Abstract
Obesity, diabetes, hypertension, and hyperlipidemia constitute risk factors for morbidity and premature mortality. Based on animal and in vitro studies, resveratrol reverts these risk factors via stimulation of silent mating type information regulation 2 homolog 1 (SIRT1), but data in human subjects are scarce. The objective of this study was to examine the metabolic effects of high-dose resveratrol in obese human subjects. In a randomized, placebo-controlled, double-blinded, and parallel-group design, 24 obese but otherwise healthy men were randomly assigned to 4 weeks of resveratrol or placebo treatment. Extensive metabolic examinations including assessment of glucose turnover and insulin sensitivity (hyperinsulinemic euglycemic clamp) were performed before and after the treatment. Insulin sensitivity, the primary outcome measure, deteriorated insignificantly in both groups. Endogenous glucose production and the turnover and oxidation rates of glucose remained unchanged. Resveratrol supplementation also had no effect on blood pressure; resting energy expenditure; oxidation rates of lipid; ectopic or visceral fat content; or inflammatory and metabolic biomarkers. The lack of effect disagrees with persuasive data obtained from rodent models and raises doubt about the justification of resveratrol as a human nutritional supplement in metabolic disorders.
Management of obesity and its complications is a major health issue (1). Because primary preventive measures often fail and therapeutic options are insufficient, novel treatment modalities are being investigated (2). As such, the polyphenolic compound resveratrol has attracted attention over the past decades (3). It is widely distributed in nature and plays an important role in plants’ defenses against external stressors and infections; it also is a constituent of grapes and wine, albeit in minute amounts (on average, 1.9 mg/L in red wine) (4).
Preclinical trials suggest that resveratrol mimics the metabolic effects of calorie restriction (CR) (5) via activation of silent mating type information regulation 2 homolog 1 (SIRT1) (6–9). By means of NAD+-dependent deacetylase activity, SIRT1 modifies various target proteins, in particular transcription factors critical for energy metabolism, such as nuclear factor-κB, peroxisome proliferator-activated receptor γ coactivator 1-α, forkhead transcription factor class O, and sterol regulatory element-binding protein 1 (10,11).
In experimental animals, these effects translate into improved glucose metabolism (5,7,12–14), reduced inflammation (15–17), cancer prevention (18–21), reversal of nonalcoholic fatty liver disease (7,17,22–24), and prevention of obesity (8,25) In accordance with CR, supplementation with resveratrol promotes longevity in several primitive species (26–28) and protects against diet-induced metabolic abnormalities in rodents (7). Whether these effects of resveratrol apply to human beings remains uncertain, even though it is widely distributed as an over-the-counter nutritional supplement.
Only one trial systematically examining metabolic effects has been performed in humans: 11 obese male participants received a daily dose of 150 mg resveratrol or placebo for 30 days in a double-blind crossover design. Significant albeit moderate improvements in insulin sensitivity, blood pressure, metabolic rate, hepatic steatosis, and pertinent biomarkers were recorded (29). Apart from this single study, only a limited number of human clinical trials of efficacy outcomes have been conducted (30–32). In the present study we tested the impact of high-dose resveratrol administration for 4 weeks on energy and substrate metabolism, insulin sensitivity, ectopic fat disposition, 24-h ambulatory blood pressure, and inflammatory as well as metabolic biomarkers in obese human subjects.
RESEARCH DESIGN AND METHODS
Ethical approval.
All participants were given oral and written information before written informed consent was obtained. The protocol was approved by the Regional Committee on Health Research Ethics and the Danish Data Protection Agency, and the study was conducted in agreement with the Declaration of Helsinki II. According to the International Committee of Medical Journal Editors, the protocol was registered at clinicaltrials.gov (NCT01150955) before recruitment was initiated.
Subjects.
Twenty-four male volunteers, aged 18–70 years, participated. All participants were obese (BMI >30 kg/m2) but otherwise healthy, were taking no prescriptive medicine, and had no overt endocrine disorders. Eligibility ultimately was based on a normal physical examination including routine clinical biochemistry and electrocardiography.
Study design.
The study was an investigator-initiated randomized, double-blinded, placebo-controlled, parallel-group trial. The subjects were treated for 4 weeks with tablets containing 500 mg trans-resveratrol (Fluxome Inc., Stenlose, Denmark) or placebo (Robinson Pharma, Santa Ana, CA) thrice daily. Randomization, blinding, packaging, and labeling was performed by the pharmacy at Aarhus University Hospital. The randomization code was unblinded once all predefined data were recorded.
During the trial period, the subjects were instructed to abstain from using nutritional supplements and consuming food suspected to contain resveratrol in significant amounts. Furthermore, the importance of maintaining their normal way of living was underscored. The compliance rate, defined as the proportion of tablets ingested relative to the intended number, was calculated when participants returned the remaining tablets during the last examination.
Overall visits and interventions.
Examinations were performed on 3 consecutive days both at baseline and after 4 weeks of treatment with the same equipment and by the same physicians and laboratory technicians on both occasions.
On the first day we performed dual-energy X-ray absorptiometry scan and initiated 24-h ambulatory blood pressure recordings. On the second day, we conducted magnetic resonance (MR) spectroscopy and imaging, and on the third day and after an overnight fast the participants underwent a full-day metabolic investigation including a hyperinsulinemic euglycemic clamp with continuous infusion of a primed glucose tracer, indirect calorimetry, repetitive blood sampling, and muscle and adipose tissue biopsies.
When completing the full-day metabolic investigation at baseline, the tablets were provided and the subjects were instructed to initiate tablet consumption in the evening and subsequently three times daily until the overnight fast before the third examination day at week 4. At weeks 2, 4, and 6, potential adverse events were recorded, and fasting blood samples were drawn for safety purposes. Urine from the first void was collected for pharmacokinetic purposes in the morning of the third examination day at week 4.
Hyperinsulinemic euglycemic clamp.
After an overnight fast (from 10:00 p.m.), the participants presented to the research unit at 7.30 a.m. and were studied in the supine position during thermoneutral conditions in the fasting state for 6 h.
For the purpose of infusions, a catheter (Venflon; Viggo, Helsingborg, Sweden) was inserted into the antecubital vein. Another catheter was placed in a heated dorsal hand vein for sampling of arterialized blood. Samples were drawn at 0, 160, 170, 180, 340, 350 and 360 min; while the clamp was in place, plasma glucose levels were determined every 10 min.
The participants were studied from 8:00 a.m. to 2:00 p.m. (0–360 min) (Supplementary Fig. 1). After a 3-h basal period (0–180 min), they were clamped at a blood glucose level of ≈5 mmol/L with a 0.5 mU/kg/min insulin infusion (Actrapid; Novo Nordisk, Bagsværd, Denmark) for the last 3 h (180–360 min) by adjusting the infusion rate of 20% glucose (GIR) in response to blood glucose measurements every 10 min. After emptying the bladder at 0 min, urine was collected at the end of both the basal and clamp periods and the volume was measured.
Muscle and adipose tissue biopsies.
Under sterile conditions and using local anesthesia (Lidocaine SAD 10 mg/mL; Amgros, Copenhagen, Denmark) skeletal muscle and adipose tissue biopsies were taken at the end of the basal period and 20 min into the clamp period (140 and 200 min, respectively). The muscle biopsy was obtained from the vastus lateralis muscle using a Bergström biopsy needle. The muscle tissue was immediately dissected free from fat and connective tissue and transferred to liquid nitrogen. Subcutaneous abdominal fat was obtained by liposuction 15 cm lateral to the umbilicus, cleaned, and subsequently snap-frozen in liquid nitrogen.
[3-3H]-glucose tracer.
A primed continuous infusion of [3-3H]-glucose was given during the entire 6-h basal/clamp procedure (bolus 20 µCi followed by 0.19 µCi/min; NEN Life Science Products, Boston, MA). To avoid rapid dilution of [3-3H]-glucose during the hyperinsulinemic euglycemic clamp, [3-3H]-glucose was added to the infused glucose (100 µCi [3-3H]-glucose/500 ml 20% glucose).
Specific activity of [3-3H]-glucose was measured (33), and glucose rate of appearance (Ra) and rate of disappearance (Rd) was calculated at 10-min intervals from 150 to 180 and 330 to 360 min using Steele’s non-steady-state equation (34).
Under basal conditions, the endogenous glucose production (EGP) equals Ra, and during the clamp period, EGP is calculated by subtracting the mean GIR from Ra. Nonoxidative glucose disposal is derived by subtracting oxidative glucose disposal from total glucose disposal (Rd).
Indirect calorimetry.
The respiratory quotient (RQ) and resting energy expenditure (REE) were estimated by indirect calorimetry (Deltatrac; Datex-Ohmeda, Helsinki, Finland) performed at 90–120 min and 270–300 min in the basal and clamp periods, respectively. Mean values taken during the last 25 min were used for calculations. Glucose and lipid oxidation rates were estimated after correction for protein oxidation, which was calculated on the basis of urea nitrogen excretion (35). Urinary urea content was measured by absorption photometry (ROCHE cobas 6000C, Roche Applied Science, Penzberg, Germany).
Blood sampling and analysis.
Plasma glucose was measured in duplicate immediately after sampling on an YSI 2300 Stat Plus (YSI Inc., Yellow Springs, OH). Glycated hemoglobin (HbA1c), alanine aminotranferase, and leukocytes were analyzed at the University Hospital Department of Clinical Biochemistry using standard methods. All other parameters were analyzed on serum that had been frozen and stored (−80°C) immediately after being drawn and centrifuged.
Total cholesterol, HDL, and triglycerides were determined by absorption photometry (ROCHE cobas 6000C, Roche Applied Science), and LDL subsequently was calculated using the Friedewald formula (36).
Insulin was analyzed using time-resolved immunofluorometric assay (AutoDELFIA Insulin kit, catalog no. B080–101, PerkinElmer, Turku, Finland) and free fatty acids (FFAs) by a commercially available kit (Wako Chemicals, Neuss, Germany). Glucagon and adiponectin were measured by in-house radioimmunoassay (37) and time-resolved immunofluorometric assay (38), respectively.
Finally, C-peptide (DakoCytomation, Cambridgeshire, UK); cortisol (Cortisol ELISA, DRG Instruments GmbH, Marburg, Germany); interleukin 6 (Human IL-6 Quantikine HS ELISA Kit [HS600B], R&D Systems, Minneapolis, MN); monocyte chemoattractant protein-1 (Human CCL2/MCP-1 Quantikine ELISA Kit (DCP00), R&D Systems); tumor necrosis factor α (Human TNF-α Quantikine HS ELISA [HSTA00D], R&D Systems); high-sensitivity C-reactive protein (CRP High Sensitive ELISA (EIA-3954), AH Diagnostics, Aarhus, Denmark); and leptin (Leptin ELISA E07, Mediagnost, Reutlingen, Germany) were analyzed using commercially available ELISA kits.
Hormones relevant to the clamp procedure (C-peptide, FFA, insulin, cortisol, adiponectin, glucagon) were measured at 0, 160, 170, 180, 340, 350, and 360 min and expressed as mean values of basal and clamp triplicates, respectively; the remainder were measured at 0 min. Homeostasis model assessment–insulin resistance (HOMA-IR) was calculated using the standard formula (39), based on fasting glucose and insulin.
Magnetic resonance spectroscopy and imaging.
Intrahepatic and intramyocellular fat content as well as visceral (VAT) and subcutaneous abdominal adipose tissue (SAAT) volumes were measured with magnetic resonance (MR) techniques using a Signa Excite 1.5 Tesla twin-speed scanner (GE Medical Systems, GE Healthcare, Little Chalfont, U.K.). MR spectroscopy of the skeletal muscle included a point-resolved spectroscopy sequence (water suppression; echo time 27 ms; repetition time 3000 ms) on a 2 × 2 × 2 cm voxel positioned in the largest cross-sectional area of the tibialis anterior muscle. Full width at half maximum was 13.4 ± 2.2 Hz. The 1H-MR liver spectroscopy technique has been described previously (40); full width at half maximum was 13.7 ± 3.3 Hz. The spectra were quantified by using the LCmodel software package (version 6.2; Stephen Provencher) by means of a dedicated muscle and liver spectroscopy fitting model. The data processing provided an estimate of the ratio of lipid to water in the tissue within the voxel (41). VAT and SAAT volumes were quantified by MR imaging (body coil; fast-spin echo sequence; echo time 8.5 ms; repetition time 600 ms; slice thickness 8 mm; field of view 48 cm). On the basis of repetitive axial slices from the proximal border of the left kidney to the femur neck, data processing was done using the software package Hippofat (42).
Dual-energy X-ray absorptiometry.
Whole-body bone mineral density and body composition were assessed by dual-energy X-ray absorptiometry (Hologic Discovery scanner S/N 80027; Hologic Inc., Waltham, MA).
Blood pressure.
Noninvasive, 24-h, ambulatory blood pressure monitoring was performed based on measurements every 20th minute (Spacelabs, model 90217).
Western blotting.
Frozen muscles biopsies (∼30 mg) were homogenized in ice-cold solubilization buffer (43), and samples were rotated for 60 min at 4°C. Insoluble materials were removed by centrifugation at 16,000g for 20 min at 4°C, and the protein content of the protein-containing supernatant was determined.
Aliquots of protein were resolved by SDS-PAGE, transferred to polyvinylidene fluoride membranes, and incubated with primary antibody. All primary antibodies were from Cell Signaling (Beverly, MA). To determine relative phosphorylation levels, phospho-specific blots were stripped in SDS buffer and reprobed with the primary antibody for the detection. Horseradish peroxidase-conjugated goat antirabbit immunoglobulin G antibody (Amersham, GE-Healthcare, Pittsburgh, PA) was used as the secondary antibody. Proteins were visualized by enhanced chemiluminescence (Pierce Supersignal West Dura; Thermo Scientific, Rockford, IL) using a ChemiDoc XRS+ CCD camera (BioRad, Hercules, CA).
Real-time RT-PCR.
Total RNA was isolated from muscle and adipose tissue using Trizol (Gibco BRL, Life Technologies, Roskilde, Denmark); RNA was quantified by measuring absorbance at 260 and 280 nm with a ratio ≥1.8 using a NanoDrop 8000 spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA). Integrity of the RNA was checked by visual inspection of the two ribosomal RNAs (18S and 28S) on an agarose gel. cDNA was synthesized with the Verso cDNA kit AB 1453 (Thermo Fisher Scientific Inc.) using random hexamers. Real-time PCR for target genes was done with β2-microglobulin levels as the internal control, and this expression did not change during intervention. Sequences of the primers used are given in Supplementary Table 1.
The PCR reactions were performed in duplicate using the KAPA SYBR FAST qPCR kit (Kapa Biosystems, Inc., Woburn, MA) in a LightCycler 480 (Roche Applied Science) using the following protocol: One step at 95°C for 3 min, then 95°C for 10 s, 60°C for 20 s, and 72°C for 10 s. The increase in fluorescence was measured in real time during the extension step. The relative gene expression was estimated using the default “Advanced Relative Quantification” mode of the software version LCS 480 1.5.0.39 (Roche Applied Science).
Plasma pharmacokinetics and urine metabolite identification.
Concentrations of urinary resveratrol metabolites were determined by high-performance liquid chromatography (HPLC) with ultraviolet/visible detection according to the following method: Aliquots of 2 mL urine were loaded onto conditioned polyamide solid phase extraction cartridges (180 mg), which were washed with 10 mL of water and 5 mL of methanol. The organic layer was evaporated to dryness, and the residues dissolved in 200 µL of 80% acetonitrile before 10 µL was injected for HPLC analysis.
Plasma samples (200 µL) were extracted with 500 µL acetonitrile (100%), mixed, and centrifuged for 3 min at 10,000 rpm. The supernatant was evaporated and redissolved in 200 µL acetonitrile (10%), and 10 µL was injected for HPLC analysis.
Metabolites were separated using aqueous ammonium formate (10 mmol/L; pH = 8.2) or aqueous formic acid (0.1%; pH 2.5) as solvent A and acetonitrile as solvent B in the mobile phase (flow rate 0.5 mL/min) with the following gradient: 10%B linear (0–10 min), 10–40%B linear (10–40 min), 40–99%B linear (40–43 min), 99%B linear (43–48 min). A HyperClone C18 reverse-phase column (length 150 mm, internal diameter4.6 mm, particle size 3 µm; Phenomenex, Torrance, CA) protected by precolumn was used. Detection of metabolites was performed at λ = 300 nm. All metabolites were quantified by external standard calibration. Recoveries of resveratrol were measured by spiking blank urine and plasma samples at the final concentration: from 7.8 µg/mL to 500 µg/mL for urine and from 0.01 µg/mL to 0.05 µg/mL for plasma. Metabolite equivalents were recalculated using standard calibration curves for resveratrol. The structures of metabolites were interpreted by negative atmospheric pressure chemical ionization liquid chromatography–mass spectrometry/mass spectrometry under the HPLC conditions described earlier; these experiments were performed on an LTQ XL (Thermo Fisher Scientific).
Statistical analysis.
Results are presented as means ± SEM when normally distributed and median (range) when not. Unless otherwise noted, the main treatment comparisons between the two groups were assessed by two-way repeated-measures ANOVA. Normality was checked by QQ-plots, and test for equal variance was assessed by the Levene’s test for equal variances. If skewed, the data were logarithmically transformed before applying ANOVA. When revealing significant differences, post hoc pairwise multiple comparison procedures were performed using the Student-Newman-Keuls method.
When appropriate, one-way ANOVA was used on normally distributed data, and the Kruskal-Wallis one-way ANOVA on ranks was used when they were not normally distributed. Baseline comparisons were done by unpaired Student t test. Before applying a nonparametric test, the data were logarithmically transformed. If the data were normally distributed, the unpaired Student t test was used. If the data were not normally distributed, the Mann-Whitney rank sum test was used.
Within-group comparisons were analyzed by paired t test or Wilcoxon signed rank test. P values <0.05 were considered significant. Power calculations were based on changes in M value. To detect a treatment difference of 1.0 mg/kg/min at a two-sided 0.05 significance level with a power of 0.90, nine participants had to be included in each group, assuming an SD of 0.6.
RESULTS
Baseline and internal validity.
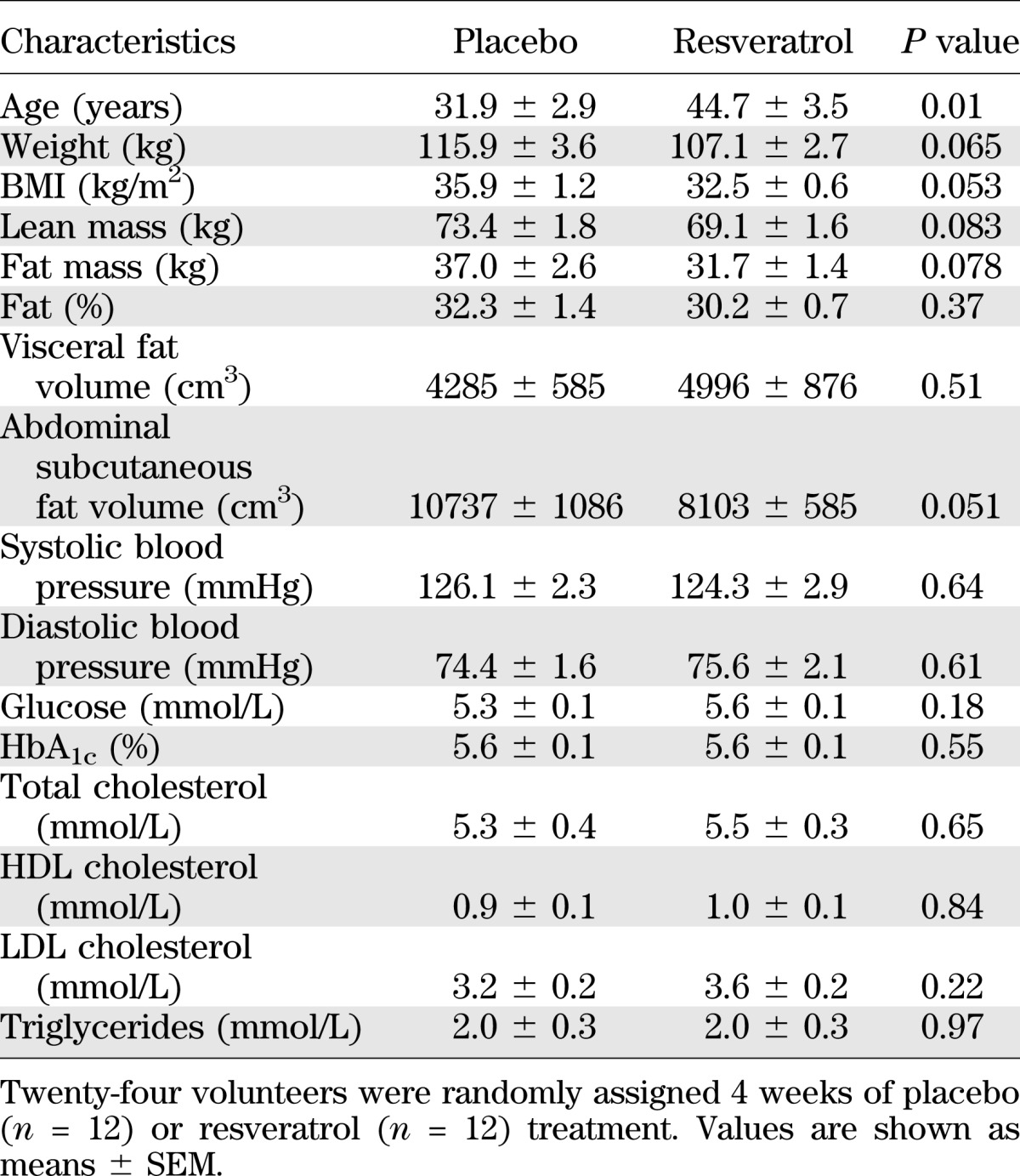
A total of 26 subjects were enrolled, but one participant dropped out because of claustrophobia in relation to MR imaging, and another developed a generalized rash after 1 week and was excluded (Supplementary Fig. 2). Both placebo and resveratrol tablets were generally well tolerated (Supplementary Table 2). The compliance rates, based on pill count, were 89.2 ± 2.9% and 88.9 ± 3.3% in the placebo and resveratrol groups, respectively. By chance the two groups differed slightly in mean age but were comparable on all other baseline parameters (Table 1).
TABLE 1.
Baseline characteristics
To document systemic absorption of the investigational product, we did a pharmacokinetic pilot study before the clinical trial. In that pilot study, three healthy subjects ingested one resveratrol tablet (from the same batch as the investigational product) of 500 mg followed by plasma samples at 0, 15, 30, 45, 60, 90, 120, 180, 240, and 300 min. The native compound reached traceable amounts at 15 min, and Cmax was reached at 90 min with 300–400 ng/mL, which is consistent with the literature (Supplementary Fig. 3) (44–48) yet distinctly below the concentration usually described in in vitro studies (50 μmol/L ≈ 11,400 ng/mL) (8). Furthermore, we compared the absorption profile of our investigational product (trans-resveratrol, Fluxome Inc., Stenlose, Denmark) with that of a similar product (500 mg resVida, DSM Nutritional Products, Ltd., Kaiseraugst, Switzerland). The two products exhibited similar absorption profiles (Supplementary Fig. 3).
The level of urinary resveratrol metabolites was determined at week 4, and no metabolites were detected in the placebo group, whereas measurable amounts were found in the resveratrol group (Supplementary Table 3).
Clinical biochemistry.
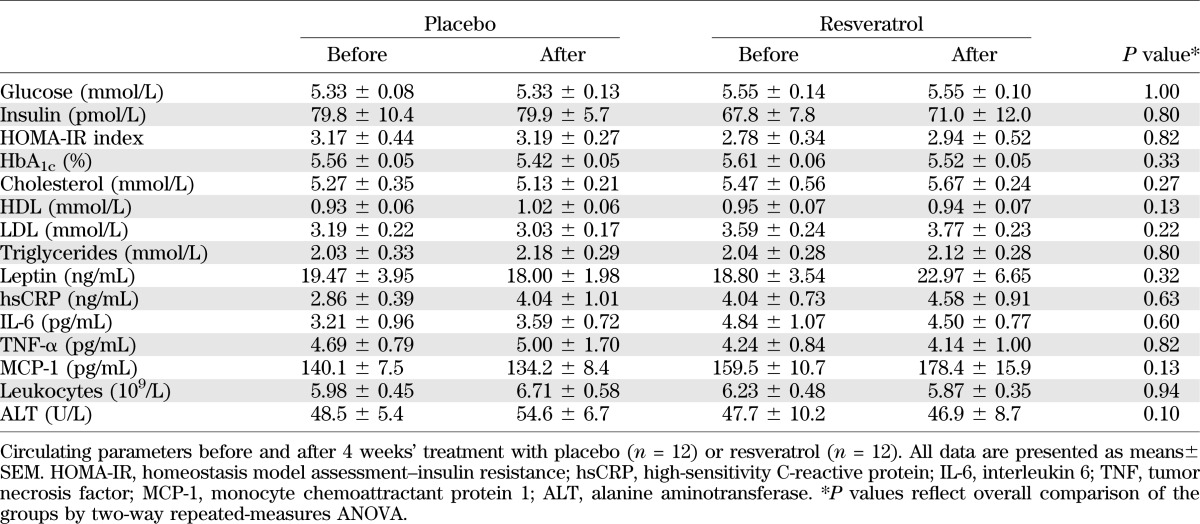
We recorded no significant effects of resveratrol on HbA1c levels or the HOMA-IR. At baseline, the lipid profile was abnormal and compatible with obesity, but we observed no changes in total cholesterol, HDL, LDL, or triglycerides during the intervention period. Moreover, we did not record significant deviations in any inflammatory biomarkers, leptin, or liver function tests (Table 2).
TABLE 2.
Plasma biochemisty
Substrate metabolism.
We did not record statistically significant changes in resting energy expenditure or respiratory quotient or in glucose or lipid oxidation rates when comparing basal and clamp values between the groups (Table 3). As expected, glucose oxidation increased during the clamp at the expense of reduced lipid oxidation. However, these changes did not differ either between or within the two groups (Table 3).
TABLE 3.
Substrate metabolism and circulating hormones
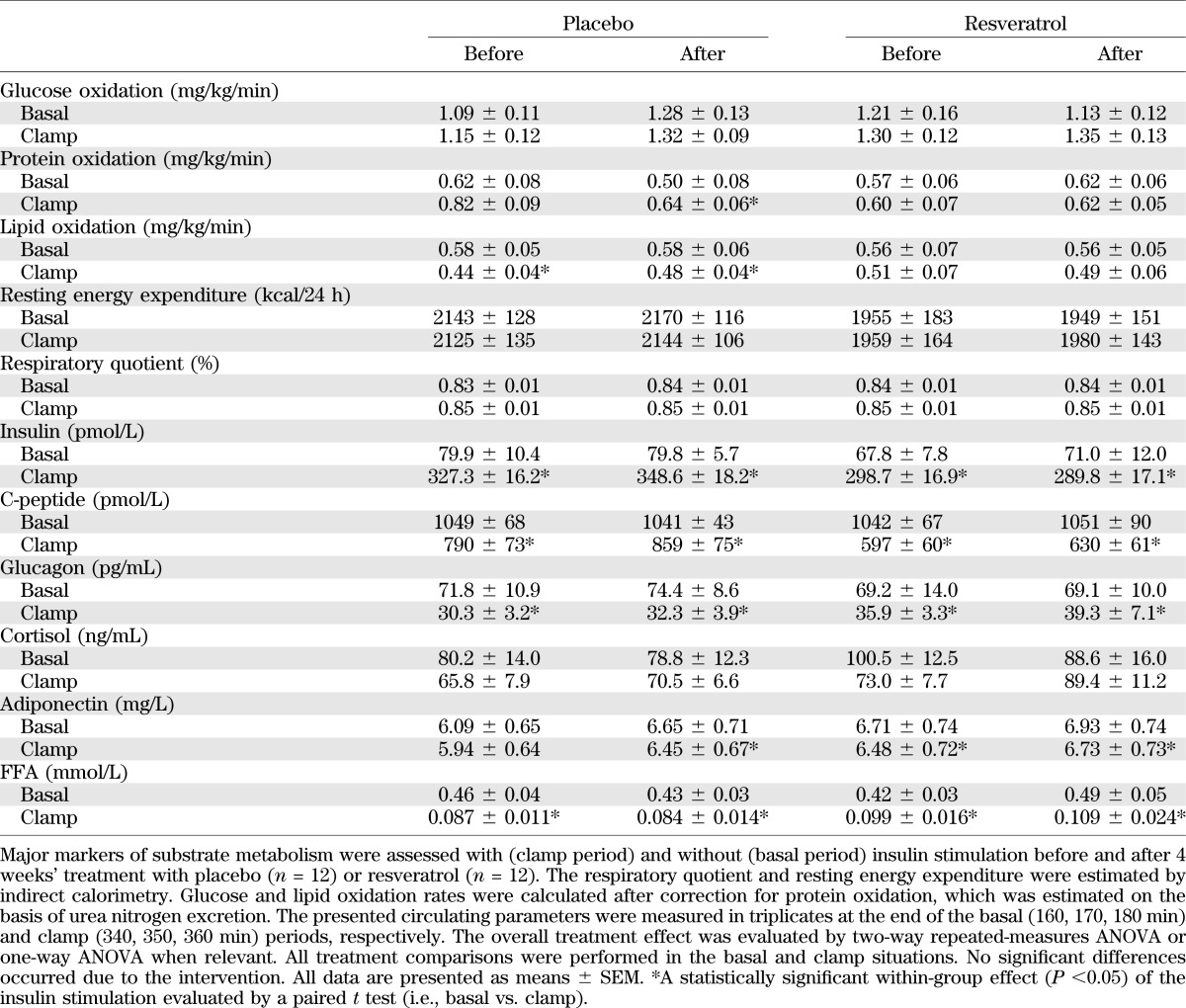
We also failed to detect significant differences in insulin, C-peptide, glucagon, cortisol, adiponectin, or FFAs between the groups. We recorded a significant within-group suppression of C-peptide, glucagon, and FFA levels during the clamp studies both before and after the intervention. We also found a statistically significant and expected decrease in insulin stimulated adiponectin. No within-group changes in cortisol levels were found during insulin stimulation (Table 3).
Insulin sensitivity.
Insulin sensitivity assessed by the hyperinsulinemic euglycemic clamp method was not significantly affected by resveratrol. Insulin levels increased from ≈75 pmol/L in the basal period to ≈315 pmol/L in the clamp period, with no significant differences between the groups regarding the overall basal and clamp periods (P = 0.66 and P = 0.07, respectively). As depicted in Fig. 1A and B, the GIR increased during the clamp and plateaued toward the end of the clamp. GIR did not differ between the groups, and the corresponding M value, defined as the mean GIR during the last 30 min of the clamp, also was comparable between the groups (Fig. 1C). As expected, insulin increased the glucose Rd approximately threefold in the clamp situation compared with the basal condition in all experimental settings, with no significant differences between the two groups (Fig. 1D). Likewise, EGP during both the basal state and the clamp period was comparable between the two groups (Fig. 1E). Finally, the rates of oxidative as well as nonoxidative glucose disposal did not differ between the groups (Fig. 1F).
FIG. 1.
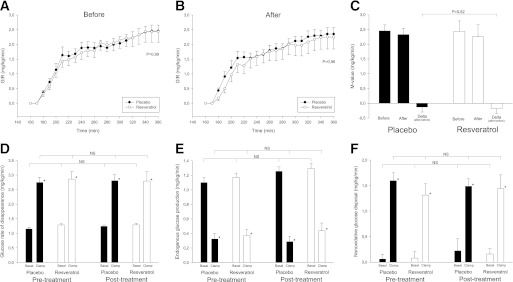
Insulin sensitivity and glucose metabolism. Glucose metabolism was examined before and after 4 weeks of resveratrol or placebo supplementation. Black bars and black dots indicate placebo group (n = 12) and white bars and white dots indicate the resveratrol group (n = 12). Results are presented as group means ± SEM. Insulin sensitivity was assessed by a hyperinsulinemic euglycemic clamp. The participants were clamped at a blood glucose level of ∼5 mmol/L with an insulin infusion of 0.5 mU/kg/min. A and B: The GIR before and after intervention, respectively. P values reflect between-group differences assessed by two-way repeated-measures ANOVA. C: The corresponding whole-body insulin sensitivity, the M value, defined as the mean GIR during the last 30 min of the clamp. P value reflects the potential treatment effect analyzed by two-way repeated-measures ANOVA. D: Glucose Rd. E: EGP. F: Nonoxidative glucose disposal. *A statistically significant within-group effect of the insulin stimulation evaluated by a paired t test. Overall comparisons of potential treatment effects were performed by two-way repeated-measures ANOVA in the basal and clamp situations, respectively. NS, nonsignificant.
Blood pressure.
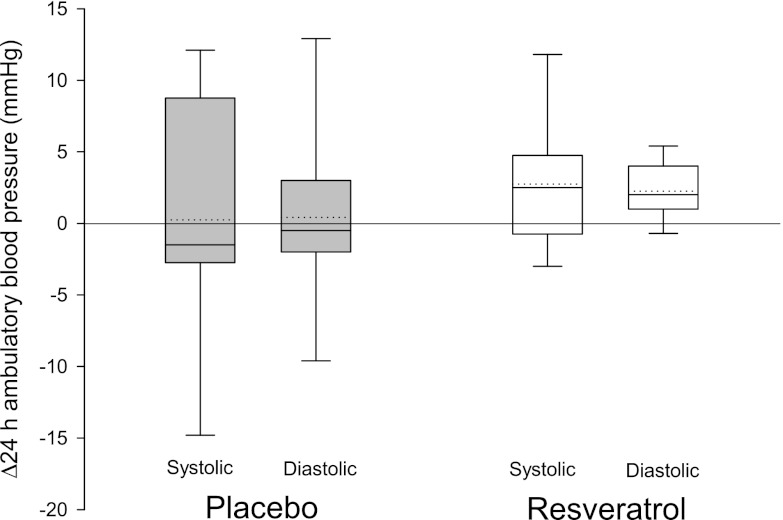
We recorded continuous ambulatory blood pressure profiles over 24 h, which were not affected by resveratrol (Fig. 2). The tendency of resveratrol to increase mean systolic (delta change 2.75 ± 1.38 mmHg) as well as mean diastolic (delta change 2.25 ± 0.58 mmHg) blood pressures was nonsignificant when compared with the placebo group (systolic, P = 0.39; diastolic, P = 0.36).
FIG. 2.
Blood pressure. Ambulatory blood pressure was measured automatically by a portable device every 20th minute over 24 h. Results are presented as box plots of the absolute changes from baseline after 4 weeks of placebo (n = 12) or resveratrol (n = 12) supplementation, respectively. The box boundaries indicate the 25th–75th percentile range, and error bars represent the 5th–95th percentiles. Solid and dotted lines represent median and mean, respectively. Resveratrol supplementation tends to increase both systolic and diastolic blood pressure; however, the elevations are not significant when compared with the placebo group by two-way repeated-measures ANOVA (systolic, P = 0.39; diastolic, P = 0.36).
Body composition.
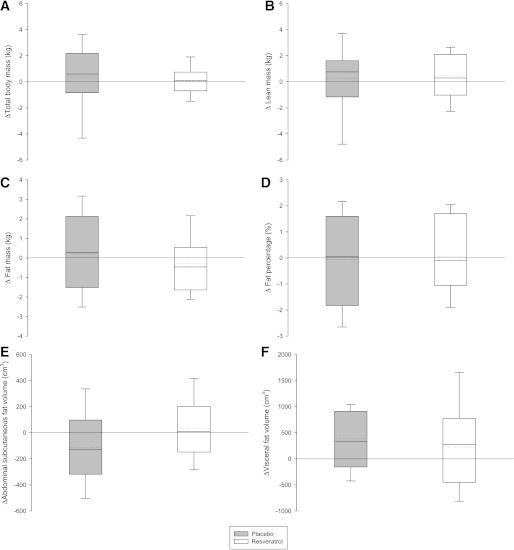
We did not demonstrate any significant differences in total body mass, lean body mass, total body fat mass, or visceral and abdominal subcutaneous fat volumes (Fig. 3).
FIG. 3.
Body composition. Absolute changes in essential body composition parameters after 4 weeks of placebo (n = 12) or resveratrol supplementation (n = 12). The box boundaries indicate the 25th–75th percentile range, and error bars represent the 5th–95th percentiles. Solid and dotted lines represent median and mean, respectively. A–D were assessed by whole-body dual-energy X-ray absorptiometry; E and F were quantified by MR imaging based on repetitive axial slices from the proximal border of the left kidney to the femur neck; nine were evaluable in each group. Two-way repeated-measures ANOVA revealed no statistically significant differences between groups.
Liver and skeletal muscle lipid content.
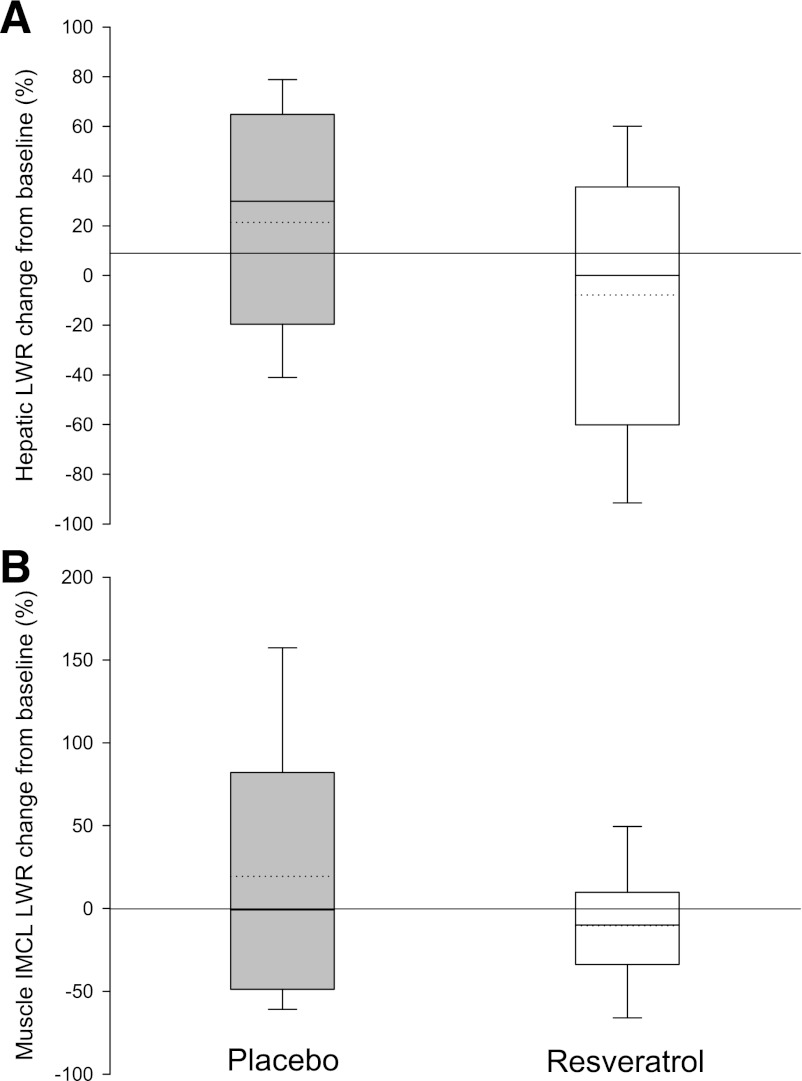
Even though considerable hepatic fat infiltration was evident at baseline, no significant changes occurred in either group during the trial. The same was true when analyzing the intramyocellular lipid content (Fig. 4).
FIG. 4.
Ectopic lipid. Relative changes in ectopic lipid deposition from baseline after 4 weeks of placebo or resveratrol supplementation, respectively. Results are generated by 1H-MR spectroscopy and raw data processing provides an estimate of the ratio of lipid to water (LWR) within the tissue. The box boundaries indicate the 25th–75th percentile range, and error bars represent the 5th–95th percentiles. Solid and dotted lines represent median and mean, respectively. Two-way repeated-measures ANOVA revealed no statistically significant differences between groups. A: Changes in hepatic lipid content; 11 were evaluable in each group. B: Changes in intramyocellular lipid (IMCL) content; 10 were evaluable in each group.
Gene expression and protein phosphorylation.
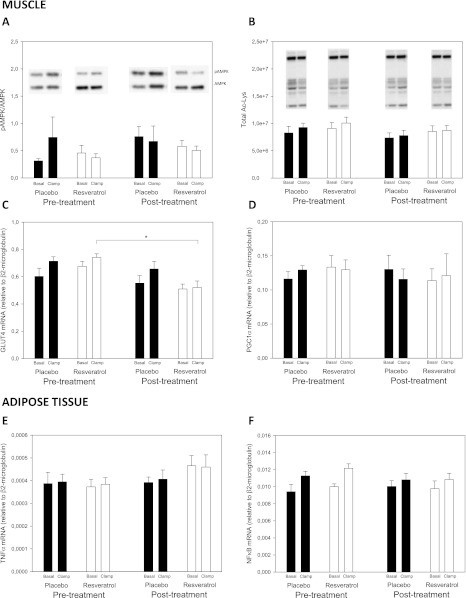
Activation of AMP-activated protein kinase (AMPK) in the muscle tissue biopsies by phosphorylation of the Thr172 residue of the catalytic α-subunit was not impacted by resveratrol. Consistent with this, phosphorylation of acetyl-CoA carboxylase, a well-known downstream target of AMPK activity, also was not affected by the intervention. Furthermore, we explored the proposed SIRT1-mediated deacetylase activity potentially induced by resveratrol by assessing the total acetylation status of lysine residues, which also was not affected (Fig. 5 and Supplementary Fig. 4).
FIG. 5.
Gene expression and protein phosphorylation. Intracellular protein levels and relative mRNA expression in muscle (A–D) and adipose (E and F) tissue biopsies taken before and after 4 weeks' treatment with placebo (n = 12) or resveratrol (n = 12). Biopsies were taken before and during (20 min after initiation) a hyperinsulinemic euglycemic clamp. Black bars indicate the placebo group and white bars indicate the resveratrol group. Results are presented as group means ± SEM, and overall comparisons of potential treatment effects were performed by two-way repeated-measures ANOVA in the basal and clamp situations, respectively. A: Phosphorylation of the intracellular kinase AMP-activated protein kinase (AMPK) assessed by Western blot analysis in muscle tissue. B: Total acetylation of lysine residues assessed by Western blot analysis in muscle tissue. C: Relative GLUT4 mRNA expression in muscle tissue assessed by RT-PCR. D: Relative peroxisome proliferator-activated receptor γ coactivator 1-α (PGC1α) mRNA expression in muscle tissue assessed by RT-PCR. E: Relative tumor necrosis factor (TNF)-α mRNA expression in subcutaneous adipose tissue assessed by RT-PCR. F: Relative nuclear factor (NF)-κB mRNA expression in subcutaneous adipose tissue assessed by RT-PCR. *In C, we found an overall treatment effect in the clamp situation, and a post hoc test revealed a statistically significant (P < 0.05) decreased expression of GLUT4 in the resveratrol group.
Apart from a slightly decreased insulin-stimulated expression of GLUT4 mRNA in muscle in the resveratrol group, gene transcription in muscle and fat did not change in any groups (Fig. 5 and Supplementary Fig. 4).
DISCUSSION
The main finding of our study is that short-term (4 weeks) supplementation with high-dose resveratrol is not associated with detectable physiological effects in obese subjects with modest insulin resistance. We consider this trial important for several reasons. First, the high incidence of obesity and type 2 diabetes calls for novel preventive and therapeutic modalities. Second, substantial evidence from preclinical work suggests that resveratrol stimulates SIRT1 activity and suppresses inflammatory pathways in human adipose tissue in vitro (49,50). Third, the compound is already widely available as an over-the-counter nutritional supplement with an array of alleged salutary effects. Fourth, resveratrol treatment may serve as a model to investigate potential biomarkers for CR and SIRT1 in human models.
Our primary outcome, insulin sensitivity, which was assessed by the hyperinsulinemic euglycemic clamp, was not significantly affected by resveratrol. The same was true for HOMA-IR, fasting glucose, and fasting insulin. Our negative findings are in conflict with the work by Timmers et al. (29), who demonstrated statistically significant improvements in HOMA-IR, suggesting a favorable effect on insulin sensitivity. However, the gold standard for the assessment of whole-body insulin sensitivity is the hyperinsulinemic euglycemic clamp technique used in our study. Furthermore, our inclusion of glucose tracer allowed for the determination of glucose turnover in the basal state and EGP during the clamp, all of which also failed to be affected by resveratrol. We also found no effects of resveratrol on either resting energy expenditure or rates of lipid oxidation.
When assessing blood pressure, we found a trend toward a moderate increase in 24-h blood pressure after resveratrol administration compared with placebo. Again, the differences were insignificant and in opposition to the findings by Timmers et al. (29), who recorded a robust decrease in both systolic and diastolic blood pressures, which were assessed by the mean of conventional clinical blood pressure triplicates. Furthermore, we examined the ectopic fat content in hepatic and muscle tissue by MR spectroscopy and, in contrast to Timmers et al., we recorded no effects of resveratrol. In preclinical settings, the finding of diminished hepatic fat induced by resveratrol is consistent, and the reason why we fail to reproduce the findings by Timmers et al. could relate to baseline differences. Because lipid content is expressed as a relative number relative to the water content, we cannot directly compare the data sets. In accordance with the ectopic fat content, we also failed to detect changes in adipose tissue assessed by MR imaging, which perhaps is less surprising considering the relatively short duration of treatment.
Consistent with the lack of physiological responses to high-dose resveratrol, we also did not find any alterations in gene expression of a panel of pivotal metabolic and inflammatory biomarkers. Nor were protein phosphorylation levels of AMPK and acetyl-CoA carboxylase or total acetylation status affected. As mentioned, our data disagree with the recently published work by Timmers et al. (29), who recorded significant effects using only one-tenth of our dose in a group of obese men. In comparison with the participants in the study by Timmers et al., ours were slightly younger (age 38.4 ± 2.6 vs. 52.5 ± 2.1 years) and more obese (BMI 34.2 ± 0.7 vs. 31.59 ± 0.7 kg/m2; fat 31.3 ± 0.8 vs. 26.4 ± 0.5%). To what degree age and extent of obesity may impact the effect of resveratrol in human subjects remains to be investigated. We have scrutinized our design to identify potential design-related problems to explain the lack of effect. We have demonstrated that the absorption of our resveratol formulation is comparable to the absorption of the compound used in the study by Timmers et al. Our study groups were well matched and of a reasonable sample size, the compliance was good, and we consider the duration of treatment sufficient. The difference in age between our two treatment groups was due to two outliers in the resveratrol group, aged 60 and 68 years. We have repeated all our analysis without these two outliers, which did not change the negative outcome (data not shown). Finally, the risk of a type 2 error due to low sample size should also be considered. In the study by Timmers et al., a sample size of 11 was sufficient to demonstrate effects in a cross-over design. Compared with that study, ours holds the potential advantage of a parallel design, which eliminates the risk of untoward seasonal changes and carry-over effects. A limitation of our study is the inability to directly demonstrate the presence of resveratrol and metabolites in plasma. However, we demonstrated the absorption and excretion of the investigational product by means of our pharmacokinetic pilot study and urinary metabolite measurements.
Based on solid preclinical evidence, it is likely that a certain degree of baseline metabolic abnormalities is a prerequisite to benefit from resveratrol treatment, and it could be speculated that our participants were “too healthy.” However, both HOMA and M values at baseline may indicate mild insulin resistance in our cohort: a HOMA value of 2.77 previously has been defined as the threshold for insulin resistance in metabolically healthy subjects (51). Moreover, in a group of lean but slightly younger men (BMI, 24.6 ± 3.2 kg/m2; age 23.0 ± 2.0 years) clamped at an identical insulin dose and with the same procedures as those used in our laboratory, we recorded an M value of 5.8 mg/kg/min after 3 h (52) compared with 2.5 mg/kg/min in the current study, which suggests a ≈60% reduction in insulin sensitivity in our participants. Nonetheless, in future studies, and before making any definitive conclusions, the therapeutic potential of resveratrol should be tested in patients with more pronounced morbidity such as type 2 diabetes, nonalcoholic fatty liver disease, and hypertension.
Little is known about the pharmacodynamic properties of resveratrol. Even though the preclinical evidence is quite substantial, basic mechanisms of action are not elucidated or agreed upon. SIRT1 seems to be a pivotal mediator of the metabolic effects of resveratrol, but the up- and downstream mechanisms are not fully understood. One of the main questions pending is whether the effects depend on AMPK and, if so, whether AMPK activation is up- or downstream of SIRT1. Regardless of our inability to demonstrate AMPK activation, resveratrol may have potential targets in addition to AMPK and SIRT1. Emerging evidence suggests certain pathways are affected differently at different dose levels, with subsequent effects on physiological outcomes, both in vitro (53) and in vivo (22). In fact, to our knowledge, the latter work is the first to report an inverse dose-response relationship in vivo; more pronounced effects apparently are observed at lower doses. Even though an inverted or J-shaped dose-response relationship seems to be an unlikely explanation for our results, this remains a possibility. Future studies should focus on dose-response relationships and preferably also compare the impact of treatment duration, including potential acute effects of resveratrol. It also remains to be reported whether alternative small-molecule activators of SIRT1 (STACs) may be more effective compared with resveratrol in clinical settings. Regardless of our findings, the scientific field of sirtuin biology remains an area of great interest.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the Danish Agency for Science Technology and Innovation (Grant 09-072323), The Novo Nordisk Foundation, Karen Elise Jensens Foundation, The Toyota Foundation, Elvira and Rasmus Riisfort Foundation, Ejnar Danielsens Foundation, and the AP Møller Maersk Foundation. The study is also part of the research program at LIRMOI Research Center (www.LIRMOI.com), which is supported by the Danish Council for Strategic Research (Grant 10-093499). No other potential conflicts of interest relevant to this article were reported.
M.M.P., N.M., N.J., S.B.P., and J.O.L.J. conceived of and designed the study. P.F.V. and H.S.-J. performed MR scans and processed the raw MR data. B.F.C. performed Western blotting analyses. Y.R. and L.P.C. performed plasma and urine pharmacokinetic analyses. M.M.P. performed all other investigations and analyses and further analyzed data. M.M.P. and J.O.L.J. wrote the manuscript, which was reviewed by all authors. M.M.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank medical laboratory technicians Pia Hornbek, Karen Mathiassen, Annette Mengel, Inge Merete Møller, Lenette Pedersen, Hanne Fjelsted Petersen, Eva Schriver, and Dorte Emilie Wulff, from the Medical Research Unit, Aarhus University Hospital, for their excellent technical assistance. The authors also thank Fluxome for providing the resveratrol and placebo tablets.
Footnotes
Clinical trial reg. no. NCT01150955, clinicaltrials.gov.
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-0975/-/DC1.
See accompanying commentary, p. 1022.
REFERENCES
- 1.World Health Organization. 2012. Obesity and overweight. Fact sheet no. 311 [report online]. Available from http://www.who.int/mediacentre/factsheets/fs311/en/index.html Accessed 12 November 2012
- 2.Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr 2001;21:323–341 [DOI] [PubMed] [Google Scholar]
- 3.Smoliga JM, Baur JA, Hausenblas HA. Resveratrol and health—a comprehensive review of human clinical trials. Mol Nutr Food Res 2011;55:1129–1141 [DOI] [PubMed] [Google Scholar]
- 4.Stervbo U, Vang O, Bonnesen C. A review of the content of the putative chemopreventive phytoalexin resveratrol in red wine. Food Chem 2007;101:449–457 [Google Scholar]
- 5.Barger JL, Kayo T, Vann JM, et al. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One 2008;3:e2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal B, Baur JA. Resveratrol and life extension. Ann N Y Acad Sci 2011;1215:138–143 [DOI] [PubMed] [Google Scholar]
- 7.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006;444:337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006;127:1109–1122 [DOI] [PubMed] [Google Scholar]
- 9.Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 2008;8:157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vang O, Ahmad N, Baile CA, et al. What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLoS One 2011;6:e19881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chachay VS, Kirkpatrick CM, Hickman IJ, Ferguson M, Prins JB, Martin JH. Resveratrol—pills to replace a healthy diet? Br J Clin Pharmacol 2011;72:27–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang W, Hong HJ, Guan J, et al. Resveratrol improves insulin signaling in a tissue-specific manner under insulin-resistant conditions only: in vitro and in vivo experiments in rodents. Metabolism 2012;61:424–433 [DOI] [PubMed] [Google Scholar]
- 13.Andersen G, Burkon A, Sulzmaier FJ, et al. High dose of dietary resveratrol enhances insulin sensitivity in healthy rats but does not lead to metabolite concentrations effective for SIRT1 expression. Mol Nutr Food Res 2011;55:1197–1206 [DOI] [PubMed] [Google Scholar]
- 14.Marchal J, Blanc S, Epelbaum J, Aujard F, Pifferi F. Effects of chronic calorie restriction or dietary resveratrol supplementation on insulin sensitivity markers in a primate, Microcebus murinus. PLoS One 2012;7:e34289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivera L, Morón R, Zarzuelo A, Galisteo M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem Pharmacol 2009;77:1053–1063 [DOI] [PubMed] [Google Scholar]
- 16.Cui X, Jin Y, Hofseth AB, et al. Resveratrol suppresses colitis and colon cancer associated with colitis. Cancer Prev Res (Phila) 2010;3:549–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bujanda L, Hijona E, Larzabal M, et al. Resveratrol inhibits nonalcoholic fatty liver disease in rats. BMC Gastroenterol 2008;8:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jang M, Cai L, Udeani GO, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997;275:218–220 [DOI] [PubMed] [Google Scholar]
- 19.Provinciali M, Re F, Donnini A, et al. Effect of resveratrol on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Int J Cancer 2005;115:36–45 [DOI] [PubMed] [Google Scholar]
- 20.Sengottuvelan M, Nalini N. Dietary supplementation of resveratrol suppresses colonic tumour incidence in 1,2-dimethylhydrazine-treated rats by modulating biotransforming enzymes and aberrant crypt foci development. Br J Nutr 2006;96:145–153 [DOI] [PubMed] [Google Scholar]
- 21.Narayanan NK, Nargi D, Randolph C, Narayanan BA. Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int J Cancer 2009;125:1–8 [DOI] [PubMed] [Google Scholar]
- 22.Cho SJ, Jung UJ, Choi MS. Differential effects of low-dose resveratrol on adiposity and hepatic steatosis in diet-induced obese mice. Br J Nutr 2012;108:2166–2175 [DOI] [PubMed] [Google Scholar]
- 23.Gomez-Zorita S, Fernandez-Quintela A, Macarulla MT, et al. Resveratrol attenuates steatosis in obese Zucker rats by decreasing fatty acid availability and reducing oxidative stress. Br J Nutr 2012;107:202–210 [DOI] [PubMed] [Google Scholar]
- 24.Shang J, Chen LL, Xiao FX, Sun H, Ding HC, Xiao H. Resveratrol improves non-alcoholic fatty liver disease by activating AMP-activated protein kinase. Acta Pharmacol Sin 2008;29:698–706 [DOI] [PubMed] [Google Scholar]
- 25.Dal-Pan A, Blanc S, Aujard F. Resveratrol suppresses body mass gain in a seasonal non-human primate model of obesity. BMC Physiol 2010;10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howitz KT, Bitterman KJ, Cohen HY, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003;425:191–196 [DOI] [PubMed] [Google Scholar]
- 27.Wood JG, Rogina B, Lavu S, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 2004;430:686–689 [DOI] [PubMed] [Google Scholar]
- 28.Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol 2006;16:296–300 [DOI] [PubMed] [Google Scholar]
- 29.Timmers S, Konings E, Bilet L, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab 2011;14:612–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brasnyó P, Molnár GA, Mohás M, et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br J Nutr 2011;106:383–389 [DOI] [PubMed] [Google Scholar]
- 31.Crandall JP, Oram V, Trandafirescu G, et al. Pilot study of resveratrol in older adults with impaired glucose tolerance. J Gerontol A Biol Sci Med Sci 2012;67:1307–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magyar K, Halmosi R, Palfi A, et al. Cardioprotection by resveratrol: a human clinical trial in patients with stable coronary artery disease. Clin Hemorheol Microcirc 2012;50:179–187 [DOI] [PubMed] [Google Scholar]
- 33.Møller N, Jørgensen JO, Schmitz O, et al. Effects of a growth hormone pulse on total and forearm substrate fluxes in humans. Am J Physiol 1990;258:E86–E91 [DOI] [PubMed] [Google Scholar]
- 34.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci 1959;82:420–430 [DOI] [PubMed] [Google Scholar]
- 35.Ferrannini E. The theoretical bases of indirect calorimetry: a review. Metabolism 1988;37:287–301 [DOI] [PubMed] [Google Scholar]
- 36.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502 [PubMed] [Google Scholar]
- 37.Orskov H, Thomsen HG, Yde H. Wick chromatography for rapid and reliable immunoassay of insulin, glucagon and growth hormone. Nature 1968;219:193–195 [DOI] [PubMed] [Google Scholar]
- 38.Frystyk J, Tarnow L, Hansen TK, Parving HH, Flyvbjerg A. Increased serum adiponectin levels in type 1 diabetic patients with microvascular complications. Diabetologia 2005;48:1911–1918 [DOI] [PubMed] [Google Scholar]
- 39.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 40.Moller L, Stodkilde-Jorgensen H, Jensen FT, Jorgensen JO. Fasting in healthy subjects is associated with intrahepatic accumulation of lipids as assessed by 1H-magnetic resonance spectroscopy. Clin Sci (Lond) 2008;114:547–552 [DOI] [PubMed] [Google Scholar]
- 41.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993;30:672–679 [DOI] [PubMed] [Google Scholar]
- 42.Positano V, Gastaldelli A, Sironi AM, Santarelli MF, Lombardi M, Landini L. An accurate and robust method for unsupervised assessment of abdominal fat by MRI. J Magn Reson Imaging 2004;20:684–689 [DOI] [PubMed] [Google Scholar]
- 43.Nielsen C, Gormsen LC, Jessen N, et al. Growth hormone signaling in vivo in human muscle and adipose tissue: impact of insulin, substrate background, and growth hormone receptor blockade. J Clin Endocrinol Metab 2008;93:2842–2850 [DOI] [PubMed] [Google Scholar]
- 44.Brown VA, Patel KR, Viskaduraki M, et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res 2010;70:9003–9011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boocock DJ, Faust GE, Patel KR, et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev 2007;16:1246–1252 [DOI] [PubMed] [Google Scholar]
- 46.Walle T, Hsieh F, DeLegge MH, Oatis JE, Jr, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos 2004;32:1377–1382 [DOI] [PubMed] [Google Scholar]
- 47.Almeida L, Vaz-da-Silva M, Falcão A, et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol Nutr Food Res 2009;53(Suppl. 1):S7–S15 [DOI] [PubMed] [Google Scholar]
- 48.Nunes T, Almeida L, Rocha JF, et al. Pharmacokinetics of trans-resveratrol following repeated administration in healthy elderly and young subjects. J Clin Pharmacol 2009;49:1477–1482 [DOI] [PubMed] [Google Scholar]
- 49.Olholm J, Paulsen SK, Cullberg KB, Richelsen B, Pedersen SB. Anti-inflammatory effect of resveratrol on adipokine expression and secretion in human adipose tissue explants. Int J Obes (Lond) 2010;34:1546–1553 [DOI] [PubMed] [Google Scholar]
- 50.Pedersen SB, Ølholm J, Paulsen SK, Bennetzen MF, Richelsen B. Low Sirt1 expression, which is upregulated by fasting, in human adipose tissue from obese women. Int J Obes (Lond) 2008;32:1250–1255 [DOI] [PubMed] [Google Scholar]
- 51.Bonora E, Kiechl S, Willeit J, et al. Prevalence of insulin resistance in metabolic disorders: the Bruneck Study. Diabetes 1998;47:1643–1649 [DOI] [PubMed] [Google Scholar]
- 52.Gormsen LC, Nielsen C, Jessen N, Jørgensen JO, Møller N. Time-course effects of physiological free fatty acid surges on insulin sensitivity in humans. Acta Physiol (Oxf) 2011;201:349–356 [DOI] [PubMed] [Google Scholar]
- 53.Park SJ, Ahmad F, Philp A, et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 2012;148:421–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.