Abstract
The specific cellular underpinnings or mechanisms of insulin resistance (IR) are not clear. Here I present evidence to support a causal association between mitochondrial energetics and IR. A large body of literature indicates that mitochondrial capacity for oxidative metabolism is lower in human obesity and type 2 diabetes. Whether or not mitochondria play a causal role in IR is hotly debated. First, IR can be caused by many factors, many of which may or may not involve mitochondria. These include lipid overload, oxidative stress, and inflammation. Thus the first tenet of an argument supporting a role for mitochondria in IR is that mitochondria derangements can cause IR, but IR does not have to involve mitochondria. The second tenet of this argument is that animal models in which oxidative metabolism are completely abolished are not always physiologically or pathologically relevant to human IR, in which small metabolic perturbations can have profound effects over a prolonged period. Lastly, mitochondria are complex organelles, with diverse functions, including links with cell signaling, oxidative stress, and inflammation, which in turn can be connected with IR. In summary, mitochondrial “deficiency” is not merely a reduced energy generation or low fatty acid oxidation; this concept should be expanded to numerous additional important functions, many of which can cause IR if perturbed.
The most common forms of human skeletal muscle insulin resistance (IR) are associated with 1) obesity, particularly abdominal obesity and excess accumulation of lipids in nonadipose tissues such as liver and skeletal muscle; and 2) physical inactivity. Identifying a common cellular basis for these conditions, however, remains elusive. Impairments in mitochondrial energetics have been linked to each of these conditions. Obesity has been reported to be associated with reduced mitochondria content and altered mitochondrial performance (1). Physical inactivity is associated with lower mitochondrial biogenesis and content (2). Conversely, exercise is a potent inducer of mitochondria biogenesis (3). Thus it is not surprising that considerable attention has been given to the possibility that mitochondria play a role in IR. But of course associations do not infer that derangements in mitochondria cause IR. Although many of these arguments can be made for other insulin-sensitive tissues such as liver, this line of reasoning to support a role for mitochondria in IR will focus on skeletal muscle.
DEVELOPING THE ARGUMENT
While there is little debate that low mitochondria content or poor mitochondrial capacity or performance is associated with IR in muscle, the current debate is centered on whether or not some aspect(s) of mitochondrial biogenesis, content, or energetics actually cause IR. I will make the case that the majority of evidence supports the claim that mitochondrial derangements can cause IR in humans. In order to set the stage for this argument, it is important to outline some fundamental truisms that support this view.
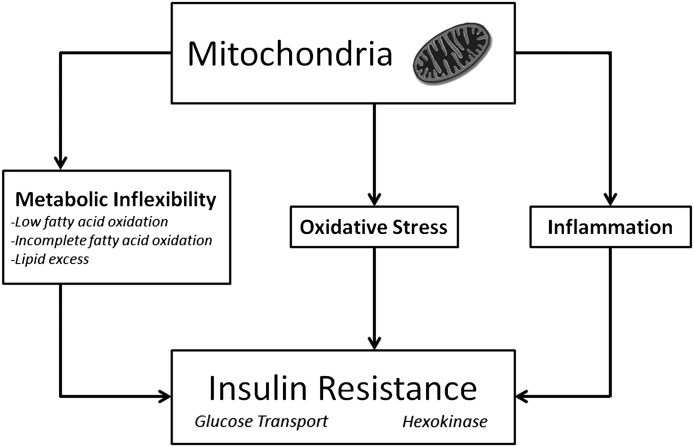
First, we must first accept that IR can be defined as a decreased insulin-stimulated glucose uptake by muscle cells or tissues, as this provides a quantitative integrated measurement of insulin action. At first this seems obvious; let’s not, however, allow that defects in insulin-signaling, GLUT4 translocation or content, or other parameters upstream of glucose uptake be held up as direct evidence. That is because—and the second consideration—impairment in insulin-stimulated glucose uptake can occur without alterations in classical insulin signaling pathways. For example, IR induced by palmitate or oxidative stress can occur without diminished Akt phosphorylation (4). In other words, there are multiple ways of inducing IR (Fig. 1).
FIG. 1.
Mitochondria are now widely recognized to have numerous complex functions, many of which have been implicated in skeletal muscle insulin resistance.
We must agree that mitochondria deficiency should not be defined as merely inadequate ATP generation or reduced fatty acid oxidation, as has been argued in previous commentaries (5,6). To the contrary, mitochondria are very dynamic organelles with many roles, including mediating oxidative stress, cellular redox state, and generating signaling molecules (Fig. 1). We must also put a heavy emphasis on human IR. While appreciating that mechanistic, causal studies are difficult—if not impossible—to perform in human subjects, we must also, however, eventually be able to translate mechanistic studies to human health and disease.
I will also argue that we are just now beginning to understand some of the other potential roles that mitochondria may have in governing metabolism, including IR. Now, although a dearth of information or poor understanding is itself not a good basis for an argument that the phenomenon is true, arguing against a causal role for mitochondria in IR without acknowledging the possibility that mitochondria can cause IR is a dangerous position to hold. I will present emerging evidence that expands the outdated simplistic view of mitochondria as merely being involved in energy generation to include a broader role for mitochondria in cell signaling, redox, oxidative stress, and likely IR.
EARLY EVIDENCE LEADING TO THE “MITOCHONDRIA HYPOTHESIS”
The landmark studies by Randle et al. (7) supported a model in which increased mitochondrial fatty acid oxidation in muscle leads to an accumulation of acetyl-CoA and citrate, thereby inhibiting pyruvate dehydrogenase and phosphofructokinase, respectively. This inhibition then increases glucose-6-phosphate concentrations, inhibiting hexokinase and resulting in reduced glucose uptake and oxidation. Although some cast doubt on whether this glucose–fatty acid cycle was operative in all tissues under all circumstances (8), these early studies spawned a series of subsequent investigations examining the biochemical mechanisms of reciprocal glucose and fatty acid oxidation.
Several groups of investigators in the 1990s led resurgence in the field to investigate the link between fatty acid oxidation and skeletal muscle IR. Kelley and Mandarino (9) reported that, in contrast to the predictions of the original glucose–fatty acid cycle, during conditions of hyperglycemia and high glucose oxidation, fat oxidation was concomitantly lower in muscle of individuals with type 2 diabetes, and this effect was exacerbated by obesity (10,11). Other studies also reported that glucose inhibits fat oxidation (12), calling it the reverse Randle cycle. These findings were also consistent with observations that skeletal muscle in obesity-induced IR has increased levels of malonyl-CoA (13), thereby inhibiting carnitine palmitoyl transferase (CPT) and thus fatty acid oxidation (14).
MITOCHONDRIA, FATTY ACID OXIDATION, AND INTRAMYOCELLULAR LIPIDS
More recently, a prevalent hypothesis has been put forth to link mitochondria with IR, whereby lower mitochondrial content or impairments in mitochondrial fatty acid oxidation lead to excess accumulation of intramyocellular lipids, most notably diacylglycerol and ceramides (15). Although attractive, there is considerable debate about whether or not this “lipotoxicity” or excess nonadipose tissue (ectopic) lipid is mechanistically linked with IR, and further, whether this can be tied to altered mitochondrial capacity for fatty acid oxidation. Human studies reported that skeletal muscle in obese subjects either without or with type 2 diabetes was inherently poor at oxidizing fatty acids (11), or had reduced mitochondrial capacity for oxidative metabolism (1).
It has been argued that mitochondrial capacity for fatty acid oxidation is not the root cause of IR because, even in IR of obesity and type 2 diabetes, mitochondria have an excess capacity to maintain adequate fatty acid oxidation and provide energy during resting conditions in which energy demand is low, for example during resting (5,6). There is no question that total energy flux is driven by energy demand. Hence the muscle mitochondria in IR and diabetes have more than adequate capacity for energy production during resting conditions. Substrate selection, on the other hand, can be independent of energy demand. For example, chronic exercise training results in higher proportion of energy generated from fatty acid oxidation at the same absolute energy demand (16). I would also argue that—at the same energy demand—reduced mitochondria content and capacity results in a proportionate shift away from fatty acid oxidation during noninsulin stimulated conditions, leading over time to lipid accumulation and IR. This metabolic inflexibility that occurs in IR muscle is characterized by both lower fatty acid oxidation in the basal, fasting state and a lower glucose uptake in the insulin-stimulated state (17).
One of the arguments made that mitochondrial deficiency is disconnected from IR derives from either pathological conditions or genetic animal models of severe mitochondrial deficiency (18). In these extreme examples, energy derived from oxidation of fatty acids is essentially abolished, so that glucose is practically the only substrate available—in both basal and insulin-stimulated conditions. Therefore, these models in which glucose is necessarily the default substrate do not provide convincing evidence against the mitochondrial link with IR.
Another argument that altered mitochondrial fatty acid oxidation may play a role in IR is suggested by genetic models in which shifting substrate selection toward fat oxidation protects against diet-induced IR. Acetyl-CoA carboxylase deficient mice exhibit the expected increase in fatty acid oxidation (presumably because of lower malonyl CoA levels to inhibit CPT-1) and protection against obesity-induced IR (19). Other studies using muscle-specific acetyl-CoA carboxylase deficient animals have not observed a similar metabolic phenotype (20), perhaps because of a compensatory decrease in overall glucose oxidation and increase in de novo lipogenesis. However, in model systems in which CPT-1 is overexpressed in skeletal muscle, fat oxidation is increased together with improved insulin sensitivity (21). Taken together, genetic manipulation in animal models or in cell systems has often led to contradictory and confusing results. Again, although these model systems have been useful to demonstrate potential causes of IR, we must look at the human data to support or refute these model systems.
Another line of evidence used to refute a causal role for mitochondria in IR is from studies in rodents in which high-fat feeding causes IR while increasing mitochondria in muscle (22). Moreover, overexpression of lipoprotein lipase in muscle provides greater free fatty acid exposure to stimulate peroxisome proliferator–activated receptor-δ and stimulate mitochondrial biogenesis (23). A counter to this is a study by Sparks et al. (24) who found that a short-term high-fat diet downregulates mitochondrial genes. This evidence can also be countered by evidence in humans. First, chronic high-fat feeding or obesity is not associated with an increase in mitochondria or oxidative capacity in humans (25). On the contrary, mitochondria capacity is decreased with obesity in humans (25). We need to consider many confounders when comparing the data from rodents fed high-fat diets with cross sectional studies of obese humans (e.g., physical activity, chronic vs. acute effects of fat overload). Second, Morino et al. (26) recently reported that reductions in lipoprotein lipase play a key role in reduced mitochondrial content in human IR through decreased peroxisome proliferator–activated receptor-δ activation by polyunsaturated fatty acids, eicosapentaenoic acid specifically. Thus we need to be careful in how we interpret the animal model data used to refute a role for mitochondria in IR.
Human evidence to support a link between mitochondria and IR may also appear to be inconsistent. It should follow from the mitochondrial-lipotoxicity hypothesis, that lower mitochondrial capacity in IR states still maintains an adequate reserve capacity to oxidize fatty acids and cannot lead to excess accumulation of intramuscular lipids. This contention can be countered by acknowledging that higher mitochondria content and capacity are associated with a higher proportion of fatty acid oxidation during resting conditions (17). Thus, although mitochondrial capacity in IR conditions should be adequate to oxidize fatty acids and maintain lower intramuscular lipids, it is likely that a lower total mitochondrial capacity can lead to a shift in substrate selection in the fasting or exercise condition away from fatty acid oxidation, which would lead to lipid accumulation and IR. Of course, although poor mitochondrial performance can lead to IR, IR can develop in the setting of lipid oversupply without low mitochondrial content or function (27). In other words, IR can develop because of lower mitochondrial capacity, but does not require it because there are many other possible causes of IR.
We have used energy restriction weight loss and exercise programs to examine how intervention-induced changes in mitochondria, muscular lipids, and IR track with one another. These interventions are reasonably good models to distinguish between increases in energy demand and reductions in energy supply. To summarize these studies: exercise increases mitochondria content and capacity for fatty acid oxidation and improves IR (28). Diet-induced weight loss, however, improves IR without increasing mitochondrial capacity (29). One interpretation is that this is solid proof that mitochondria are not in a causal pathway to IR. It is quite likely, however, that just as there are many paths to develop IR, there are several means to improve IR. Exercise improves mitochondria content and capacity, decreases ceramides (30), and improves IR. Although it is possible that these are simply phenomena all occurring as an adaption to exercise, this leaves the door ajar to the possibility that exercise improves IR by increasing the proportion of energy derived from mitochondrial fatty acid oxidation during resting conditions, thereby partitioning fatty acids away from lipotoxic moieties. An alternative compatible explanation linking mitochondria to IR is that reductions in oxidative stress with either exercise or energy restriction can improve IR. In this next section, I will argue that this and other facets of mitochondrial energetics are likely causally linked with IR.
MITOCHONDRIA, METABOLIC OVERLOAD, OXIDATIVE STRESS, AND INFLAMMATION
During the past few years, interesting insights have come to light from genetic models of IR in which high rates of incomplete fat oxidation and by-products of fatty acid catabolism are associated with IR (31). In these models, IR was associated with elevated β-oxidation with no change in overall mitochondrial respiration. This might suggest that in the setting of low energy demand, e.g., a sedentary lifestyle, a metabolic overload stress on the mitochondria contributes to IR. It remains to be determined whether an increase in mitochondria content or capacity—even in low energy demand states—may act as a buffer to reduce this metabolic stress and maintain insulin sensitivity.
Glucose transport is a key defect in systemic glucose disposal, and some have argued that this is the primary derangement governing IR in muscle (32). Recent studies in animal models have challenged the concept by demonstrating a more distributed control of glucose flux involving hexokinase during conditions in which glucose transport is maximized (33). This should be viewed in the context of studies demonstrating hexokinase binding to mitochondria (34) and its link to thioredoxin-interacting protein (TXNIP) as well as TXNIP effects to inhibit glucose uptake in humans (35). Thus it is quite possible that reduced mitochondrial content or remodeling in IR states results in decreased overall hexokinase binding and subsequently a reduced phosphorylation of glucose and diminished glucose uptake.
Another attractive paradigm gaining acceptance is that mitochondria-derived reactive oxygen species (ROS) act as signaling molecules (36). Anderson et al. (37) demonstrated that in obese rodents and humans that elevated mitochondrial-mediated oxidative stress was related to IR, and that antioxidant scavenging improved IR. In support of a role of mitochondrial oxidative stress in IR, Lee et al. (38) reported that overexpression of a mitochondria-targeted catalase resulted in a reduction in diacylglycerol and protection against aging-induced IR. Moreover, it is well accepted that ROS activate several aspects of cell signaling related to insulin action (39). An interesting study by Shi and colleagues (40) reported that remodeling of cardiolipin, a phospholipid specific to mitochondria, plays a key role in mediating mitochondrial O2 consumption, oxidative stress and IR.
Inflammation has steadily gained acceptance as a likely cause of IR. The links among inflammation, mitochondria and IR have only recently begun to be appreciated. Mitochondrial TXNIP may also be an important link between mitochondria and inflammation. TXNIP has also been implicated in type 2 diabetes, and is associated with ROS-dependent Nod-like receptor 3 inflammasome activation after its detachment from thioredoxin (41). Zhou et al. (41) found that inhibition of mitochondrial respiratory chain activity, which causes ROS generation, activates the Nod-like receptor 3 inflammasome, and further, that the mitochondria voltage-dependent anion channels are crucial for inflammasome activation. These data place mitochondrial signaling squarely in the path of inflammation. This inflammatory response has also been shown to contribute to changes in the extracellular matrix, an altered mechanosignal transduction, and reduced mitochondrial content and function associated with IR. Taken together, these studies indicate that oxidative stress and inflammation are important aspects of mitochondrial energetics and play a role in IR.
In summary, human type 2 diabetes and obesity are associated with a reduced mitochondrial capacity and IR. The etiology and underlying mechanisms of skeletal muscle IR are not clear, in part because there are many cellular causes of IR. Lipid overload, derangements in fatty acid oxidation, oxidative stress, and inflammation have all been shown to cause IR. Further, the majority of the available evidence indicates that mitochondria play a role in these forms of human skeletal muscle IR. Mitochondrial deficiency, as articulated previously from the perspective of reduced oxidative capacity or low fatty acid oxidation, should be more broadly defined to include mitochondria’s various complex roles in health and disease. By accepting that mitochondria are complex organelles with many functions and that there are multiple pathways leading to IR, we will more fully appreciate that mitochondria may cause IR. Then we will be able to advance a clearer understanding of this complex pathology.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
Footnotes
REFERENCES
- 1.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 2002;51:2944–2950 [DOI] [PubMed] [Google Scholar]
- 2.Rimbert V, Boirie Y, Bedu M, Hocquette J-F, Ritz P, Morio B. Muscle fat oxidative capacity is not impaired by age but by physical inactivity: association with insulin sensitivity. FASEB J 2004;18:737–739 [DOI] [PubMed] [Google Scholar]
- 3.Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci 2006;61:534–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoehn KL, Hohnen-Behrens C, Cederberg A, et al. IRS1-independent defects define major nodes of insulin resistance. Cell Metab 2008;7:421–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holloszy JO. Skeletal muscle “mitochondrial deficiency” does not mediate insulin resistance. Am J Clin Nutr 2009;89:463S–466S [DOI] [PubMed] [Google Scholar]
- 6.Muoio DM, Neufer PD. Lipid-induced mitochondrial stress and insulin action in muscle. Cell Metab 2012;15:595–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963;1:785–789 [DOI] [PubMed] [Google Scholar]
- 8.Rennie MJ, Holloszy JO. Inhibition of glucose uptake and glycogenolysis by availability of oleate in well-oxygenated perfused skeletal muscle. Biochem J 1977;168:161–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelley DE, Mandarino LJ. Hyperglycemia normalizes insulin-stimulated skeletal muscle glucose oxidation and storage in noninsulin-dependent diabetes mellitus. J Clin Invest 1990;86:1999–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelley DE, Mokan M, Simoneau JA, Mandarino LJ. Interaction between glucose and free fatty acid metabolism in human skeletal muscle. J Clin Invest 1993;92:91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mandarino LJ, Consoli A, Jain A, Kelley DE. Interaction of carbohydrate and fat fuels in human skeletal muscle: impact of obesity and NIDDM. Am J Physiol 1996;270:E463–E470 [DOI] [PubMed] [Google Scholar]
- 12.Sidossis LS, Wolfe RR. Glucose and insulin-induced inhibition of fatty acid oxidation: the glucose-fatty acid cycle reversed. Am J Physiol 1996;270:E733–E738 [DOI] [PubMed] [Google Scholar]
- 13.Winder WW, Arogyasami J, Elayan IM, Cartmill D. Time course of exercise-induced decline in malonyl-CoA in different muscle types. Am J Physiol 1990;259:E266–E271 [DOI] [PubMed] [Google Scholar]
- 14.McGarry JD. The mitochondrial carnitine palmitoyltransferase system: its broadening role in fuel homoeostasis and new insights into its molecular features. Biochem Soc Trans 1995;23:321–324 [DOI] [PubMed] [Google Scholar]
- 15.Coen PM, Goodpaster BH. Role of intramyocelluar lipids in human health. Trends Endocrinol Metab 2012;23:391–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coggan AR, Kohrt WM, Spina RJ, Bier DM, Holloszy JO. Endurance training decreases plasma glucose turnover and oxidation during moderate-intensity exercise in men. J Appl Physiol 1990;68:990–996 [DOI] [PubMed] [Google Scholar]
- 17.Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol 1999;277:E1130–E1141 [DOI] [PubMed] [Google Scholar]
- 18.Zechner C, Lai L, Zechner JF, et al. Total skeletal muscle PGC-1 deficiency uncouples mitochondrial derangements from fiber type determination and insulin sensitivity. Cell Metab 2010;12:633–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abu-Elheiga L, Oh W, Kordari P, Wakil SJ. Acetyl-CoA carboxylase 2 mutant mice are protected against obesity and diabetes induced by high-fat/high-carbohydrate diets. Proc Natl Acad Sci USA 2003;100:10207–10212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoehn KL, Turner N, Swarbrick MM, et al. Acute or chronic upregulation of mitochondrial fatty acid oxidation has no net effect on whole-body energy expenditure or adiposity. Cell Metab 2010;11:70–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perdomo G, Commerford SR, Richard AM, et al. Increased beta-oxidation in muscle cells enhances insulin-stimulated glucose metabolism and protects against fatty acid-induced insulin resistance despite intramyocellular lipid accumulation. J Biol Chem 2004;279:27177–27186 [DOI] [PubMed] [Google Scholar]
- 22.Turner N, Bruce CR, Beale SM, et al. Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes 2007;56:2085–2092 [DOI] [PubMed] [Google Scholar]
- 23.Levak-Frank S, Radner H, Walsh A, et al. Muscle-specific overexpression of lipoprotein lipase causes a severe myopathy characterized by proliferation of mitochondria and peroxisomes in transgenic mice. J Clin Invest 1995;96:976–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sparks LM, Xie H, Koza RA, et al. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes 2005;54:1926–1933 [DOI] [PubMed] [Google Scholar]
- 25.Simoneau JA, Veerkamp JH, Turcotte LP, Kelley DE. Markers of capacity to utilize fatty acids in human skeletal muscle: relation to insulin resistance and obesity and effects of weight loss. FASEB J 1999;13:2051–2060 [DOI] [PubMed] [Google Scholar]
- 26.Morino K, Petersen KF, Sono S, et al. Regulation of mitochondrial biogenesis by lipoprotein lipase in muscle of insulin-resistant offspring of parents with type 2 diabetes. Diabetes 2012;61:877–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nair KS, Bigelow ML, Asmann YW, et al. Asian Indians have enhanced skeletal muscle mitochondrial capacity to produce ATP in association with severe insulin resistance. Diabetes 2008;57:1166–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubé JJ, Amati F, Toledo FG, et al. Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia 2011;54:1147–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toledo FG, Menshikova EV, Azuma K, et al. Mitochondrial capacity in skeletal muscle is not stimulated by weight loss despite increases in insulin action and decreases in intramyocellular lipid content. Diabetes 2008;57:987–994 [DOI] [PubMed] [Google Scholar]
- 30.Dubé JJ, Amati F, Stefanovic-Racic M, Toledo FG, Sauers SE, Goodpaster BH. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete’s paradox revisited. Am J Physiol Endocrinol Metab 2008;294:E882–E888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koves TR, Ussher JR, Noland RC, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 2008;7:45–56 [DOI] [PubMed] [Google Scholar]
- 32.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest 2000;106:171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wasserman DH, Ayala JE. Interaction of physiological mechanisms in control of muscle glucose uptake. Clin Exp Pharmacol Physiol 2005;32:319–323 [DOI] [PubMed] [Google Scholar]
- 34.da-Silva WS, Gómez-Puyou A, de Gómez-Puyou MT, et al. Mitochondrial bound hexokinase activity as a preventive antioxidant defense: steady-state ADP formation as a regulatory mechanism of membrane potential and reactive oxygen species generation in mitochondria. J Biol Chem 2004;279:39846–39855 [DOI] [PubMed] [Google Scholar]
- 35.Parikh H, Carlsson E, Chutkow WA, et al. TXNIP regulates peripheral glucose metabolism in humans. PLoS Med 2007;4:e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D’Autréaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol 2007;8:813–824 [DOI] [PubMed] [Google Scholar]
- 37.Anderson EJ, Lustig ME, Boyle KE, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 2009;119:573–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee HY, Choi CS, Birkenfeld AL, et al. Targeted expression of catalase to mitochondria prevents age-associated reductions in mitochondrial function and insulin resistance. Cell Metab 2010;12:668–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bloch-Damti A, Bashan N. Proposed mechanisms for the induction of insulin resistance by oxidative stress. Antioxid Redox Signal 2005;7:1553–1567 [DOI] [PubMed] [Google Scholar]
- 40.Li J, Romestaing C, Han X, et al. Cardiolipin remodeling by ALCAT1 links oxidative stress and mitochondrial dysfunction to obesity. Cell Metab 2010;12:154–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011;469:221–225 [DOI] [PubMed] [Google Scholar]