Abstract
NKG2D ligands (NKG2DLs) are widely expressed on ovarian cancers to various degrees, making them attractive targets for immunotherapy. Here, we applied a chimeric antigen receptor (CAR) approach for the targeting of NKG2DLs expressed on human ovarian cancer cells and evaluated the impact of pharmacological upregulation of NKG2DLs on immune recognition. Various NKG2DLs, including MICA/B and ULBP-1, -2, -3, and -4, were expressed at various levels on the surface of all established ovarian cancer cell lines and primary ovarian cancer samples tested. To redirect human T cells against NKG2DLs, an NKG2DL-specific CAR was generated by fusing the extracellular domain of the NKG2D receptor to the 4-1BB costimulatory and CD3-ζ chain signaling domains. In vitro expansion of chimeric NKG2D CAR T cells was delayed compared with untransduced T cells and control CAR T cells; the likely result of fratricide among activated T cells expressing NKG2DLs. However, NKG2D CAR T cells did expand and were selectively enriched during prolonged culture. In coculture, CD4+ and CD8+ NKG2D CAR T cells specifically recognized and killed NKG2DL-expressing ovarian cancer cell lines but not NKG2DL-negative cells. Notably, pretreatment of ovarian cancer cells expressing moderate to low levels of NKG2DLs with the histone deacetylase inhibitor sodium valproate (VPA) upregulated NKG2DL cell surface expression and consequently enhanced their immune recognition by chimeric NKG2D CAR T cells. Our results demonstrate that VPA-induced upregulation of NKG2DL expression enhances the immune recognition of ovarian cancer cells by engineered NKG2D CAR T cells, and rationalizes the use of VPA in combination with NKG2DL-targeted immunotherapy in ovarian cancer.
Song and colleagues target NKG2D ligands (NGK2DLs) expressed on various human ovarian cancer cells, using a chimeric antigen receptor (CAR) approach. NGK2DL-specific CAR T cells selectively kill NKG2DL-expressing cells, and this immune recognition is enhanced by increasing surface NGK2DL expression, using a histone deacetylase inhibitor.
Introduction
Despite significant advances in surgical procedures and chemotherapy regimens, ovarian cancer remains the fifth leading cause of cancer in women, and the most lethal gynecological malignancy in the United States (Jemal et al., 2010). The demonstrated association between accumulation of immune cell infiltrates, particularly T lymphocytes, within the tumor islet, and progress-free and overall survival supports the notion that ovarian cancer cells can be naturally immunogenic (Zhang et al., 2003) and rationalizes the application of immune-based therapy in advanced disease (Kandalaft et al., 2011). Indeed, adoptive transfer of autologous tumor-infiltrating lymphocytes (TILs) has been shown to be a promising antitumor therapy for ovarian cancer in pilot clinical trials (Aoki et al., 1991; Fujita et al., 1995) but is challenged by limitations in identification and expansion of tumor-reactive TILs. An alternative strategy for the rapid generation of tumor antigen-specific T cells is through genetic modification of nonreactive T cells to express a chimeric antigen receptor (CAR): a fusion gene encoding an extracellular single-chain variable fragment (scFv) antibody domain that specifically binds to tumor antigens in an MHC-unrestricted fashion that is linked to intracellular signaling modules that mediate T cell activation (Eshhar, 2008). Adoptive T cell therapy using CD19-redirected CAR T cells has shown substantial promise in the treatment of patients with CD19-expressing hematological malignancies (Kochenderfer et al., 2010; Porter et al., 2011). CAR T cells were first tested in ovarian cancer, where targeting of folate receptor-α (FR) with CAR T cells was shown to be feasible but did not induce tumor regression because of the poor persistence of the gene-modified T cells (Kershaw et al., 2006).
We have demonstrated that incorporation of costimulatory signaling domains, such as CD137 (4-1BB), CD28, or CD27, in a FR-specific CAR overcomes the limitations of past CAR approaches by improving the persistence and antitumor activity of transferred CAR T cells in vivo (Song et al., 2011, 2012). On the basis of these promising preclinical results in ovarian cancer, we sought to broadly apply and test this approach for targeting other tumor-associated antigens (TAAs) that are commonly expressed in human ovarian cancer. In humans, NKG2D ligands (NKG2DLs) comprise two members of the MIC (MHC class I-related chain) family and six members of the ULBP/RAET (UL16-binding protein, or retinoic acid early transcript) family (Nausch and Cerwenka, 2008), which are generally absent or expressed at low levels by healthy tissues but widely expressed on ovarian cancers to various degrees (Barber et al., 2007; Nausch and Cerwenka, 2008). We therefore constructed a novel NKG2DL-specific CAR containing the extracellular domain of the NKG2D receptor, to allow its recognition of ligands on the cancer cell surface, and also the intracellular domain of CD137 in tandem with CD3ζ for enhanced T cell activation. NKG2D CAR T cells were tested for their ability to recognize and respond against human ovarian cancer cell lines expressing high or low levels of surface NKG2DLs. To further improve the potency of engineered NKG2D CAR T cells against human ovarian cancer cells expressing low levels of NKG2DLs, we investigated whether sodium valproate (VPA), a histone deacetylase (HDAC) inhibitor, upregulates various NKG2DLs on human ovarian cancer cells and increases their susceptibility to CAR T cell-mediated attack.
Materials and Methods
Cell lines
Lentivirus packaging was performed in the immortalized normal fetal renal 293T cell line purchased from the American Type Culture Collection (ATCC, Manassas, VA). Human cell lines used in immune-based assays included the established human ovarian cancer cell lines SKOV3, A1847, OVCAR2, OVCAR3, OVCAR4, OVCAR5, A2780, A2008, C30, and PEO-1. For human cells, donors entered into an institutional review board-approved clinical protocol and signed an informed consent before tumor collection. For solid tumors (PT#1909, 1911, and 1913) or normal ovarian cells (#1744 and 1804), specimen was diced in RPMI 1640, washed and centrifuged at room temperature at 800 rpm for 5 min, and resuspended in enzymatic digestion buffer (collagenase [0.2 mg/ml] and DNase [30 units/ml] in RPMI 1640) for overnight rotation at room temperature. Ascites (PT#1801 and 1888) collections were washed and cryopreserved before study. For bioluminescence assays, target cancer cell lines were transfected to express firefly luciferase (fLuc). The mouse malignant mesothelioma cell line AE17 (kindly provided by S. Albelda, University of Pennsylvania, Philadelphia, PA) was used as negative control. All cell lines were maintained in R10 medium: RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS), penicillin (100 U/ml), streptomycin sulfate (100 mg/ml), and HEPES (10 mmol/liter).
Construction of NKG2DL-specific CAR
The extracellular portion of human NKG2D (amino acids 82–216) was amplified by PCR from T cell cDNA, using the primers 5′-gcgggatccttcaaccaagaagttcaaattccc-3′ (forward primer, BamHI site underlined) and 5′-gcggctagccacagtcct ttgcatgcagatgtacg-3′ (reverse primer, NheI site underlined), and then cloned into the 4-1BB-CD3z-CAR lentiviral backbone previously described (Milone et al., 2009; Song et al., 2011), in which the CAR sequences were preceded in-frame by a green fluorescent protein (GFP) sequence followed by the 2A ribosomal skipping sequence. MOv19 scFv-based FR-specific CAR was used as an antigen-specific positive control (Song et al., 2011).
Production of lentivirus
High-titer replication-defective lentiviral vectors were produced and concentrated as previously described (Parry et al., 2003). Briefly, 293T cells were seeded in a 150-cm2 flask and transfected using Express-In (Open Biosystems/Thermo Scientific, Waltham, MA) according to the manufacturer's instructions. Fifteen micrograms of NKG2DL-specific CAR transgene plasmid was cotransfected with 7 μg of pVSV-G (VSV glycoprotein expression plasmid), 18 μg of pRSV.REV (Rev expression plasmid), and 18 μg of pMDLg/p.RRE (Gag/Pol expression plasmid) with 174 μl of Express-In (1 μg/μl) per flask. Supernatants were collected 24 and 48 hr after transfection, concentrated by ultracentrifugation for 2 hr at 28,000 rpm with a Beckman SW 32 Ti rotor (Beckman Coulter, Brea, CA), and then resuspended in 0.75 ml of R10 medium.
Human T cells and transfection
Primary human CD4+ and CD8+ T cells, purchased from the Human Immunology Core at the University of Pennsylvania, were isolated from healthy volunteer donors after leukapheresis by negative selection. All specimens were collected under a protocol approved by a university institutional review board, and written informed consent was obtained from each donor. T cells were cultured in R10 medium and stimulated with anti-CD3 and anti-CD28 monoclonal antibody (mAb)-coated beads (Invitrogen, Carlsbad, CA). Eighteen to 24 hr after activation, human T cells were transduced by a spinoculation procedure. Briefly, 0.5×106 cells were incubated with 0.75 ml of vector supernatant in 24-well plates in the presence of Polybrene (8 μg/ml). Mixtures of cells and vectors were centrifuged at room temperature for 90 min (2500 rpm) in a table-top centrifuge (Sorvall ST 40; Thermo Scientific). Human recombinant interleukin-2 (IL-2; Novartis, Basel, Switzerland) was added every 2–3 days to a 100-IU/ml final concentration, and a cell density of 0.5×106 to 1×106 cells/ml was maintained. Once engineered T cell cultures appeared to rest down, as determined by both decreased growth kinetics and cell-sizing determined with a Multisizer 3 Coulter Counter (Beckman Coulter), the T cells were used for functional analysis.
Reagents and antibodies
Sodium valproate (VPA) was purchased from Sigma-Aldrich (St. Louis, MO). The following antibodies were used for T cell analysis: allophycocyanin (APC)–Cy7 mouse anti-human CD3, phycoerythrin (PE)–anti-human CD8, and APC–anti-human NKG2D. Expression of NKG2DL-specific CAR was detected by intracellular GFP and surface NKG2D expression was detected with anti-NKG2D antibody. NKG2DLs were analyzed with PE–anti-MICA/B (clone 6D4; BD Biosciences), PE–anti-ULBP1 (clone 170818; R&D Systems, Minneapolis, MN), PE–anti-ULBP2 (clone 165903; R&D Systems), anti-ULBP3 (clone 2F9; Santa Cruz Biotechnology, Santa Cruz, CA), and polyclonal peridinin–chlorophyll protein complex (PerCP)–anti-human ULBP4 (R&D Systems). Recombinant human NKG2D Fc chimera and a control recombinant human HER2 Fc chimera were purchased from R&D Systems. Pacific blue-labeled anti-human CD326 (EpCAM) (clone 9C4; BioLegend, San Diego, CA) was used as a marker of primary ovarian cancer samples. Matched secondary and isotype antibodies were used in all analyses. Flow cytometry was performed with a BD FACSCanto II flow cytometer (BD Biosciences) and flow cytometric data were analyzed with FlowJo version 7.2.5 software (Tree Star, Ashland, OR).
Cytokine release assays
Cytokine release assays were performed by coculture of 5×104 T cells with 5×104 target cells per well in triplicate in 96-well flat-bottom plates in a 200-μl volume of R10 medium. After ∼20 hr, cell-free supernatants were assayed for the presence of interferon (IFN)-γ, using an ELISA kit (BioLegend). Values represent the mean of triplicate wells. To block NKG2D receptors, T cells were preincubated at 37°C for 2 hr with anti-human NKG2D (clone 1D11, 20 μg/ml) or isotype control antibodies before addition of target cells.
Cytotoxicity assays
For the cell-based bioluminescence assays, 5×104 firefly luciferase (fLuc)-expressing tumor cells were cultured with R10 medium in the presence of various ratios of transduced T cells with the use of a 96-well microplate (BD Biosciences). After incubation for ∼20 hr at 37°C, each well was filled with 50 μl of Dulbecco's phosphate-buffered saline (DPBS) resuspended with 1 μl of d-luciferin (0.015 g/ml) and imaged with a Xenogen IVIS Spectrum system (PerkinElmer, Waltham, MA). Percent tumor cell viability was calculated as the mean luminescence of the experimental sample minus background divided by the mean luminescence of the input number of target cells used in the assay minus background times 100. All data are represented as a mean of triplicate wells.
Pretreatment of cancer cell lines with VPA
Ovarian cancer cell lines OVCAR5, PEO-1, A2780, and AE17 and normal primary ovarian epithelial cells (#1744 and 1804) were seeded in 48-well plates at a cell density of 1×105 cells per well. After overnight attachment, cells were starved for 4 hr and then incubated for 48 or 96 hr with increasing concentrations of VPA. NKG2DL expression was detected via antibody-based flow cytometry as described previously. Cell viability was measured by 7-aminoactinomycin D (7-AAD) staining, using flow cytometry.
Statistical analysis
A two-tailed Student t test was used to evaluate differences in T cell expansion and cytokine secretion. GraphPad Prism 5.0 (GraphPad, San Diego, CA) was used for the statistical calculations. P<0.05 was considered significant.
Results
Expression of NKG2D ligands on ovarian cancer cells
To investigate NKG2DL expression in human ovarian cancer, we screened 11 ovarian cancer cell lines for NKG2DL expression via flow cytometry, using a recombinant NKG2D receptor–human IgG1-Fc fusion protein that recognizes all ligands for this receptor. Although varied in level of expression, all ovarian cancer cell lines tested expressed cell-surface NKG2DL. The majority of lines expressed a high to moderate level of NKG2DL; however, lower levels of expression were detected on A2780, C30, and PEO-1. AE17, a mouse malignant mesothelioma cell line, served as a negative control and did not express detectable human NKG2DL. We next determined the expression distribution of the various NKG2DL family members in human ovarian cancer by flow cytometry, using antibodies specific for MICA/B or for ULBP-1, -2, -3, or -4 (Fig. 1). Expression of the ULBPs varied; ULBP1 was expressed only on six cell lines and at low levels, whereas ULBP-2, -3, and -4 were more often strongly expressed in the ovarian cancer cell lines. Six ovarian cancer cell lines tested expressed surface MICA/B.
FIG. 1.
Surface expression of NKG2D ligands in ovarian cancer cells. A panel of human ovarian cancer cell lines was stained with specific antibodies that recognize MICA; MICB; ULBP-1, -2, -3, or -4; or with a soluble human NKG2D–human IgG1 fusion protein (open histogram) or matched isotype controls (shaded histogram) and analyzed by flow cytometry. A murine mesothelioma cell line, AE17, was used as negative control.
Because results from cell lines may be misleading, we analyzed three primary ovarian cancer samples and two ascites samples for expression of each NKG2D ligand on EpCAM+ cancer cells (Supplementary Fig. S1; supplementary data are available online at www.liebertonline.com/hum). We observed that all primary cancer samples expressed NKG2DLs on the surface, although the amount of each individual ligand expressed varied between samples. Thus, NKG2DLs appear to be broadly expressed by ovarian cancer cells, with some cells displaying a relatively low level of ligand expression.
Construction and expression of anti-NKG2DL-specific CAR
CARs expressed on T cells are composed of an extracellular binding portion fused to a transmembrane and intracellular tail to facilitate antigen/ligand-triggered T cell activation. To redirect human T cells against NKG2DLs on cancer cells, we constructed a human NKG2DL-specific CAR (referred to as an NKG2D CAR) composed of the extracellular portion of the human NKG2D receptor linked to a CD8α hinge and transmembrane region, followed by an intracellular CD137 (4-1BB) costimulatory domain and a CD3ζ signaling motif. An anti-FR-specific CAR was constructed as an antigen-specific control CAR for assays. Bicistronic CAR vectors incorporated an upstream green fluorescent protein (GFP) sequence to confirm and facilitate transduced T cell detection. The CAR constructs, and structures of the engineered NKG2D CAR and endogenous NKG2D receptor, are diagrammed in Fig. 2A.
FIG. 2.
Construction, expression of an NKG2D ligand (NKG2DL)-specific chimeric antigen receptor (CAR), and expansion of CAR T cells in vitro. (A) Schematic illustration of the lentiviral construct for the NKG2DL- and folate receptor-α (FR)-specific CARs (left) and domain architecture of endogenous NKG2D and engineered NKG2DL CAR (right). The chimeric NKG2D (chNKG2D) CAR contains the extracellular portion of the human NKG2D receptor, which linked to a CD8α hinge and transmembrane region, followed by an intracellular CD137 (4-1BB) costimulatory domain and a CD3ζ signaling motif. A similar FR-specific CAR using an anti-FR single-chain variable fragment (scFv) was constructed as an antigen-specific control CAR for assays. (B) NKG2DL-specific CAR and GFP coexpressed by both transduced human CD4+ and CD8+ T cells 14 days after transduction. (C) Comparison of expansion of chNKG2D CAR T cells, untransduced and FR-CAR T cells. (D) Temporal induction of apoptosis in untransduced (UNT), chNKG2D CAR, and FR-transduced CAR T cells after stimulation and transduction. (E) NKG2DLs are expressed on activated CD4+ and CD8+ T cells after activation in vitro. Shown is the mean±SD NKG2D+ T cell frequency from three independent assays. (F) NKG2DL-specific CARpos (GFP-expressing) T cells are enriched over time. Results for three independent donors are shown. 7-AAD, 7-aminoactinomycin D; ECD, extracellular domain; ICD, intracellular domain. Color images available online at www.liebertpub.com/hum
Primary human T cells were activated with anti-CD3/CD28 antibody-coated beads, transduced with NKG2D CAR lentiviral particles, and evaluated for the surface expression of the engineered NKG2D CAR by fluorescence-activated cell sorting (FACS) ∼1 week after transduction. Bicistronic expression vectors incorporating a 2A peptide sequence permitted dual-expression analysis of GFP and the NKG2D CAR. Engineered NKG2D CAR and GFP displayed a linear coexpression pattern on both CD4+ and CD8+ transduced T cells (Fig. 2B), indicating that GFP is a suitable marker for detection of engineered NKG2D CAR surface expression on T cells. Only a small percentage of untransduced (GFPneg) CD4+ T cells expressed endogenous NKG2D (GFPnegNKG2Dpos), whereas the majority of GFPnegCD8+ T cells expressed endogenous NKG2D (Fig. 2B), consistent with activation-induced upregulation of endogenous NKG2D known to occur in human CD8+ T lymphocytes (Verneris et al., 2004).
NKG2DLs expressed on activated T cells promote fratricide but enrich for NKG2D-redirected CAR T cells
To prepare CAR T cells for testing, human T cells were stimulated with anti-CD3/CD28 antibody-coated beads, transduced with lentivirus, and expanded for 14 days in the presence of IL-2 (100 IU/ml). NKG2D CAR T cells grew slowly over 2 weeks compared with untransduced T cells (data not shown), suggesting that transduction with CAR-encoding lentivirus may have a toxic effect on T cell expansion. Therefore, we carefully compared in parallel the expansion of T cells, from three different donors, that were engineered to express either the NKG2D CAR, an FR-specific CAR, or left untransduced. After 14 days of expansion, FR-specific CAR T cells and untransduced T cells showed comparable fold expansion (594±44 and 540±40-fold, respectively; p=0.25), indicating that transduction alone had limited impact on cell growth. However, NKG2D CAR T cells showed only 295±90-fold expansion, which was significantly less than observed in untransduced and FR-specific CAR T cells (p<0.05) (Fig. 2C). Consistent with their delayed expansion, increased frequencies of T cells in early and late stages of apoptosis were observed in NKG2D CAR T cell populations on days 3 and 5 after transduction, compared with controls (Fig. 2D). NKG2DL expression on T cells can be activation-induced (Molinero et al., 2002; Cerboni et al., 2007), suggesting that fratricide among NKG2D-redirected T cells, but not FR-specific T cells, may account for poor expansion of the former. We therefore tested for cell surface expression of NKG2DLs on activated T cells after stimulation with anti-CD3/CD28 beads in vitro according to our CAR transduction protocol. Expression analysis performed on T cells from three different donors showed that unstimulated CD4+ and CD8+ T cells on day 0 did not express surface NKG2DLs; however, NKG2DL expression was upregulated 4 days after T cell stimulation, with persistent expression on day 5 with a gradual decline over days 6 to 10 (Fig. 2E and Supplementary Fig. S2A). CD4+ T cells expressed a higher level of NKG2DLs than did CD8+ T cells. Together, these results implicate activated NKG2DL+ T cells as potential targets of NKG2D CAR T cell-mediated fratricide in vitro.
Activated CD8+ T cells generally proliferate faster than activated CD4+ T cells, and thus activation can promote a relative enrichment of CD8+ T cells in culture (Lo et al., 2010). We therefore monitored CD4+ and CD8+ subsets of T cells during gene transduction by flow cytometry over 2 weeks of expansion in vitro after initial anti-CD3/CD28 stimulation. At the start of culture, the CD8+ subset represented ∼30% of the CD3+ T cell population. By day 14 poststimulation, the NKG2D CAR T cell group contained 50.1±4.44% CD8+ T cells, which was statistically similar to the untransduced T cell group (59.3±5.86%) and the control FR CAR T cell group (57.5±7.99%) (p=0.22 and p=0.09, respectively; Supplementary Fig. S2B). Thus, no strong skewing in CD4/CD8 ratio was noted and the final CD4/CD8 ratio of NKG2D CAR T cells was approximately 1:1 after in vitro culture, which is reported to be favorable for antitumor response in vivo (Gyobu et al., 2004; Moeller et al., 2007).
Despite their delay, GFP-expressing NKG2D CAR T cells from three different donors did expand in vitro and were highly enriched for CAR+ cells during prolonged culture. Consistently, only 65–68% of T cells were positive for GFP on day 7 posttransduction, but were preferentially enriched to 96–98% after 14 days of culture (Fig. 2F). Next, independent kinetic monitoring of surface CAR expression on NKG2D CAR T cells was performed, using anti-FR CAR T cells as control (Supplementary Fig. S3A and B). The NKG2D CAR-expressing T cell frequency increased from 49 to 81% during the period from day 3 to day 16 of culture. In contrast, the percentage of anti-FR CAR-expressing T cells was stable at ∼48% over this time, suggestive of a dependence on NKG2D–NKG2DL interaction in the selective longitudinal enrichment of NKG2D-redirected CARpos T cells.
NKG2D CAR T cells recognize NKG2DL-positive ovarian cancer cells in an NKG2D-dependent manner
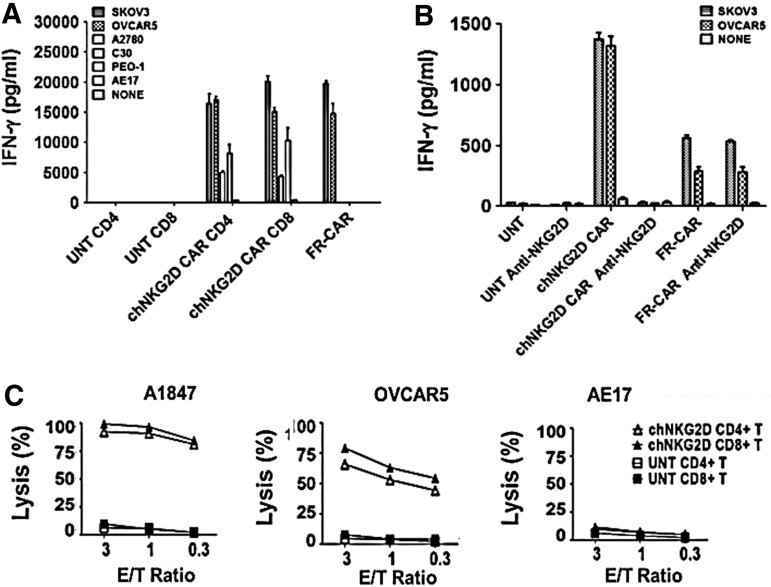
To detect recognition of NKG2DLs on cancer cells by engineered T cells, we used a panel of established human ovarian cancer cell lines that express surface NKG2DLs at various levels for assays (shown in Fig. 1). Primary human CD4+ and CD8+ NKG2D CAR T cells recognized NKG2DL-positive tumor lines and secreted high levels of IFN-γ in overnight cultures, but not when stimulated with the NKG2DL-negative cell line, AE17 (Fig. 3A). The level of IFN-γ response generally trended toward being associated with the level of NKG2DL expressed on the target cell surface. Anti-FR CAR T cells served as positive assay controls for IFN-γ release in response to FRpos cell lines SKOV3 and OVCAR5, but not FRneg cells as previously described (Song et al., 2011). Similar to established cell lines, all primary ovarian cancer samples (CD45-depleted cells) tested were recognized by NKG2D CAR T cells in accordance with their positive NKG2DL expression (Supplementary Fig. S1A and B). Untransduced T cells did not secrete IFN-γ in response to any of the above-described cancer cells. To further evaluate whether NKG2D CAR recognition and activation were dependent on NKG2D–NKG2D ligand interactions, we tested whether blockade of NKG2D reduced IFN-γ release by engineered T cells in short-term cocultures with NKG2DLpos cancer cells. As shown in Fig. 3B, the amount of IFN-γ released by NKG2D CAR T cells in response to culture with SKOV3 or OVCAR5 cells was reduced to background levels in the presence of NKG2D-blocking antibody. In contrast, the amount of IFN-γ released by FR-CAR T cells in response to SKOV3 and OVCAR5 tumor cells was unaltered by addition of NKG2D-blocking antibody.
FIG. 3.
Recognition of human ovarian cancer cells by NKG2DL-specific CAR T cells. (A) Chimeric NKG2D (chNKG2D) CAR-modified T cells secrete IFN-γ during overnight culture with all NKG2DL-expressing ovarian cancer cells, but not NKG2D-negative AE17 mesothelioma cells. Mean IFN-γ concentration±SEM (pg/ml) from triplicate cultures is shown. (B) To show NKG2D dependence, NKG2DL-specific CAR T cells were incubated with anti-NKG2D antibodies or with isotype control IgG antibodies before incubation with tumor cells for 6 hr. Blocking NKG2D markedly reduced the amount of IFN-γ released by NKG2DL-specific CAR T cells against tumor cells at all ratios compared with control. (C) Lysis of NKG2DL-expressing tumor cells (A1847 and OVCAR5) by CAR CD4+ and CD8+ T cells in an 18-hr bioluminescence assay at the indicated effector-to-target (E/T) ratios. Untransduced CD4+ and CD8+ human T cells and AE17 mesothelioma cells served as negative effector and target cell controls, respectively.
We next measured the killing of NKG2DLpos cancer cells by NKG2D CAR T cells in a cell-based bioluminescence assay. Consistent with cytokine production results, primary human CD4+ and CD8+ T cells expressing the NKG2D CAR directly and efficiently lysed NKG2DLpos human ovarian cancer cell lines A1847 and OVCAR5, but not the NKG2DLneg line AE17 (Fig. 3C).
VPA treatment increases surface expression of NKG2D ligands on ovarian cancer cells but not normal ovarian epithelial cells
Cytokine release results from cocultures of NKG2D CAR T cells and human ovarian cancer cells (Fig. 3A) suggest that pharmacological enhancement of NKG2DL expression may augment tumor cell recognition and susceptibility to CAR-mediated immune response. Sodium valproate (VPA) is a histone deacetylase (HDAC) inhibitor known to enhance natural killer (NK) cell-mediated antitumor activity by upregulating the surface expression of NKG2DLs on various cancer cells (Armeanu et al., 2005; Zhang et al., 2009; Chavez-Blanco et al., 2011; Tanic et al., 2012). To assess the capacity of VPA to sensitize ovarian cancer cells to immune attack, we assayed for dose of VPA in vitro at which most ovarian cancer cells remained viable and upregulate surface NKG2D expression. A panel of human ovarian cancer cells was treated with VPA at various concentrations (0, 0.5, 1, 2, 4, 8, 16, or 32 mM) for 48 or 96 hr. At concentrations of 0.5, 1, 2, and 4 mM, most ovarian cancer cells remained viable cells at these time points, although C30 and A1847 cells were particularly sensitive at the 4 mM concentration (Fig. 4A). We hypothesized that viable ovarian cancer cells expressing NKG2DL at low levels could be pharmacologically enhanced by low-dose VPA treatment. The expression levels of NKG2DLs on the cancer cell surface were examined after exposure to 2 mM VPA, a dose that had limited toxic effect on cancer cells with >90% cancer cell viability. Viable OCAR5, A2780, and PEO-1 cell lines, which express moderate to low levels of NKG2DLs when untreated, showed an increase in NKG2DL expression when treated with VPA (Fig. 4B). Specifically, all lines displayed enhanced MICA/B and ULBP-2 expression. ULBP-1 and ULBP-3 expression was slightly increased on A2780 and PEO-1 cells, and ULBP-4 expression was slightly elevated on OVCAR5 and A2780 cells. Consistent with other reports (Armeanu et al., 2005; Langenkamp et al., 2009), the surface expression of NKG2DLs on normal primary ovarian epithelial cells, which occurred at low levels, remained stable after VPA treatment (Fig. 4C). Primary normal ovarian cells did not show any response to treatment with VPA, even at high concentrations of 8 and 16 mM, which induce toxic effects on various cancer cells (Supplementary Fig. S4).
FIG. 4.
Increased NKG2DL surface expression by human ovarian cancer cells after sodium valproate (VPA) treatment. (A) Effect of VPA on cell viability. The viability of ovarian cancer cells was analyzed via 7-aminoactinomycin D (7-AAD) staining 48 and 96 hr after VPA (final concentration, 0–32 mM) treatment. (B) Histograms show the surface expression of the respective NKG2DLs on OVCAR5, A2780, and PEO-1 ovarian cancer cells treated with VPA (dashed histograms) or without treatment (open histograms). Shaded histograms represent matched isotype controls. (C) Histograms show the surface expression of the respective NKG2DLs on normal primary ovarian cells from donors #1744 and #1804 with VPA (dashed histograms) or without VPA (open histograms). Ovarian cancer cells from patient PT#1801 are shown for comparison. Shaded histograms represent matched isotype controls.
VPA sensitizes human ovarian cancer cells to NKG2D-redirected T cell attack
To determine whether augmented levels of NKG2DLs on the tumor cell surface are associated with their improved recognition by NKG2D-redirected CAR T cells, we measured the IFN-γ release of NKG2D CAR T cells after culture with VPA-treated or untreated NKG2DLpos ovarian cancer cells. OVCAR5, A2780, and PEO-1 cell lines were VPA pretreated (2 mM) or untreated for ∼20 hr before coculture with an equivalent number of NKG2D CAR T cells. As predicted, VPA-induced NKG2DL upregulation improved antigen-specific recognition and activity by NKG2D CAR T cells, with significantly higher amounts of secreted IFN-γ observed in cocultures with VPA-treated ovarian cancer cell lines compared with untreated cells (Fig. 5). Untransduced T cells did not release IFN-γ when incubated with either VPA-treated or untreated cancer cells. These data suggest that VPA treatment significantly increased the susceptibility of ovarian cancer cell lines to antitumor immune response by T cells redirected against NKG2DL.
FIG. 5.
Upregulation of NKG2DL expression after VPA treatment enhances the susceptibility of ovarian cancer cells to NKG2DL-specific CAR T cell-mediated attack. The antitumor effect of NKG2DL-specific CAR T cells against untreated or VPA-pretreated ovarian cancer cells was assessed by IFN-γ release after overnight coculture, using lymphocytes from three different donors. Mean values of triplicate assays are shown. p<0.05 for all VPA versus untreated conditions for all cancer cell lines and donor lymphocytes tested.
Discussion
The goal of our study was to further apply CD137 (4-1BB)-costimulated CAR for the targeting of various tumor-associated antigens (TAAs) in ovarian cancer. Ligands for the human NKG2D receptor include MHC class I-related protein A (MICA), MICB, and UL-16-binding proteins (ULBPs) 1–6 (González et al., 2008; Nausch and Cerwenka, 2008; Eagle et al., 2009). Surface expression of NKG2D ligands, which are prevalently found in ovarian cancer and many other types of human malignancies, thus represent important therapeutic targets. Moreover, ULBP-2 and ULBP-4 (lymphocyte effector cell toxicity-activating ligand, Letal) are independent predictors of poor prognosis in patients with ovarian cancer, rationalizing the targeting of cancer cells expressing these ligands.
Cross-linking of NKG2D by NKG2DLs leads to an activating signal, resulting in release of cytokines and cytotoxicity. The feasibility of an NKG2DL-specific CAR T cell approach has been demonstrated by Sentman and colleagues using the full-length NKG2D fused to the cytoplasmic domain of CD3ζ, where the orientation of the CD3ζ portion was reversed because NKG2D is a type II receptor, in which the NH2 terminal is located intracellularly, whereas the signaling motifs of CARs are derived from type I proteins with the COOH terminus in the cytoplasm. In our study, we directly fused the extracellular domain of NKG2D to a CD8α hinge and transmembrane, followed by the 4-1BB and CD3ζ-derived signaling domains with type I orientation. Unlike most conventional CARs that use a scFv derived from mouse monoclonal antibodies, our NKG2D CAR incorporates the extracellular domain of the human NKG2D receptor, and thus it would not be expected to induce xenogeneic human anti-mouse antibody (HAMA) or T cell responses in humans (Kershaw et al., 2006; Jensen et al., 2010; Lamers et al., 2011), which have limited their success.
Lehner and colleagues (2012) constructed a similar NKG2D-based CAR with the ectodomain of NKG2D followed by an integrated costimulatory CD28 domain for enhanced T cell activation. CD28 costimulation augments anti-apoptotic Bcl-XL expression (Boise et al., 1995), increases IL-2-independent proliferation, enhances the resistance of CAR T cells to T regulatory cells (Loskog et al., 2006), and is the costimulatory domain of choice for most CARs. In the clinical setting, proliferation and persistence of CAR T cells can be modestly enhanced by addition of the CD28 costimulatory domain compared with CAR T cells that lack CD28 (Savoldo et al., 2011). Kochenderfer and colleagues (2012) showed that six of eight patients treated with CD28-costimulated CD19 CAR T cells obtained remissions of advanced, progressive B cell malignancies, but the persistence of CAR T cells varied widely. Evidence supports the notion that the cytoplasmic domain of CD137 (4-1BB) may provide better proliferation and persistence of CAR T cells compared with the CD28 moiety. Porter and colleagues (2011) and Kalos and colleagues (2011) have reported more extensive proliferation and long-term persistence of transferred CAR T cells bearing the 4-1BB motif in three patients. Under comparative analysis in preclinical models of cancer, we (Song et al., 2011, 2012) and Carpenito and colleagues (2009) have shown that CAR T cells containing the 4-1BB domain were more multifunctional and persisted at greater number in tumor-bearing mice after adoptive transfer, compared with mice receiving CD28-costimulated CAR T cells. However, a larger group of patients and randomization would be required before any firm conclusions can be drawn about distinctive roles of these costimulatory molecules in CARs.
Human NKG2DLs are expressed not only on tumor cells but also on activated CD4+ and CD8+ T cells. Cerboni and colleagues (2007) have shown that MICA and ULBP-1, ULBP-2, and ULBP-3, but not ULBP-4, are expressed on T cells in response to superantigen, alloantigen, or to a specific antigenic peptide. In our study, we confirm that anti-CD3/CD28 bead-stimulated T cells transiently express NKG2DLs. NKG2DL expression was detected at a high level on day 4, persisted on day 5, and then gradually declined. CD4+ T cells expressed a higher level of NKG2DLs than did CD8+ T cells. This fratricide-promoting expression pattern would be predicted to be a major limitation in the development of NKG2D-directed T cell-based immunotherapy. For example, Leisegang and colleagues (2010) showed that survivin-specific T cells underwent fratricide when activated, because the target antigen, survivin, is also expressed in activated T cells. We previously reported a similar HLA-restricted affect in p53-redirected T cells (Theoret et al., 2008). In our current study, fratricide was observed among NKG2D CAR T cells during the first week after transduction, resulting in delayed T cell expansion. Despite this early delay, these cultures could then be expanded for up to 8–10 weeks, without evident fratricide observed. Consistent with the study by Lehner and colleagues (2012), NKG2D CARpos T cells were enriched during prolonged culture, suggesting an autostimulatory effect possibly through endogenous expression of NKG2DLs induced after T cell stimulation. This is congruous with NKG2D expression restoration to near baseline (nadir) levels by day 10 poststimulation, cessation of NKG2D-targeted fratricide, and the ensuing preferential expansion of T cells bearing NKG2D CAR. Importantly, expanded NKG2D CAR T cells do express CAR that is fully functional on the T cell surface as both engineered CD4+ and CD8+ CAR T cells mediated antigen-specific immune responses and could efficiently kill human ovarian cancer cells expressing NKG2DLs in culture.
Because of the variable levels of NKG2DL expression observed on ovarian cancer cells, we explored pharmacological means of molecule upregulation to bolster CAR responses. HDAC inhibitors are a structurally diverse group of agents, comprising both natural and synthetic compounds (Dokmanovic and Marks, 2005). Studies have demonstrated that HDAC inhibitors including VPA, sodium butyrate (SB), trichostatin A (TsA), MS-275, and suberoylanilide hydroxamic acid (SAHA) can enhance NK cell-mediated cytotoxicity against cancer cells through upregulation of NKG2D ligands (Armeanu et al., 2005; Zhang et al., 2009; Chavez-Blanco et al., 2011; Berghuis et al., 2012). VPA is of great interest because it is a well-established and tolerated drug for long-term therapy of patients with convulsions. In our study, VPA exposure had differing effects on the expression of the various NKG2DLs. MIC A/B, ULBP-2, and ULBP-4 expression, but not ULBP-1 and −3 expression, was slightly upregulated on OVCAR5 cells after 2 days of pretreatment with 2 mM VPA. ULBP-2 surface expression on A2780 cells was markedly enhanced, whereas MICA/B and ULBP-1, -3, and -4 expression was modestly increased. ULBP-1, -2, and -3 were moderately increased on PEO-1 cells, but not MICA/B or ULBP-4. With the possible exception of ULBP-2, it appears that VPA-induced expression of each NKG2D ligand may be differentially regulated according to cell line even under similar stimulation conditions, although more detailed investigation is required. Importantly, we find that our novel NKG2D CAR T cells secrete IFN-γ when incubated with ovarian cancer cells expressing low levels of NKG2DL and that this recognition can be significantly enhanced after treatment of cancer cells with VPA. This recognition was dependent on NKG2D–NKG2DL interaction, because it was abolished by NKG2D blockade. In considering the potential of HDAC inhibitors in combination with CAR T cells therapy, it was important to ascertain whether VPA treatment also induces NKG2DL expression on normal cells, which might then be rendered more sensitive to CAR-mediated attack. Surface expression of NKG2DLs on primary normal ovarian epithelial cells treated with or without VPA treatment was maintained at the same low level. These cells also tolerated treatment with VPA well, unlike malignant cells. Previous studies (Armeanu et al., 2005; Langenkamp et al., 2009) have demonstrated that VPA induces NKG2DL expression in malignant but not normal cells in hepatocellular carcinoma and acute myeloid leukemia models, respectively. However, the mechanism of preferential VPA-induced upregulation of NKG2DL expression by malignant cells is not well understood.
Although these results rationalize the combination of VPA and NKG2D-directed therapies, limitations may exist. Cancer cells have evolved sophisticated mechanisms to elude NK and T cell recognition and killing. For example, it has been shown that the CD3ζ chain of the T cell receptor is often downregulated in ovarian cancer, thus contributing to the lack of a host T cell response to the tumor (Lai et al., 1996; Lockhart et al., 2001). Further, tumor cells can shed a soluble form of MICA (sMICA), which can then serve to downregulate NKG2D expression and impair NK cell and CD8+ T cell functions (Groh et al., 2002). As a novel chimeric protein with expression stably enforced by an EF-1α promoter, it is possible that the chNKG2D receptor, consisting of the NKG2D receptor fused to the CD3ζ chain and incorporating 4-1BB costimulation in tandem, may resist natural tumor-induced downregulation of CD3ζ signaling in the tumor microenvironment. It is, however, also possible that soluble NKG2DL protein may also limit CAR T cell activity in vivo; formal testing is required. Regional administration such as intratumoral or intraperitoneal injection of these CAR T cells may in part resolve these issues and further minimize the potential for “on target, off organ” toxicity trigged by low-level expression of NKG2DLs on normal tissues. Indeed, Parente-Pereira and colleagues (2011) demonstrated that a majority of gene-modified T cells remained at the site of injection after subcutaneous and intraperitoneal injection. These results suggest that intraperitoneal delivery of NKG2D CAR T cells represents a viable treatment option for ovarian cancer. However, one must consider that costimulated CAR T cells may persist long term in vivo and ultimately migrate to important organs, resulting in “on-target, off-tumor” toxicity. In this case, a restricted dose escalation scheme of CAR T cells should be considered for future clinical trials (Junghans, 2010) with possible incorporation of a suicide gene system for inducible deletion of CAR T cells in vivo and thus increased safety of the CAR T cell therapy approach (Di Stasi et al., 2011). Alternatively, the use of an RNA electroporation approach that results in transient CAR expression on electroporated T cells may provide a safer platform (Barrett et al., 2011).
Our results demonstrate that VPA increases the susceptibility of human ovarian cancer cells to NKG2D CAR T cell-mediated immune attack. Our novel NKG2D CAR T cells undergo fratricide over the first week of culture followed by a remarkably selective enrichment of CAR-expressing T cells during prolonged culture, which are both the likely effect of autostimulation resulting from induced NKG2DL expression on activated T cells. Nevertheless, NKG2D CAR T cells at the time of cell harvest maintain robust and specific antitumor activity suitable for use in therapy. Taken together, our results suggest that pharmacological treatment of ovarian cancer with VPA can favorably sculpt the immune landscape of tumor cells in vivo and allow for the improved efficacy of NKG2DL-directed immunotherapeutics in patients with tumors with low NKG2DL expression.
Supplementary Material
Acknowledgments
This work was supported in part by grants from the Ovarian Cancer Research Fund (OCRF), the Sandy Rollman Ovarian Cancer Foundation, the NIH (R01-CA168900) and the Joint Fox Chase Cancer Center and University of Pennsylvania Ovarian Cancer SPORE (P50 CA083638).
Author Disclosure Statement
The authors declare that there are no conflicts of interest.
References
- Aoki Y. Takakuwa K. Kodama S., et al. Use of adoptive transfer of tumor-infiltrating lymphocytes alone or in combination with cisplatin-containing chemotherapy in patients with epithelial ovarian cancer. Cancer Res. 1991;51:1934–1939. [PubMed] [Google Scholar]
- Armeanu S. Bitzer M. Lauer U.M., et al. Natural killer cell-mediated lysis of hepatoma cells via specific induction of NKG2D ligands by the histone deacetylase inhibitor sodium valproate. Cancer Res. 2005;65:6321–6329. doi: 10.1158/0008-5472.CAN-04-4252. [DOI] [PubMed] [Google Scholar]
- Barber A. Zhang T. Demars L.R., et al. Chimeric NKG2D receptor–bearing T cells as immunotherapy for ovarian cancer. Cancer Res. 2007;67:5003–5008. doi: 10.1158/0008-5472.CAN-06-4047. [DOI] [PubMed] [Google Scholar]
- Barrett D.M. Zhao Y. Liu X., et al. Treatment of advanced leukemia in mice with mRNA engineered T cells. Hum. Gene Ther. 2011;22:1575–1586. doi: 10.1089/hum.2011.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghuis D. Schilham M.W. Vos H.I., et al. Histone deacetylase inhibitors enhance expression of NKG2D ligands in Ewing sarcoma and sensitize for natural killer cell-mediated cytolysis. Clin. Sarcoma Res. 2012;2:8. doi: 10.1186/2045-3329-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boise L.H. Minn A.J. Noel P.J., et al. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- Carpenito C. Milone M.C. Hassan R., et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc. Natl. Acad. Sci. U.S.A. 2009;106:3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerboni C. Zingoni A. Cippitelli M., et al. Antigen-activated human T lymphocytes express cell-surface NKG2D ligands via an ATM/ATR-dependent mechanism and become susceptible to autologous NK-cell lysis. Blood. 2007;110:606–615. doi: 10.1182/blood-2006-10-052720. [DOI] [PubMed] [Google Scholar]
- Chavez-Blanco A. de la Cruz-Hernandez E. Dominguez G.I., et al. Upregulation of NKG2D ligands and enhanced natural killer cell cytotoxicity by hydralazine and valproate. Int. J. Oncol. 2011;39:1491–1499. doi: 10.3892/ijo.2011.1144. [DOI] [PubMed] [Google Scholar]
- di Stasi A. Tey S.K. Dotti G., et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokmanovic M. Marks P.A. Prospects: Histone deacetylase inhibitors. J. Cell Biochem. 2005;96:293–304. doi: 10.1002/jcb.20532. [DOI] [PubMed] [Google Scholar]
- Eagle R.A. Traherne J.A. Hair J.R., et al. ULBP6/RAET1L is an additional human NKG2D ligand. Eur. J. Immunol. 2009;39:3207–3216. doi: 10.1002/eji.200939502. [DOI] [PubMed] [Google Scholar]
- Eshhar Z. The T-body approach: Redirecting T cells with antibody specificity. Handb. Exp. Pharmacol. 2008:329–342. doi: 10.1007/978-3-540-73259-4_14. [DOI] [PubMed] [Google Scholar]
- Fujita K. Ikarashi H. Takakuwa K., et al. Prolonged disease-free period in patients with advanced epithelial ovarian cancer after adoptive transfer of tumor-infiltrating lymphocytes. Clin. Cancer Res. 1995;1:501–507. [PubMed] [Google Scholar]
- González S. López-Soto A. Suarez-Alvarez B., et al. NKG2D ligands: Key targets of the immune response. Trends Immunol. 2008;29:397–403. doi: 10.1016/j.it.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Groh V. Wu J. Yee C. Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- Gyobu H. Tsuji T. Suzuki Y., et al. Generation and targeting of human tumor-specific Tc1 and Th1 cells transduced with a lentivirus containing a chimeric immunoglobulin T-cell receptor. Cancer Res. 2004;64:1490–1495. doi: 10.1158/0008-5472.can-03-2780. [DOI] [PubMed] [Google Scholar]
- Jemal A. Siegel R. Xu J. Ward E. Cancer statistics, 2010. CA Cancer J. Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Jensen M.C. Popplewell L. Cooper L.J., et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol. Blood Marrow Transplant. 2010;16:1245–1256. doi: 10.1016/j.bbmt.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghans R.P. Strategy escalation: An emerging paradigm for safe clinical development of T cell gene therapies. J. Transl. Med. 2010;8:55. doi: 10.1186/1479-5876-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalos M. Levine B.L. Porter D.L., et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandalaft L.E. Powell D.J., Jr. Singh N. Coukos G. Immunotherapy for ovarian cancer: What's next? J. Clin. Oncol. 2011;29:925–933. doi: 10.1200/JCO.2009.27.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw M.H. Westwood J.A. Parker L.L., et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin. Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer J.N. Wilson W.H. Janik J.E., et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer J.N. Dudley M.E. Feldman S.A., et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai P. Rabinowich H. Crowley-Nowick P.A., et al. Alterations in expression and function of signal-transducing proteins in tumor-associated T and natural killer cells in patients with ovarian carcinoma. Clin. Cancer Res. 1996;2:161–173. [PubMed] [Google Scholar]
- Lamers C.H.J. Willemsen R. van Elzakker P., et al. Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood. 2011;117:72–82. doi: 10.1182/blood-2010-07-294520. [DOI] [PubMed] [Google Scholar]
- Langenkamp U. Siegler U. Jorger S., et al. Human acute myeloid leukemia CD34+CD38− stem cells are susceptible to allorecognition and lysis by single KIR-expressing natural killer cells. Haematologica. 2009;94:1590–1594. doi: 10.3324/haematol.2009.005967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner M. Gotz G. Proff J., et al. Redirecting T cells to Ewing's sarcoma family of tumors by a chimeric NKG2D receptor expressed by lentiviral transduction or mRNA transfection. PLoS One. 2012;7:e31210. doi: 10.1371/journal.pone.0031210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisegang M. Wilde S. Spranger S., et al. MHC-restricted fratricide of human lymphocytes expressing survivin-specific transgenic T cell receptors. J. Clin. Invest. 2010;120:3869–3877. doi: 10.1172/JCI43437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo A.S. Ma Q. Liu D.L. Junghans R.P. Anti-GD3 chimeric sFv-CD28/T-cell receptor ζ designer T cells for treatment of metastatic melanoma and other neuroectodermal tumors. Clin. Cancer Res. 2010;16:2769–2780. doi: 10.1158/1078-0432.CCR-10-0043. [DOI] [PubMed] [Google Scholar]
- Lockhart D.C. Chan A.K. Mak S., et al. Loss of T-cell receptor-CD3ζ and T-cell function in tumor-infiltrating lymphocytes but not in tumor-associated lymphocytes in ovarian carcinoma. Surgery. 2001;129:749–756. doi: 10.1067/msy.2001.114554. [DOI] [PubMed] [Google Scholar]
- Loskog A. Giandomenico V. Rossig C., et al. Addition of the CD28 signaling domain to chimeric T-cell receptors enhances chimeric T-cell resistance to T regulatory cells. Leukemia. 2006;20:1819–1828. doi: 10.1038/sj.leu.2404366. [DOI] [PubMed] [Google Scholar]
- Milone M.C. Fish J.D. Carpenito C., et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol. Ther. 2009;17:1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller M. Kershaw M.H. Cameron R., et al. Sustained antigen-specific antitumor recall response mediated by gene-modified CD4+ T helper-1 and CD8+ T cells. Cancer Res. 2007;67:11428–11437. doi: 10.1158/0008-5472.CAN-07-1141. [DOI] [PubMed] [Google Scholar]
- Molinero L.L. Fuertes M.B. Rabinovich G.A., et al. Activation-induced expression of MICA on T lymphocytes involves engagement of CD3 and CD28. J. Leukoc. Biol. 2002;71:791–797. [PubMed] [Google Scholar]
- Nausch N. Cerwenka A. NKG2D ligands in tumor immunity. Oncogene. 2008;27:5944–5958. doi: 10.1038/onc.2008.272. [DOI] [PubMed] [Google Scholar]
- Parente-Pereira A.C. Burnet J. Ellison D., et al. Trafficking of CAR-engineered human T cells following regional or systemic adoptive transfer in SCID beige mice. J. Clin. Immunol. 2011;31:710–718. doi: 10.1007/s10875-011-9532-8. [DOI] [PubMed] [Google Scholar]
- Parry R.V. Rumbley C.A. Vandenberghe L.H., et al. CD28 and inducible costimulatory protein Src homology 2 binding domains show distinct regulation of phosphatidylinositol 3-kinase, Bcl-xL, and IL-2 expression in primary human CD4 T lymphocytes. J. Immunol. 2003;171:166–174. doi: 10.4049/jimmunol.171.1.166. [DOI] [PubMed] [Google Scholar]
- Porter D.L. Levine B.L. Kalos M., et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoldo B. Ramos C.A. Liu E., et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J. Clin. Invest. 2011;121:1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D.G. Ye Q. Carpenito C., et al. In vivo persistence, tumor localization, and antitumor activity of CAR-engineered T cells is enhanced by costimulatory signaling through CD137 (4-1BB) Cancer Res. 2011;71:4617–4627. doi: 10.1158/0008-5472.CAN-11-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D.G. Ye Q. Poussin M., et al. CD27 costimulation augments the survival and antitumor activity of redirected human T cells in vivo. Blood. 2012;119:696–706. doi: 10.1182/blood-2011-03-344275. [DOI] [PubMed] [Google Scholar]
- Tanic M. Yanowsky K. Rodriguez-Antona C., et al. Deregulated miRNAs in hereditary breast cancer revealed a role for miR-30c in regulating KRAS oncogene. PLoS One. 2012;7:e38847. doi: 10.1371/journal.pone.0038847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoret M.R. Cohen C.J. Nahvi A.V., et al. Relationship of p53 overexpression on cancers and recognition by anti-p53 T cell receptor-transduced T cells. Hum. Gene Ther. 2008;19:1219–1232. doi: 10.1089/hum.2008.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verneris M.R. Karami M. Baker J., et al. Role of NKG2D signaling in the cytotoxicity of activated and expanded CD8+ T cells. Blood. 2004;103:3065–3072. doi: 10.1182/blood-2003-06-2125. [DOI] [PubMed] [Google Scholar]
- Zhang C. Wang Y. Zhou Z., et al. Sodium butyrate upregulates expression of NKG2D ligand MICA/B in HeLa and HepG2 cell lines and increases their susceptibility to NK lysis. Cancer Immunol. Immunother. 2009;58:1275–1285. doi: 10.1007/s00262-008-0645-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. Conejo-Garcia J.R. Katsaros D., et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.