Abstract
Objective
Iron plays a key role in brain function, and a deficiency of iron has been implicated in various cognitive, motor, and psychiatric disorders. Because of recent evidence that iron deficiency may be related to attention-deficit/hyperactivity disorder (ADHD) and other psychiatric disorders, the goal of this study was to compare the iron status of children and youth seen in a community mental health clinic with a national sample of same-aged subjects.
Methods
In this study, a consecutive series of 108 patients (79 males) referred to a community mental health clinic was compared with a National Health and Nutrition Examination Survey (NHANES) sample on measures of iron status. Wilcoxon sign rank and median tests were used to compare distributions of ferritin. Quantile regression was performed to compare the ferritin level in the two samples while adjusting for demographic differences. Chi squared (χ2) was used to compare rates of low hemoglobin in the two samples.
Results
The iron status of the clinic sample, as measured by ferritin levels (median=23 μg/L), was significantly lower than that of the national sample (median=43 μg/L). After adjustment for age, gender, and race, the clinic sample was found to have 19.2 μg/L lower ferritin than the national sample (95%CI from 7.6 to 30.9, p value=0.001). There were also significantly more subjects in the clinic sample with low hemoglobin than in the national sample. There were no differences in ferritin levels between those patients in the clinic sample with and without an ADHD or other specific psychiatric diagnosis.
Conclusions
The ferritin levels of children and youth in a mental health clinic sample were significantly lower than those of the same-aged subjects in a national sample. Therefore, compromised iron status may be an additional biological risk factor for cognitive, behavioral, and psychiatric problems in pediatric populations served by the community mental health clinic.
Introduction
Iron-deficiency is the most common single nutrient deficiency in the United States and the world (World Health Organization 2008). Iron has been shown to be necessary for proper cognitive, emotional, and motor development (Nokes et al. 1998; Grantham-McGregor and Ani 2001; Beard 2007). Additionally, iron-deficiency anemia (IDA) and iron-deficiency without anemia (ID) are associated with a broad spectrum of cognitive and behavioral problems in animals and humans (Yager and Hartfield 2002; McCann and Ames 2007). Because of ethical concerns regarding study design and the generally disadvantaged background in which this deficiency naturally occurs, it is difficult to prove cause-and-effect relationships between IDA and/or ID and cognitive and behavioral deficits in humans. However, in animal studies, clear motor and behavioral changes caused by ID and IDA have been documented (Golub et al. 2006; McCann and Ames 2007). Furthermore, changes to brain structure and function are evident in both IDA and ID in animals (Erikson et al. 2000, 2001; Beard 2003; Connor et al. 2008). ID impairs neurotransmitter regulation including gamma-aminobutyric acid (GABA), dopamine, serotonin, and norepinephrine (Yager and Hartfield 2002). Iron is a necessary co-factor for tyrosine hydroxylase, an enzyme that is the rate-limiting step in the production of dopamine, norepinephrine, and serotonin (Nelson et al. 1997; Erikson et al. 2000; Youdim and Yehuda 2000; Beard 2003; Gorin, and Vali 2007; Kaushik, et al. 2007). Iron is also needed in the brain for myelin protein formation (Ortiz et al. 2004), synaptic strengthening (Jorgenson et al. 2005), and energy generation (Pinero et al. 2000).
IDA has long been associated with increased irritability and behavioral problems in children (Werkman et al. 1964; Orii et al. 2002). A well-controlled 20 year longitudinal study of Costa Rican children with a history of severe chronic IDA in infancy found that history of IDA was associated with lower cognitive scores and increased anxiety and depression, as well as increased behavioral problems (Lozoff et al. 2000; Corapci et al. 2010). In multiple studies, ID has been associated with various cognitive deficits and with psychiatric disorders (Lozoff et al. 2000; Corapci et al. 2006; Golub et al. 2006; Lozoff and Georgieff 2006; Lozoff et al. 2006; Lozoff 2007; Lozoff et al. 2007a,b; Murray-Kolb and Beard 2007; Lozoff et al. 2008). Poorer cognitive outcomes associated with ID have included lower math scores and poor performance on verbal memory tasks (Bruner et al. 1996; Halterman et al. 2001).
Although IDA is recognized as a clear target for clinical intervention and is screened for routinely in infants and young children, ID is not screened for regularly and has not gained widespread recognition as a potentially reversible cause of cognitive and behavioral problems in children and adolescents. Reviewers have also suggested that the cognitive and behavioral consequences of IDA and ID may be different depending upon the environment in which the deficiency occurs (Nokes et al. 1998). Because the brain may be the first organ to suffer when a more subtle ID is present and symptoms may be vague and nonspecific, the role of ID in cognition and mental disorder may be an under-recognized but correctible etiologic factor in some children. Furthermore, like most normal values of nutrient status, normal iron status is not based on cognitive or behavioral outcomes (Benton 2008). Therefore, it is possible that a low-normal iron status could be associated with deficits in cognition and behavioral problems particularly in individuals with other risk factors.
Two brain disorders that have been the subject of recent research regarding a potential link to ID are restless legs syndrome (RLS) and attention-deficit/hyperactivity disorder (ADHD). A brain disorder that disturbs sleep, RLS has been closely linked to ID and most notably to low ferritin (a measure of adequate iron stores) (Kryger et al. 2002; Allen and Earley 2007; Earley et al. 2008, 2009). Further, RLS is associated with poor performance on tasks of executive functioning (Pearson et al. 2006). Additionally, RLS and ADHD have been shown to be associated, and in youth with both disorders, iron status is more likely to be low (Picchietti et al. 1998,1999; Konofal et al. 2007; Picchietti 2007; Cortese et al. 2009).
Studies using ferritin as a peripheral measure of iron stores in youth with ADHD and that include a control group are summarized in Table 1 (Konofal et al. 2007; Konofal et al. 2004; Millichap et al. 2006; Juneja et al. 2010; Menegassi et al. 2010; Donfransesco et al. 2012). Additionally, one study linked a history of low iron status to poor stimulant response in children with ADHD (Calarge et al. 2010). In the summarized studies there is a 10-fold difference between the highest (59) and lowest (6) mean ferritin levels in the ADHD samples, whereas the control group means have a much smaller range (33–59; see Table 1). One explanation for these disparate findings in ferritin levels is that most of the studies made, at best, minimal attempts to control for differences in demographic characteristics of the samples, although age, sex, race, and income status have all been shown to be significant factors associated with iron status (Brotanek et al. 2007). Income has been characterized in only one of the previous studies looking at the ferritin levels of youth with and without ADHD, although in non-ADHD samples, income has been shown to be positively associated with iron status in that those with higher income have more iron stores (Looker et al. 1997; Crowell et al. 2005; Lozoff et al. 2007). Unlike the other studies, that by Juneja et al. (2010) matched the controls to cases by age and sex. They reported that the income was lower in the control group than in the ADHD group (84% of controls and 44% of ADHD participants were from “low socioeconomic status”) (2010). As observed in Table 1, Juneja et al. found much lower ferritin levels in the ADHD group than in the control group, a finding that argues against income being the cause of the significant difference between ferritin levels. Millichap et al. also controlled for age and sex but not income and found that ferritin levels were not significantly different in ADHD patients from a national sample of same-aged youth (2006).
Table 1.
Summary of Previous Studies that Included ADHD and Serum Ferritin
| Study | Participant characteristics | Ages (yrs) | M:F | ADHD group ferritin level μg/L±SD (n) | Control group ferritin level μg/L±SD (n) | p value |
|---|---|---|---|---|---|---|
| Konofal et al. 2004, France | ADHD-excluding other psych diagnoses and behavioral disordersa | 4–14 | 6:1 | 23±13 (53) | 44±22 (27) | <0.001 |
| Controls-have reading disorders | ||||||
| Konofal et al. 2007, France | ADHD+RLS-excluding other psych and physical disordersa | 5–9 | 3:1 | 16±6 (12) | 46±18 (10) | <0.0005 |
| Controls referred but no psych disordersa | ||||||
| Millichap et al. 2006, U.S. | ADHD: no mention of comorbidities Controls – national sample (NHANES) | 5–16 | 4:1 | 40±41 (68) | NHANES grouped by age | >0.2 |
| Juneja et al. 2010, India | ADHD: with comorbidities Controls: age and sex matched | 6–14 | 6:1 | 6.0±3.9 (25) | 49±42 (25) | <0.001 |
| Menegassi et al., 2010, Brazil | ADHD: on stimulant ADHD: not on stimulant | 6–15 | 4:1 | 59±21 (19)54±17 (22) | 59±29 (21) | 0.72 |
| Controls: no diagnosis | ||||||
| Donfrancesco et al. 2012, Italy | ADHD: stimulant naïve Controls: group matched for age and gender | 6–14 | 10:1 | 33±11 (101) | 33±19 (93) | 1.00 |
Diagnosed with standardized interview.
ADHD, attention-deficit/hyperactivity disorder; RLS, restless leg syndrome; NHANES, National Health and Nutrition Examination Survey.
Psychosocial adversity is another potential risk factor that is important in the relationship between iron status and mental disorder, not only because psychosocial adversity increases the risk of ADHD, but also because psychosocial adversity may negatively impact the absorption and metabolism of iron. A recent study showed that psychological stress in rats diminished iron absorption and iron accumulation in the brain (Yu et al. 2011). Additionally, ADHD and other mental disorders may change nutritional intake as mental disorders and the medications to treat them have been shown to impact appetite. Further, it has been suggested that, at least in infants and young children, the effects on behavior and cognition of ID may be mitigated by the availability of a responsive caregiver, something that may be less likely in the context of elevated psychosocial adversity (Nokes et al. 1998). Therefore, the negative impacts of low iron status may only be evident in those children with the additional element of psychosocial adversity.
Psychosocial adversity, such as mental disorder in a parent, incarceration of a parent, and domestic violence, increase the risk for mental disorder in youth (Szatmari et al. 1994; Grant et al. 2006). A high level of psychosocial adversity often exists in the context of low socioeconomic status (Evans 2004; Hatch and Dohrenwend 2007; Amone-P'Olak et al. 2009), and low socioeconomic status is associated with low nutritional status, in general (Langevin et al. 2007), and low iron status, specifically (Looker et al. 1997). Therefore, low socioeconomic status, high psychosocial adversity, low iron status, and mental disorder, may often occur together. If iron does play a role in mental disorders of children with high levels of psychosocial adversity, it would be a potential target for the treatment for children whose response to other interventions is not optimal.
The current study aimed to characterize the iron status of consecutively referred children and youth in a community mental health clinic. The ferritin levels of the sample of youth in the clinic were compared with those of a national sample of same-aged subjects from the National Health and Nutrition Examination Survey (NHANES), to determine if the iron status of the two samples were different from each other. The community mental health clinic represented in the current study serves a population with predominately low socioeconomic status and Medicaid insurance. In the clinic sample, a measure of psychosocial adversity was used to assess the effect of the variable on the association between iron status and mental disorder. In addition, the relationships between the iron status of children and youth in the clinic sample with specific disorders were compared with the iron status of those in the clinic without these disorders to determine if there were significant differences in iron status.
Methods
The methods of the present study were approved by the Johns Hopkins University Institutional Review Board.
Participants
Data were obtained from the charts of a consecutive series of outpatients referred to the Children's Outpatient Clinic of the Community Psychiatry Program at Johns Hopkins Bayview Medical Center in Baltimore, Maryland from June, 2008 to June, 2010. The Community Psychiatry Program serves Southeast Baltimore, a community that includes low-income neighborhoods. Seventy-five percent of the patient population in the program is between 5 and 18 years old; 45% are Caucasian, 23% are African American, 12% are Hispanic, 2% are multiracial, and 18% are of other or unknown racial background. Seventy-eight percent of patients in the clinic are insured through medical assistance (Medicaid). In Maryland, a family's adjusted income must be ≤2 times the official United States poverty level to qualify for Medicaid. Therefore, the majority of children and youth in the sample are from a disadvantaged socioeconomic background.
Psychiatric and laboratory assessments were obtained from all patients referred to the clinic. All fully assessed patients with available charts within the timeframe were included in the sample. Information on demographics, psychiatric diagnoses, psychosocial adversity, and blood analyses was extracted from the medical records. Sample data were compared with data for children and youth 5–18 years of age from the NHANES 2001–2002 data set. The NHANES is an ongoing survey that collects data from a sample of the United States population. Detailed information regarding data collection methods can be obtained from the Center for Disease Control and Prevention (CDC) (Centers for Disease Control and Prevention 2011). For the NHANES sample, ferritin levels were measured using a single-incubation two-site immunoradiometric assay (BioRad Laboratories, Hercules, CA).
Psychiatric assessment
Psychiatric diagnoses were established in the outpatient clinic by a standard diagnostic evaluation completed by board-certified, child and adolescent psychiatrists using American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) diagnostic criteria for ADHD, oppositional defiant disorder (ODD), conduct disorder, major depressive disorder, dysthymia, bipolar disorder, posttraumatic stress disorder, panic disorder, social phobia, generalized anxiety disorder, autistic spectrum disorders, reading disorder, and mathematics disorder (American Psychiatric Association 1994). Patients and their parents were interviewed on separate occasions. School assessments, psychological evaluations, and occupational therapy evaluations all contributed to a best-estimate diagnosis. For the NHANES sample, 38% of the participants' caregivers were asked if the subject had a lifetime diagnosis of ADHD and 28% were asked if the participant had a lifetime diagnosis of a learning disability, and the answers were recorded in a yes/no fashion.
Assessment of iron status
For the clinic sample, serum ferritin was used to assess iron status. Ferritin concentration is an indicator of the amount of iron stored in the liver and is recommended by the World Health Organization (WHO) as the best means to assess iron status (Joint World Health Organization/Centers for Disease Control and Prevention Technical Consultation on the Assessment of Iron Status at the Population Level 2004). All patients referred to the outpatient clinic completed a blood analysis as a part of standard assessment procedures, which included complete blood cell count (including hemoglobin and red blood cell distribution width [RDW]), comprehensive metabolic panel, thyroid stimulating hormone, lead, ferritin, and erythrocyte sedimentation rate (ESR). Serum ferritin levels were determined by the enzyme immunoassay method, using commercial kits. The ferritin method used in the Johns Hopkins laboratories during the time was ST AIA-PACK ferritin assay on the Tosoh AIA-1800 analyzer (Tosoh Bioscience, S. San Francisco, CA). Hemoglobin and RDW were obtained by the electrical impedance method; blood lead was obtained by atomic absorption spectrophotometry; and ESR was obtained using Wintergreen's method. The blood tests were random and non-fasting.
Assessment of psychosocial adversity
In the outpatient clinic, psychosocial adversity was measured on the eight-point Modified Psychosocial Adversity Scale developed by Brown and colleagues (Brown et al. 1981) and described by Szatmari et al. (1994) and by Vasa et al. (2004). The number of psychosocial risk factors was enumerated in the psychosocial history of each clinic patient. The 8 risk factors are: 1) single-parent household; 2) presence or history of domestic violence; 3) past or current mental disorder of one parent; 4) past or current mental disorder in the other parent; 5) past or current substance abuse or dependence in one parent; 6) past or substance abuse or dependence in the other parent; 7) past or current criminal conviction of one parent; and 8) past or current criminal conviction of the other parent. One point was given for each risk factor present.
There is no measure of psychosocial adversity in the NHANES data set, but there are household income measures. To assess for income in the NHANES data, the income level of the household was converted to a poverty index ratio (PIR) by dividing household income by the poverty threshold income for the number of people in the household. For example, if the household income was $25,000 and the threshold for poverty for the number of people in the house is $20,000 then the PIR is 25/20 or 1.25.The PIR was used to assess whether income was associated with ferritin levels in the NHANES sample.
Data analysis
As ferritin values appear to be right skewed, medians were used to compare the clinic sample to the NHANES sample. Wilcoxon sign rank and median tests to compare the distributions of ferritin values and the medians in the two samples were conducted, respectively. To test the hypothesis that ferritin levels are lower in the clinic than in the general United States population, it was necessary to account for demographic differences between the two samples. Ferritin levels have been shown to increase with age (Deinard et al. 1983; Elmlinger et al. 2002), and racial and gender differences in ferritin levels have also been documented in the NHANES (Zacharski et al. 2000). To check for differences in demographics between the clinic sample and the NHANES sample, the clinic sample was compared to the NHANES sample using the χ2 test for race and gender and the t test for age. Quantile regression was performed and median ferritin was modeled as a function of sample (clinic vs. NHANES), age, gender, and race (Koenker and Hallock 2001). Pearson's χ2 was used to test the hypothesis that a greater proportion of children and youth in the clinic have lower than normal hemoglobin level compared with the general United States population.
A Spearman's correlation was used to test the hypothesis that those with higher psychosocial adversity have lower iron status. Logistic regression was performed to assess whether the probability of ADHD is associated with lower iron status within the clinic sample. The same analysis compared those with other specific diagnoses with those without those diagnoses to assess whether any specific diagnosis within the clinic had lower ferritin levels than did those without that diagnosis. All analyses were conducted in STATA 11.0 (StataCorp 2009).
Results
Data were collected on 108 patients (79 boys) 5–17 years of age. Table 2 illustrates the demographic and clinical characteristics of the clinic sample and the NHANES 2001–2002 comparison group of 5–18-year-old males and females. These data are publicly available at the CDC website.
Table 2.
Demographic and Clinical Characteristics of the Samples
| |
Clinic sample |
NHANES sample |
||||
|---|---|---|---|---|---|---|
| Males | Females | Total | Males | Females | Total | |
| n (%) | 79 (73) | 29 (27) | 108 | 1307 (49) | 1345 (51) | 2652 |
| Age (years), mean (SD) | 8.8 (2.7) | 9.6 (3.2) | 9.04 (2.8) | 11.8 (3.8) | 11.7 (3.7) | 11.8 (3.7) |
| Race, n (%) | ||||||
| Caucasian | 35 (44) | 13 (45) | 48 (44) | 389 (30) | 408 (31) | 797 (31) |
| African American | 24 (30) | 8 (28) | 32 (30) | 439 (32) | 407 (30) | 846 (31) |
| Hispanic | 7 (9) | 2 (7) | 9 (8) | 426 (33) | 474 (35) | 900 (34) |
| Other | 11 (14) | 3 (10) | 14 (13) | 53 (4) | 56 (4) | 109 (4) |
| Missing/Unknown | 2 (3) | 3 (10) | 5 (5) | 0 (0) | 0 (0) | 0 (0) |
| Family PIR, mean (SD) | Unknown | Unknown | Unknown | 2.1 (1.5) | 2.2 (1.6) | 2.1 (1.6) |
| Psychosocial adversity, n (%) | ||||||
| Category 1 (0 points) | 2 (2) | 0 (0) | 2 (2) | Unknown | Unknown | Unknown |
| Category 2 (1–2 points) | 17 (22) | 5 (19) | 22 (21) | Unknown | Unknown | Unknown |
| Category 3 (3–4 points) | 32 (42) | 9 (33) | 41 (40) | Unknown | Unknown | Unknown |
| Category 4 (5–8 points) | 25 (33) | 13 (48) | 38 (37) | Unknown | Unknown | Unknown |
| Missing/Unknown | 3 (4) | 2 (7) | 5 (5) | Unknown | Unknown | Unknown |
| Psychiatric disorders, n (%) | ||||||
| ADHD | 60 (77) | 22 (76) | 82 (77) | 34 (7) | 37 (7) | 71 (7) |
| ODD | 28 (35) | 13 (45) | 41 (38) | Unknown | Unknown | Unknown |
| Other DBD | 21 (27) | 4 (14) | 25 (24) | Unknown | Unknown | Unknown |
| Any mood disorder | 23 (29) | 9 (31) | 32 (30) | Unknown | Unknown | Unknown |
| Any anxiety disorder | 30 (38) | 8 (28) | 38 (35) | Unknown | Unknown | Unknown |
| PDD/Autism | 2 (3) | 0 (0) | 2 (2) | Unknown | Unknown | Unknown |
| Intellectual disability | 4 (5) | 1 (4) | 5 (5) | Unknown | Unknown | Unknown |
| Speech disorder | 15 (20) | 3 (12) | 18 (18) | Unknown | Unknown | Unknown |
| Learning disorder | 13 (17) | 3 (11) | 16 (15) | 46 (13) | 35 (10) | 81 (11) |
| Serum ferritin, mean (SD) | 27.2 (14.7) | 23.3 (12.0) | 26.2 (14.1) | 82.3 (105.2) | 82.7 (112.2) | 82.5 (108.8) |
| Normal range: 10–300 μg/L | ||||||
| Hemoglobin, mean (SD) | 12.6 (1.0) | 12.2 (0.6) | 12.5 (0.9) | 14.1 (1.35) | 13.2 (1.04) | 13.9 (1.54) |
| Normal range: age<11=11.7–13.8 g/dL; age ≥11=12.4–14.8 g/dL | ||||||
| Low hemoglobin, n, (%) | 13 (16) | 8 (28) | 21 (19) | 34 (2.6) | 151 (11) | 185 (7.0) |
| RDW, mean (SD) | 12.9 (0.6) | 12.9 (0.9) | 12.9 (0.8) | 12.5 (0.673) | 12.4 (0.918) | 12.4 (0.807) |
| Normal range:11.5–14.5% | ||||||
| ESR, mean (SD) | 6.8 (5.7) | 11.6 (11.2) | 8.2 (7.9) | 12.5 (0.673) | 12.4 (0.918) | 12.4 (0.807) |
| Normal range: 4–20 mm/hour | ||||||
| Lead | 1.0 (1.9) | 0.3 (1.1) | 0.8 (1.7) | 1.9 (1.8) | 2.0 (2.4) | 2.0 (2.1) |
| C-reactive protein, mean (SD) | Unknown | Unknown | Unknown | 0.299 (0.502) | 0.364 (0.860) | 0.332 (0.709) |
| Normal range: <1 mg/dL | ||||||
NHANES, National Health and Nutrition Examination Survey; PIR, poverty index ratio; ADHD, attention-deficit/hyperactivity disorder; ODD: oppositional defiant disorder; DBD, disruptive behavior disorder; PDD: pervasive developmental disorder; RDW, red blood cell distribution width; ESR, erythrocyte sedimentation rate.
Low hemoglobin, as defined by the clinical laboratory, was <11.7 g/dL for those <11 years of age and <12.4 g/dL for those ≥11 years of age. As seen in Table 2, the percentage of subjects with hemoglobin below these cutoffs was 19% and 7% of the clinic and NHANES sample, respectively. Using Pearson's χ2, it was found that the percentage of low hemoglobin was significantly different between the two samples for males, females, and combined (males: Pearson χ2[1]=43.6 p<0.001; females: Pearson χ2[1]=7.4, p=0.006; combined: Pearson χ2[1]=23.4 p<0.001).
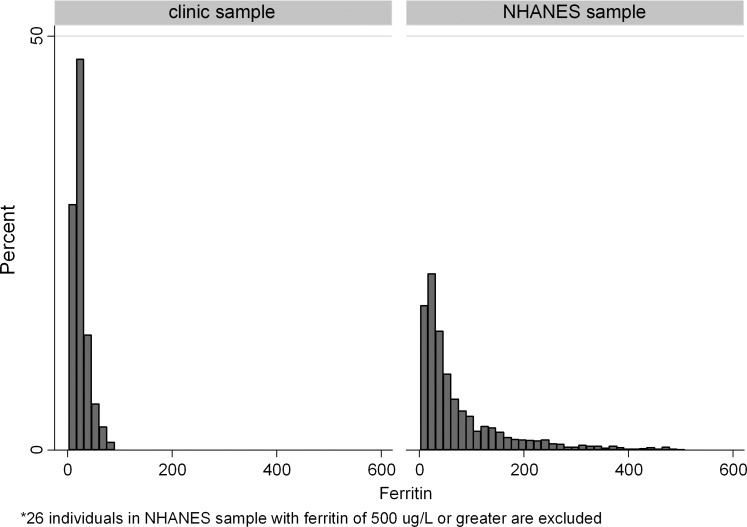
The distributions of ferritin values in the clinic and national samples are presented in Figure 1. The median ferritin values in the clinic sample and in the NHANES sample were 23 and 43 μg/L, respectively. The results of Wilcoxon sign rank test indicated significant differences in distributions of ferritin values (p<0.0001). Analogously, a test of medians indicated that the medians were significantly different in the two samples (p<0.0001).
FIG 1.
Ferritin distribution in outpatient clinic and National Health and Nutrition Examination Survey(NHANES) samples.
The clinic and NHANES samples also had significantly different age distributions (average age in the clinic sample was 9.0 vs. 11.8 in the NHANES sample, p<0.0001); gender distributions (proportion of boys in the clinic sample was 73% vs. 49% in the NHANES sample, p<0.0001); and race distributions (proportion of Caucasian race in the clinic sample was 44% vs. 31% in the NHANES sample, p<0.0001) (Table 2). After adjustment for age, gender, and race, the difference in median ferritin values between the clinic sample and the NHANES sample was estimated to be 19.2 μg/L lower in the clinic sample than in the NHANES sample (95%CI from 7.6 to 30.9, p<0.001). Table 3 shows the median ferritin levels for the clinic and NHANES samples for the various demographic characteristics.
Table 3.
Median Ferritin by Sample, Gender, Race, and Age
| |
Female |
Male |
Female |
Male |
Female |
Male |
Female |
Male |
|---|---|---|---|---|---|---|---|---|
| Age (yrs) | Caucasian | African American | Hispanic | Other | ||||
| Clinic sample: Median ferritin level μg/L (n) | ||||||||
| 5–8 | 24 (3) | 19.5 (20) | 16 (4) | 27 (11) | 29 (1) | 18 (1) | 15.5 (2) | 22.5 (8) |
| 9–12 | 18 (9) | 29 (10) | 42 (3) | 27 (11) | 55 (1) | 21 (5) | 19 (1) | 27 (3) |
| 13–18 | 5 (1) | 27 (5) | 8 (1) | 52.5 (2) | * | 25 (1) | * | * |
| NHANES 2001–2002: Median ferritin level μg/L (n) | ||||||||
| 5–8 | 47 (103) | 43 (103) | 40 (107) | 41 (100) | 47 (103) | 44 (104) | 44 (23) | 30 (13) |
| 9–12 | 32 (108) | 47.5 (116) | 39 (109) | 41 (134) | 47 (122) | 54 (118) | 78.5 (14) | 52.5 (10) |
| 13–18 | 48 (197) | 47 (170) | 44 (191) | 43 (205) | 41 (249) | 40.5 (204) | 48 (19) | 26 (30) |
NHANES, National Health and Nutrition Examination Survey.
No data for these cells.
When limiting the NHANES sample to a PIR of ≤2 (the income below which families are Medicaid eligible), and comparing it with the clinic sample (which is largely made up of those with PIR<2), the results do not change significantly. The clinic sample has 18.8 μg/L lower ferritin levels than the NHANES sample (95%CI: 8.3–29.2 p<0.001).Within the NHANES sample, PIR does not show a significant association with ferritin level (coef=0.0067 and p value=0.813 for the natural log of ferritin regressed to PIR). The hemoglobin in the NHANES sample is significantly positively associated with PIR (coef=0.184 and p value <0.0001). (This correlation means that within the NHANES sample, those with higher incomes relative to household size had higher hemoglobin levels.)
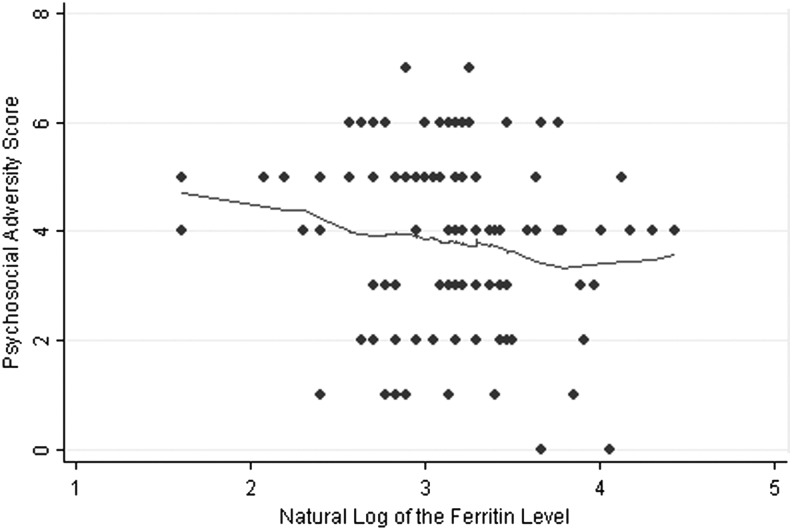
We attempted to explore the role of psychosocial adversity as a potential confounder. As seen in Figure 2, there is a slight negative trend toward an association between psychosocial adversity and the natural log of the ferritin levels (Spearman's ρ=−0.15, p=0.12) because 1) most of the subjects in the clinic sample had moderate to high psychosocial adversity, and 2) as only two in the sample had no psychosocial adversity, no further analysis controlling for psychosocial adversity in the clinic sample was undertaken.
FIG. 2.
Psychosocial adversity and the natural log of the ferritin levels.
Most of the clinic sample, 82/108 (76%), had a diagnosis of ADHD. Six charts recorded ADHD as a rule-out diagnosis, leaving 20 without this diagnosis as a comparison sample to the 82 with the diagnosis. With and without controlling for sex, age, race, and psychosocial adversity, regression of the clinic sample demonstrated that there were no significant differences in ferritin levels for those with and without ADHD, and that the difference was the opposite of what was expected (with ADHD ferritin mean=26.7 μg/L and without ADHD ferritin mean=22.3 μg/L, p value=0.21). Similar comparisons were done for other psychiatric disorders, and none resulted in significant differences between the ferritin levels of those with and without the disorder.
Discussion
The main findings in the current study are that ferritin levels are lower in the community mental health clinic sample than the NHANES sample after adjusting for demographic differences, and that there is a greater proportion of subjects with low hemoglobin in the clinic than in the NHANES sample. Given the previous research reviewed in the Introduction regarding the association of ADHD and other psychiatric disorders with low ferritin, the current study results add a dimension to that discussion. The dimension is the added variables of psychosocial adversity and socioeconomic status. Although this study could not fully explore the effect of either of these variables because of lack of sufficient variation in socioeconomic and adversity scores, the current findings suggest that the addition of these variables will be important in future exploration of this topic. The findings are important, not because they refute or explain an observed association between mental disorders such as ADHD and low ferritin, but because the findings inform future exploration of this topic. The current study presents the intriguing result that a vulnerable population of children and youth in a community mental health clinic may have compromised iron status regardless of the diagnostic status. This finding is hypothesis generating rather than explanatory.
There are at least five ways the finding–that compromised iron status is more common in the community mental health clinic than in a national sample of children and youth–can be understood: 1) Compromised iron status is a contributing etiologic factor to mental disorder; 2) compromised iron status is a biological marker indicating poor nutritional status in general and thereby increasing the risk of mental disorder; 3) mental disorder and/or its treatment causes low iron status; 4) compromised iron status and mental disorder are independent and co-occurring and are both related to clinic sample characteristics such as low socioeconomic status; and 5) there is an unknown variable causing the compromised iron status in the clinic sample.
There is some evidence that compromised iron status could be a contributing etiologic factor in the development and/or maintenance of multiple mental disorders. Though certain diagnoses such as ADHD have received the most attention in the literature, compromised iron status may carry a generalized risk for mental disorder rather than a risk for a specific disorder. An early study reported that IDA in children requiring hospitalization was associated with increased behavioral problems post-hospitalization compared with children who were hospitalized for other reasons (Werkman et al. 1964). Infants and preschoolers with IDA have measurably worse behavior and more problems with affect when compared with iron-sufficient controls (Perez et al. 2005; Lozoff et al. 2007). Several studies have found a negative association between ADHD symptom severity and ferritin levels (those with lower ferritin levels had more ADHD symptoms) (Sever et al. 1997; Starobrat-Hermelin 1998; Konofal et al. 2004, 2007; Oner et al. 2007; Cortese et al. 2008; Oner and Oner 2008; Oner et al. 2008). ODD, frequently comorbid with ADHD and a common diagnosis in the current study sample, has been linked to ID (Juneja et al. 2010). Some symptoms of IDA, such as low energy, fatigue, and poor concentration, overlap with symptoms of a major depressive episode. In some studies lower iron status has been linked to depression symptoms (Beard et al. 2005; Vahdat Shariatpanaahi et al. 2007). In animal models of anxiety, ID has been shown to increase anxiety-like behaviors (Beard et al. 2002). Therefore, it could be that the lower ferritin levels in the clinic sample may be prevalent because compromised iron status increases the risk of mental disorder broadly rather than increasing the risk of a specific mental disorder.
The second and third explanations for the findings in this study are similar, except that the cause and effect are reversed. It could be that multiple deficiencies of macro and micro nutrients, not just iron, are etiologic agents for psychiatric disorder. It is also true that psychiatric disorders themselves change nutritional intake and thereby affect nutritional status. As stated earlier, the sampled children and youth from the mental health clinic are from a low socioeconomic background and at greater risk for poor nutrition. However, when compared with a NHANES subsample with low income, the clinic sample still has lower ferritin levels than the NHANES sample. Future studies will need to include food intake and other laboratory measures of nutritional status to test whether low nutritional status and poor nutritional intake quality can explain low iron status in clinical samples tested. Additionally, a longitudinal study could establish the temporal relationship between ID and mental disorder, answering the question “which came first?”
The fourth explanation for the findings is that low ferritin and participation in a mental health clinic could be independent, co-occurring problems both related to other common risk factors. Although we did not find that income, as measured by the PIR, was associated with ferritin level in the national sample, in other studies iron status has been linked to income, those with lower incomes being at higher risk for IDA and ID (Looker et al. 1997; Park et al. 2009). However, limiting the NHANES sample to <2 PIR did not change the comparison results in the current study. The clinic patients had lower ferritin levels than the NHANES patients with low incomes.
The final explanation for the findings in this study is that there may be something unique to the clinic sample that results in finding lower ferritin levels in the clinic than the national sample. For example, high lead levels can lead to low iron absorption and are a potential confounder if exposure were different in the two groups. (Lead levels were obtained in both samples in this study and were not significantly different.) Asa control sample from the same region was not available, there is the possibility that local differences in an unknown variable influencing iron status could account for the measured difference between the clinic and the national sample.
Limitations
A limitation of this study is the lack of a control group in the community proximate to the clinic. In addition, the clinic sample is a convenience sample of consecutive patients rather than a random sample, whereas the NHANES population sample has a purposeful over representation of minorities. The method of determining ferritin in the NHANES sample is different from that used with the clinic sample, and published data comparing the two methods are lacking. Although these methodological differences could explain a small difference, it is not expected that the magnitude of difference between the median ferritin levels of the two samples could be explained solely on the basis of differing methods of analysis. The NHANES researchers have made available comparisons between two methods of ferritin analysis used in that study showing that the differences are small (National Center for Health Statistics 2010). Because of these and other limitations, this study must be considered hypothesis generating. Findings are consistent with previous research that suggests the possibility that iron status is a factor contributing to childhood mental disorders. This is an exciting possibility because iron status improvement could be an adjunct to treatment influencing outcomes of children and youth with not only ADHD but also a range of mental disorders. Other limitations include the retrospective design, lack of structured diagnostic interviews to determine psychiatric diagnoses, and lack of cognitive measures.
A caveat, to the possible conclusion that ID is a factor in mental disorder, is that the research in the field shows that the effect of early ID is not entirely reversible and is different depending upon the timing and severity of the deficiency (Georgieff 2011). Because this is a cross-sectional study, nothing is known about the iron status history of either the clinic patients or the NHANES population. It would be ideal for future research to follow the iron status of clinic patients longitudinally rather assessing it only at one point in time.
Another obstacle is that an ideal measure for identifying ID has not been characterized, and that the optimal level of iron stores in the body is open to debate. In this study, ferritin was used as the main measure of iron status, and although ferritin levels are thought to indicate total body iron stores and are recommended by the WHO as the single best measure for the detection of ID (World Health Organization 2008), the use of ferritin presents limitations in interpretation. For example, ferritin levels are influenced not only by iron stores but also by inflammation (Thurnham et al. 2010). If inflammation is present, ferritin levels will be elevated and are no longer reflective of iron status. The only measure of inflammation available from the clinic sample was ESR, a measure of inflammation that is not sensitive enough to assess the various stages of inflammation. Other measures of inflammation such as C-reactive protein and α-1-acid glycoprotein are more specific and would provide a means to adjust for the stages of inflammation, but these measures were not available. The inability to correct ferritin levels that were falsely elevated by inflammatory status is an additional limitation of the study (Thurnham et al. 2010). Exclusion of subjects with elevated ESR in the sample did not change the results of comparison analyses. Because inflammation inflates ferritin levels and there were a significant number of subjects with elevated ESR as compared with the few subjects in the NHANES sample with elevated C-reactive protein, a correction would only strengthen the results of the comparative analysis between the clinic sample and the NHANES sample.
Conclusions
Although ferritin is a less than ideal measure of iron status, it is felt that ferritin levels of <12 μg/L indicate ID. However, some have used ferritin cutoffs as low as 10 and as high as 50 μg/L to indicate ID. Because of this wide variation in “normal,” it is difficult to characterize low iron status by using a cutoff value. The “iron transferrin receptor to ferritin ratio” has also been used to detect ID, but soluble transferrin receptor was not available from the standard laboratory measures in the clinic or national samples. Other measures of iron status and iron metabolism are not widely available to clinicians (Ullrich et al. 2005; Earley et al. 2008). The blood tests used to obtain ferritin levels in the clinic sample were random and non-fasting. Although some have raised a concern about the diurnal variation of iron status measures, at least one study has shown that restricting sample collection to certain times of day does not improve the reliability of results (Dale et al. 2002).
Clinical Significance
The finding that children and youth in the community mental health clinic had significantly lower ferritin levels than those in the NHANES sample is important because it provides support that ID may play an etiologic role in some cases of mental disorder. If ID is an etiologic factor contributing to cognitive and emotional deficits, its presence may exacerbate the health disparities between at-risk and not at-risk children and youth. In a previous study in urban high schools, iron supplementation of teen girls with ID improved some measures of cognitive functioning (Bruner et al. 1996). Ferritin is a measure of total body iron stores. This measure is increased with inflammation and decreased in ID. Measuring the iron status of children and youth 3–18 years of age is not routine. Making iron status measurement routine for psychiatric assessment of children and youth at risk for ID may be a prudent way to identify those that could benefit from iron supplementation. Measuring ferritin and soluble transferrin receptor together is the best means of assessing iron status and can be made available for as little as $1 per sample (Erhardt et al. 2004). An ultimate goal is to determine whether improving the iron status as a preventative measure and/or as a treatment will reduce mental disorder. This research points to ID as a potentially modifiable risk factor for mental disorder in children and adolescents.
Disclosures
Dr. Gottfried had a research grant from the American Academy of Child and Adolescent Psychiatry supported by Lilly USA. Dr. Yenokyan is supported by grant UL1 RR 025005 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research. Dr. Riddle is principal investigator of a National Institute for Mental Health (NIMH)-funded study that receives aripirazole from Bristol-Myers Squibb. He also serves on the National Institute of Child Health and Human Development (NICHD)-sponsored Data Safety Management Board (DSMB) for studies conducted under the Best Pharmaceuticals for Children Act (BPCA). Ms. Machell and Dr. Gerring have no conflicts of interest or financial ties to disclose.
References
- Allen RP. Earley CJ. The role of iron in restless legs syndrome. Mov Disord. 2007;18:440S–448S. doi: 10.1002/mds.21607. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. 4th. Washington, DC: American Psychiatric Association; 1994. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Amone–P'Olak K. Ormel J. Huisman M. Verhulst FC. Oldehinkel AJ. Burger H. Life stressors as mediators of the relation between socioeconomic position and mental health problems in early adolescence: The TRAILS study. J Am Acad Child Adolesc Psychiatry. 2009;48:1031–1038. doi: 10.1097/CHI.0b013e3181b39595. [DOI] [PubMed] [Google Scholar]
- Beard J. Iron deficiency alters brain development and functioning. J Nutr. 2003;133:1468S–1472S. doi: 10.1093/jn/133.5.1468S. [DOI] [PubMed] [Google Scholar]
- Beard J. Recent evidence from human and animal studies regarding iron status and infant development. J Nutr. 2007;137:524S–530S. doi: 10.1093/jn/137.2.524S. [DOI] [PubMed] [Google Scholar]
- Beard J. Erikson KM. Jones BC. Neonatal iron deficiency results in irreversible changes in dopamine function in rats. J Nutr. 2003;133:1174–1179. doi: 10.1093/jn/133.4.1174. [DOI] [PubMed] [Google Scholar]
- Beard JL. Erikson KM. Jones BC. Neurobehavioral analysis of developmental iron deficiency in rats. Behav Brain Res. 2002;134:517–524. doi: 10.1016/s0166-4328(02)00092-x. [DOI] [PubMed] [Google Scholar]
- Beard JL. Hendricks MK. Perez EM. Murray–Kolb LE. Berg A. Vernon–Feagans L. Irlam J. Isaacs W. Sive A. Tomlinson M. Maternal iron deficiency anemia affects postpartum emotions and cognition. J Nutr. 2005;135:267–272. doi: 10.1093/jn/135.2.267. [DOI] [PubMed] [Google Scholar]
- Benton D. ILSI Europe a.i.s.b.l: Micronutrient status, cognition and behavioral problems in childhood. Eur J Nutr. 2008;47(Suppl 3):38–50. doi: 10.1007/s00394-008-3004-9. [DOI] [PubMed] [Google Scholar]
- Brotanek JM. Gosz J. Weitzman M. Flores G. Iron deficiency in early childhood in the United States: Risk factors and racial/ethnic disparities. Pediatrics. 2007;120:568–575. doi: 10.1542/peds.2007-0572. [DOI] [PubMed] [Google Scholar]
- Brown G. Chadwick O. Shaffer D. Rutter M. Traub M. A prospective study of children with head injuries: III. Psychiatric sequelae. Psychol Med. 1981;11:63–78. doi: 10.1017/s0033291700053289. [DOI] [PubMed] [Google Scholar]
- Bruner AB. Joffe A. Duggan AK. Casella JF. Brandt J. Randomised study of cognitive effects of iron supplementation in non-anaemic iron-deficient adolescent girls. Lancet. 1996;348:992–996. doi: 10.1016/S0140-6736(96)02341-0. [DOI] [PubMed] [Google Scholar]
- Calarge C. Farmer C. DiSilvestro R. Arnold LE. Serum ferritin and amphetamine response in youth with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2010;20:495–502. doi: 10.1089/cap.2010.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, National Health and Nutrition Examination Survey. Atlanta, GA: [Jun 20;2011 ]. In Center for Disease Control and Prevention (database online) pp. 2009–2011. [Google Scholar]
- Connor JR. Wang XS. Neely EB. Ponnuru P. Morita H. Beard J. Comparative study of the influence of Thy1 deficiency and dietary iron deficiency on dopaminergic profiles in the mouse striatum. J Neurosci Res. 2008;86:3194–3202. doi: 10.1002/jnr.21758. [DOI] [PubMed] [Google Scholar]
- Corapci F. Calatroni A. Kaciroti N. Jimenez E. Lozoff B. Longitudinal evaluation of externalizing and internalizing behavior problems following iron deficiency in infancy. J Pediatr Psychol. 2010;35:296–305. doi: 10.1093/jpepsy/jsp065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corapci F. Radan AE. Lozoff B. Iron deficiency in infancy and mother–child interaction at 5 years. J Dev. Behav Pediatr. 2006;27:371–378. doi: 10.1097/00004703-200610000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese S. Konofal E. Bernardina BD. Mouren MC. Lecendreux M. Sleep disturbances and serum ferritin levels in children with attention–deficit/hyperactivity disorder. Eur Child Adolesc Psychiatry. 2009;18:393–399. doi: 10.1007/s00787-009-0746-8. [DOI] [PubMed] [Google Scholar]
- Cortese S. Lecendreux M. Bernardina BD. Mouren MC. Sbarbati A. Konofal E. Attention–deficit/hyperactivity disorder, Tourette's syndrome, and restless legs syndrome: The iron hypothesis. Med Hypotheses. 2008;70:1128–1132. doi: 10.1016/j.mehy.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Crowell R. Pierce MB. Ferris AM. Slivka H. Joyce P. Bernstein BA. Russell–Curtis S. Managing anemia in low-income toddlers: Barriers, challenges and context in primary care. J Health Care Poor Underserved. 2005;16:791–807. doi: 10.1353/hpu.2005.0092. [DOI] [PubMed] [Google Scholar]
- Dale JC. Burritt MF. Zinsmeister AR. Diurnal variation of serum iron, iron-binding capacity, transferrin saturation, and ferritin levels. Am J Clin Pathol. 2002;117:802–808. doi: 10.1309/2YT4-CMP3-KYW7-9RK1. [DOI] [PubMed] [Google Scholar]
- Deinard AS. Schwartz S. Yip R. Developmental changes in serum ferritin and erythrocyte protoporphyrin in normal (nonanemic) children. Am J Clin Nutr. 1983;38:71–76. doi: 10.1093/ajcn/38.1.71. [DOI] [PubMed] [Google Scholar]
- Donfransesco R. Parisi P. Vanacore N. Martines F. Sargentini V. Cortese S. Iron, ADHD: Time to move beyond serum ferritin levels. J Atten Disord. 2012 Jan 30; doi: 10.1177/1087054711430712. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Earley CJ. Horska A. Mohamed MA. Barker PB. Beard JL. Allen RP. A randomized, double-blind, placebo-controlled trial of intravenous iron sucrose in restless legs syndrome. Sleep Med. 2009;10:206–211. doi: 10.1016/j.sleep.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley CJ. Ponnuru P. Wang X. Patton SM. Conner JR. Beard JL. Taub DD. Allen RP. Altered iron metabolism in lymphocytes from subjects with restless legs syndrome. Sleep. 2008;31:847–852. doi: 10.1093/sleep/31.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmlinger MW. Kuhnel W. Ranke MB. Reference ranges for serum concentrations of lutropin (LH), follitropin (FSH), estradiol (E2), prolactin, progesterone, sex hormone-binding globulin (SHBG), dehydroepiandrosterone sulfate (DHEAS), cortisol and ferritin in neonates, children and young adults. Clin Chem Lab Med. 2002;40:1151–1160. doi: 10.1515/CCLM.2002.202. [DOI] [PubMed] [Google Scholar]
- Erikson KM. Jones BC. Beard JL. Iron deficiency alters dopamine transporter functioning in rat striatum. J Nutr. 2000;130:2831–2837. doi: 10.1093/jn/130.11.2831. [DOI] [PubMed] [Google Scholar]
- Erikson KM. Jones BC. Hess EJ. Zhang Q. Beard JL. Iron deficiency decreases dopamine D1 and D2 receptors in rat brain. Pharmacol Biochem Behav. 2001;69:409–418. doi: 10.1016/s0091-3057(01)00563-9. [DOI] [PubMed] [Google Scholar]
- Erhardt JG. Estes JE. Pfeiffer CM. Biesalski HK. Craft NE. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J Nutr. 2004;134:3127–3132. doi: 10.1093/jn/134.11.3127. [DOI] [PubMed] [Google Scholar]
- Evans GW. The environment of childhood poverty. Am Psychol. 2004;59:77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- Georgieff M MK. Long-term brain and behavioral consequences of early iron deficiency. Nutr Rev. 2011;69(Suppl 1):S43–438. doi: 10.1111/j.1753-4887.2011.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS. Hogrefe CE. Germann SL. Capitanio JP. Lozoff B. Behavioral consequences of developmental iron deficiency in infant rhesus monkeys. Neurotoxicol Teratol. 2006;28:3–17. doi: 10.1016/j.ntt.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KE. Compas BE. Thurm AE. McMahon SD. Gipson PY. Campbell AJ. Krochock K. Westerholm RI. Stressors and child and adolescent psychopathology: Evidence of moderating and mediating effects. Clin Psychol Rev. 2006;26:257–283. doi: 10.1016/j.cpr.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Grantham–McGregor S. Ani C. A review of studies on the effect of iron deficiency on cognitive development in children. J Nutr. 2001;131:649S–668S. doi: 10.1093/jn/131.2.649S. [DOI] [PubMed] [Google Scholar]
- Halterman JS. Kaczorowski JM. Aligne CA. Auinger P. Szilagyi PG. Iron deficiency and cognitive achievement among school-aged children and adolescents in the United States. Pediatrics. 2001;107:1381–1386. doi: 10.1542/peds.107.6.1381. [DOI] [PubMed] [Google Scholar]
- Hatch SL. Dohrenwend BP. Distribution of traumatic and other stressful life events by race/ethnicity, gender, SES and age: A review of the research. Am J Community Psychol. 2007;40:313–332. doi: 10.1007/s10464-007-9134-z. [DOI] [PubMed] [Google Scholar]
- Joint World Health Organization/Centers for Disease Control, Prevention Technical Consultation on the Assessment of Iron Status at the Population Level: Assessing the iron status of populations: Including literature reviews. WHO Library Cataloguing-in-Publication Data; 2004. 2004. Report of a Joint World Health Organization/Centers for Disease Control and Prevention technical consultation on the assessment of iron status at the population level; pp. 6–8. [Google Scholar]
- Jorgenson LA. Sun M. O'Connor M. Georgieff MK. Fetal iron deficiency disrupts the maturation of synaptic function and efficacy in area CA1 of the developing rat hippocampus. Hippocampus. 2005;15:1094–1102. doi: 10.1002/hipo.20128. [DOI] [PubMed] [Google Scholar]
- Juneja M. Jain R. Singh V. Mallika V. Iron deficiency in Indian children with attention deficit hyperactivity disorder. Indian Pediatr. 2010;47:955–958. doi: 10.1007/s13312-010-0160-9. [DOI] [PubMed] [Google Scholar]
- Kaushik P. Gorin F. Vali S. Dynamics of tyrosine hydroxylase mediated regulation of dopamine synthesis. J Comput Neurosci. 2007;22:147–160. doi: 10.1007/s10827-006-0004-8. [DOI] [PubMed] [Google Scholar]
- Koenker R. Hallock KF. Quantile regression. J Econ Perspect. 2001;15:143–156. [Google Scholar]
- Konofal E. Cortese S. Marchand M. Mouren MC. Arnulf I. Lecendreux M. Impact of restless legs syndrome and iron deficiency on attention-deficit/hyperactivity disorder in children. Sleep Med. 2007;8:711–715. doi: 10.1016/j.sleep.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Konofal E. Lecendreux M. Arnulf I. Mouren MC. Iron deficiency in children with attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med. 2004;158:1113–1115. doi: 10.1001/archpedi.158.12.1113. [DOI] [PubMed] [Google Scholar]
- Kryger MH. Otake K. Foerster J. Low body stores of iron and restless legs syndrome: A correctable cause of insomnia in adolescents and teenagers. Sleep Med. 2002;3:127–132. doi: 10.1016/s1389-9457(01)00160-5. [DOI] [PubMed] [Google Scholar]
- Langevin DD. Kwiatkowski C. McKay MG. Maillet JO. Touger–Decker R. Smith JK. Perlman A. Evaluation of diet quality and weight status of children from a low socioeconomic urban environment supports “at risk” classification. J Am Diet Assoc. 2007;107:1973–1977. doi: 10.1016/j.jada.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Looker AC. Dallman PR. Carroll MD. Gunter EW. Johnson CL. Prevalence of iron deficiency in the United States. JAMA. 1997;277:973–976. doi: 10.1001/jama.1997.03540360041028. [DOI] [PubMed] [Google Scholar]
- Lozoff B. Iron deficiency and child development. Food Nutr Bull. 2007;28:560S–571S. doi: 10.1177/15648265070284S409. [DOI] [PubMed] [Google Scholar]
- Lozoff B. Beard J. Connor J. Barbara F. Georgieff M. Schallert T. Long-lasting neural, behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64:34S–43S. doi: 10.1301/nr.2006.may.S34-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B. Clark KM. Jing Y. Armony–Sivan R. Angelilli ML. Jacobson SW. Dose–response relationships between iron deficiency with or without anemia and infant social-emotional behavior. J Pediatr. 2008;152:696–702. doi: 10.1016/j.jpeds.2007.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B. Corapci F. Burden MJ. Kaciroti N. Angulo–Barroso R. Sazawal S. Black M. Preschool-aged children with iron deficiency anemia show altered affect and behavior. J Nutr. 2007a;137:683–689. doi: 10.1093/jn/137.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B. Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13:158–165. doi: 10.1016/j.spen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Lozoff B. Jimenez E. Hagen J. Mollen E. Wolf AW. Poorer behavioral, developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105:E51. doi: 10.1542/peds.105.4.e51. [DOI] [PubMed] [Google Scholar]
- Lozoff B. Lu Angelilli M. Zatakia J. Jacobson SW. Calatroni A. Beard J. Iron status of inner-city African-American infants. Am J Hematol. 2007b;82:112–121. doi: 10.1002/ajh.20782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann JC. Ames BN. An overview of evidence for a causal relation between iron deficiency during development and deficits in cognitive or behavioral function. Am J Clin Nutr. 2007;85:931–945. doi: 10.1093/ajcn/85.4.931. [DOI] [PubMed] [Google Scholar]
- Menegassi M. Mello ED. Guimaraes LR. Matte BC. Driemeier F. Pedroso GL. Rohde LA. Schmitz M. Food intake and serum levels of iron in children and adolescents with attention-deficit/hyperactivity disorder. Rev Bras Psiquiatr. 2010;32:132–138. doi: 10.1590/s1516-44462009005000008. [DOI] [PubMed] [Google Scholar]
- Millichap JG. Yee MM. Davidson SI. Serum ferritin in children with attention-deficit hyperactivity disorder. Pediatr Neurol. 2006;34:200–203. doi: 10.1016/j.pediatrneurol.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Murray–Kolb LE. Beard JL. Iron treatment normalizes cognitive functioning in young women. Am J Clin Nutr. 2007;85:778–787. doi: 10.1093/ajcn/85.3.778. [DOI] [PubMed] [Google Scholar]
- Nelson C. Erikson K. Pinero DJ. Beard JL. In vivo dopamine metabolism is altered in iron-deficient anemic rats. J Nutr. 1997;127:2282–2288. doi: 10.1093/jn/127.12.2282. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics. National Health and Nutrition Examination Survey 2003–2004. Documentation, codebook, and frequencies. Laboratory component. Ferritin and transferrin receptor. http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/l06tfr_c.pdf. Jan 18, 2010. http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/l06tfr_c.pdf
- Nokes C. van den Bosch C. Bundy DAP: The effects of iron deficiency, anemia on mental, motor performance, educational achievement, behavior in children: An annotated bibliography. 1998. http://www.idpas.org/pdf/119AEffectsofIronDeficiency.pdf. [Dec 10;2009 ]. http://www.idpas.org/pdf/119AEffectsofIronDeficiency.pdf
- Oner O. Alkar OY. Oner P. Relation of ferritin levels with symptom ratings and cognitive performance in children with attention deficit-hyperactivity disorder. Pediatr Int. 2008;50:40–44. doi: 10.1111/j.1442-200X.2007.02496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oner P. Dirik EB. Taner Y. Caykoylu A. Anlar O. Association between low serum ferritin and restless legs syndrome in patients with attention deficit hyperactivity disorder. Tohoku J Exp Med. 2007;213:269–276. doi: 10.1620/tjem.213.269. [DOI] [PubMed] [Google Scholar]
- Oner P. Oner O. Relationship of ferritin to symptom ratings children with attention deficit hyperactivity disorder: Effect of comorbidity. Child Psychiatry Hum Dev. 2008;39:323–330. doi: 10.1007/s10578-007-0095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orii KE. Kato Z. Osamu F. Funato M. Kubodera U. Inoue R. Shimozawa N. Kondo N. Changes of autonomic nervous system function in patients with breath-holding spells treated with iron. J Child Neurol. 2002;17:337–340. doi: 10.1177/088307380201700505. [DOI] [PubMed] [Google Scholar]
- Ortiz E. Pasquini JM. Thompson K. Felt B. Butkus G. Beard J. Connor JR. Effect of manipulation of iron storage, transport, or availability on myelin composition and brain iron content in three different animal models. J Neurosci Res. 2004;77:681–689. doi: 10.1002/jnr.20207. [DOI] [PubMed] [Google Scholar]
- Park K. Kersey M. Geppert J. Story M. Cutts D. Himes JH. Household food insecurity is a risk factor for iron-deficiency anaemia in a multi-ethnic, low-income sample of infants and toddlers. Public Health Nutr. 2009;12:2120–2128. doi: 10.1017/S1368980009005540. [DOI] [PubMed] [Google Scholar]
- Pearson VE. Allen RP. Dean T. Gamaldo CE. Lesage SR. Earley CJ. Cognitive deficits associated with restless legs syndrome (RLS) Sleep Med. 2006;7:25–30. doi: 10.1016/j.sleep.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Perez EM. Hendricks MK. Beard JL. Murray–Kolb LE. Berg A. Tomlinson M. Irlam J. Isaacs W. Njengele T. Sive A. Vernon–Feagans L. Mother-infant interactions and infant development are altered by maternal iron deficiency anemia. J Nutr. 2005;135:850–855. doi: 10.1093/jn/135.4.850. [DOI] [PubMed] [Google Scholar]
- Picchietti D. Is iron deficiency an underlying cause of pediatric restless legs syndrome and of attention-deficit/hyperactivity disorder? Sleep Med. 2007;8:693–694. doi: 10.1016/j.sleep.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Picchietti DL. England SJ. Walters AS. Willis K. Verrico T. Periodic limb movement disorder and restless legs syndrome in children with attention-deficit hyperactivity disorder. J Child Neurol. 1998;13:588–594. doi: 10.1177/088307389801301202. [DOI] [PubMed] [Google Scholar]
- Picchietti DL. Underwood DJ. Farris WA. Walters AS. Shah MM. Dahl RE. Trubnick LJ. Bertocci MA. Wagner M. Hening WA. Further studies on periodic limb movement disorder and restless legs syndrome in children with attention-deficit hyperactivity disorder. Mov Disord. 1999;14:1000–1007. doi: 10.1002/1531-8257(199911)14:6<1000::aid-mds1014>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Pinero DJ. Li NQ. Connor JR. Beard JL. Variations in dietary iron alter brain iron metabolism in developing rats. J Nutr. 2000;130:254–263. doi: 10.1093/jn/130.2.254. [DOI] [PubMed] [Google Scholar]
- Sever Y. Ashkenazi A. Tyano S. Weizman A. Iron treatment in children with attention deficit hyperactivity disorder. A preliminary report. Neuropsychobiology. 1997;35:178–180. doi: 10.1159/000119341. [DOI] [PubMed] [Google Scholar]
- Starobrat–Hermelin B. The effect of deficiency of selected bioelements on hyperactivity in children with certain specified mental disorders. Ann Acad Med Stetin. 1998;44:297–314. [PubMed] [Google Scholar]
- Szatmari P. Shannon HS. Offord DR. Models of multiple risk: Psychiatric disorder and poor school performance. Int J Method Psychiatr Res. 1994;4:231–240. [Google Scholar]
- Thurnham DI. McCabe LD. Haldar S. Wieringa FT. Northrop–Clewes CA. McCabe GP. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: A meta-analysis. Am J Clin Nutr. 2010;92:546–555. doi: 10.3945/ajcn.2010.29284. [DOI] [PubMed] [Google Scholar]
- Ullrich C. Wu A. Armsby C. Rieber S. Wingerter S. Brugnara C. Shapiro D. Bernstein H. Screening healthy infants for iron deficiency using reticulocyte hemoglobin content. JAMA. 2005;294:924–930. doi: 10.1001/jama.294.8.924. [DOI] [PubMed] [Google Scholar]
- Vahdat Shariatpanaahi M. Vahdat Shariatpanaahi Z. Moshtaaghi M. Shahbaazi SH. Abadi A. The relationship between depression and serum ferritin level. Eur J Clin Nutr. 2007;61:532–535. doi: 10.1038/sj.ejcn.1602542. [DOI] [PubMed] [Google Scholar]
- Vasa RA. Grados M. Slomine B. Herskovits EH. Thompson RE. Salorio C. Christensen J. Wursta C. Riddle MA. Gerring JP. Neuroimaging correlates of anxiety after pediatric traumatic brain injury. Biol Psychiatry. 2004;55:208–216. doi: 10.1016/s0006-3223(03)00708-x. [DOI] [PubMed] [Google Scholar]
- Werkman SL. Shifman L. Skelly T. Psychosocial correlates of iron deficiency anemia in early childhood. Psychosom Med. 1964;26:125–134. doi: 10.1097/00006842-196403000-00004. [DOI] [PubMed] [Google Scholar]
- World Health Organization: Micronutrient deficiencies. World Health Organization [database online] 2008. www.who.int/nutrition/topics/ida/en/index.html. [Nov 15;2008 ]. www.who.int/nutrition/topics/ida/en/index.html
- Yager JY. Hartfield DS. Neurologic manifestations of iron deficiency in childhood. Pediatr Neurol. 2002;27:85–92. doi: 10.1016/s0887-8994(02)00417-4. [DOI] [PubMed] [Google Scholar]
- Youdim MB. Yehuda S. The neurochemical basis of cognitive deficits induced by brain iron deficiency: Involvement of dopamine-opiate system. Cell Mol Biol. 2000;46:491–500. [PubMed] [Google Scholar]
- Yu S. Feng Y. Shen Z. Li M. Diet supplementation with iron augments brain oxidative stress status in a rat model of psychological stress. Nutrition. 2011;27:1048–1052. doi: 10.1016/j.nut.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Zacharski LR. Ornstein DL. Woloshin S. Schwartz LM. Association of age, sex, and race with body iron stores in adults: Analysis of NHANES III data. Am Heart J. 2000;140:98–104. doi: 10.1067/mhj.2000.106646. [DOI] [PubMed] [Google Scholar]