol Chem 2004;279:49704–49715 [DOI] [PubMed] [Google Scholar]
- 16.Colombo JP, Cervantes H, Kokorovic M, Pfister U, Perritaz R. Effect of different protein diets on the distribution of amino acids in plasma, liver and brain in the rat. Ann Nutr Metab 1992;36:23–33 [DOI] [PubMed] [Google Scholar]
- 17.McBean GJ, Roberts PJ. Neurotoxicity of L-glutamate and DL-threo-3-hydroxyaspartate in the rat striatum. J Neurochem 1985;44:247–254 [DOI] [PubMed] [Google Scholar]
- 18.Fujisawa H, Dawson D, Browne SE, MacKay KB, Bullock R, McCulloch J. Pharmacological modification of glutamate neurotoxicity in vivo. Brain Res 1993;629:73–78 [DOI] [PubMed] [Google Scholar]
- 19.Weiss JH, Hartley DM, Koh J, Choi DW. The calcium channel blocker nifedipine attenuates slow excitatory amino acid neurotoxicity. Science 1990;247:1474–1477 [DOI] [PubMed] [Google Scholar]
- 20.Felig P. Amino acid metabolism in man. Annu Rev Biochem 1975;44:933–955 [DOI] [PubMed] [Google Scholar]
- 21.Lam TK, Pocai A, Gutierrez-Juarez R, et al. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med 2005;11:320–327 [DOI] [PubMed] [Google Scholar]
- 22.Xia H, Mao Q, Eliason SL, et al. RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat Med 2004;10:816–820 [DOI] [PubMed] [Google Scholar]
- 23.McCrimmon RJ, Shaw M, Fan X, et al. Key role for AMP-activated protein kinase in the ventromedial hypothalamus in regulating counterregulatory hormone responses to acute hypoglycemia. Diabetes 2008;57:444–450 [DOI] [PubMed] [Google Scholar]
- 24.Knight CM, Gutierrez-Juarez R, Lam TK, et al. Mediobasal hypothalamic SIRT1 is essential for resveratrol’s effects on insulin action in rats. Diabetes 2011;60:2691–2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes 2002;51:271–275 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.