Abstract
The tumor-homing property of mesenchymal stem cells (MSC) has lead to their use as delivery vehicles for therapeutic genes. The application of the sodium iodide symporter (NIS) as therapy gene allows noninvasive imaging of functional transgene expression by 123I-scintigraphy or PET-imaging, as well as therapeutic application of 131I or 188Re. Based on the critical role of the chemokine RANTES (regulated on activation, normal T-cell expressed and presumably secreted)/CCL5 secreted by MSCs in the course of tumor stroma recruitment, use of the RANTES/CCL5 promoter should allow tumor stroma-targeted expression of NIS after MSC-mediated delivery. Using a human hepatocellular cancer (HCC) xenograft mouse model (Huh7), we investigated distribution and tumor recruitment of RANTES-NIS-engineered MSCs after systemic injection by gamma camera imaging. 123I-scintigraphy revealed active MSC recruitment and CCL5 promoter activation in the tumor stroma of Huh7 xenografts (6.5% ID/g 123I, biological half-life: 3.7 hr, tumor-absorbed dose: 44.3 mGy/MBq). In comparison, 7% ID/g 188Re was accumulated in tumors with a biological half-life of 4.1 hr (tumor-absorbed dose: 128.7 mGy/MBq). Administration of a therapeutic dose of 131I or 188Re (55.5 MBq) in RANTES-NIS-MSC-treated mice resulted in a significant delay in tumor growth and improved survival without significant differences between 131I and 188Re. These data demonstrate successful stromal targeting of NIS in HCC tumors by selective recruitment of NIS-expressing MSCs and by use of the RANTES/CCL5 promoter. The resulting tumor-selective radionuclide accumulation was high enough for a therapeutic effect of 131I and 188Re opening the exciting prospect of NIS-mediated radionuclide therapy of metastatic cancer using genetically engineered MSCs as gene delivery vehicles.
Knoop and colleagues demonstrate selective recruitment of mesenchymal stem cells (MSCs) expressing sodium iodide symporter (NIS) under the control of the RANTES/CCL5 promoter (RANTES-NIS-MSCs) into xenografted tumors in a human hepatocellular cancer mouse model. Administration of a therapeutic dose of either 131I or 188Re radionuclide results in significantly delayed tumor growth and improves survival in mice treated with RANTES-NIS-MSCs.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignancy and the third leading cause of cancer-related death worldwide (Parkin et al., 2005). The only available, potentially curative treatment options, such as liver transplantation, surgical resection, or radiofrequency ablation, are reserved for patients with early-stage HCC. However, more than half of the patients with HCCs are diagnosed at an intermediate or advanced tumor stage with only limited, palliative treatment options, leading to a poor prognosis for these patients (Parkin et al., 2005; Pinter et al., 2012). Growing HCC requires an active tumor stroma with extensive vasculature with high endothelial cell turnover, numerous cancer-associated fibroblasts, inflammatory cells, and increased levels of cytokines and chemokines such as TNF-α, TGF-β, IL-6, IL-10, CCL2/MCP-1, CCL3, and RANTES (regulated on activation, normal T-cell expressed and presumably secreted)/CCL5 for effective tumor growth (Niess et al., 2011; Braunersreuther et al., 2012). The tumor stroma is therefore recognized as an important therapeutic target in the treatment of HCCs.
Mesenchymal stem cells (MSC) play a key role in the maintenance and regeneration of diverse tissues. In the course of tissue injury, or during chronic inflammation, MSCs contribute to tissue remodeling by their recruitment to sites of tissue injury (Aquino et al., 2010). We, and others, have shown that MSCs are strongly recruited into the stroma of many malignant tumors. It is thought that the growing tumor is seen by the body as a “chronic wound,” and MSCs act as progenitor cells for components of the tumor stroma (Conrad et al., 2007; Zischek et al., 2009; Aquino et al., 2010; Dwyer et al., 2010; Conrad et al., 2011; Knoop et al., 2011; Niess et al., 2011). The tropism of MSCs for tumors represents the basis for the paradigm of the “Trojan horse” approach. Due to their intrinsic tumor-homing capacity, MSCs are under development as cellular vehicles for the targeted delivery of therapeutic genes into the stroma of malignant tumors (Klopp et al., 2007; Zischek et al., 2009; Knoop et al., 2011; Niess et al., 2011).
Central issues that must be addressed with this therapeutic approach include the development of restricted transgene expression to spare potential damage to nontumor tissues, enhanced noninvasive in vivo imaging techniques that could be applied to patients, and the development of more potent therapy gene strategies.
Karnoub et al. (2007) recently demonstrated recruitment of MSCs into the tumor stroma of breast cancer, followed by their induced expression of the CC-chemokine RANTES/CCL5. CCL5 is a chemoattractant of monocytes, eosinophils, and activated CD4 T cells, which signals through the G protein-coupled receptors (GPCR) CCR1, CCR3, and CCR5 (Zlotnik and Yoshie, 2000). CCL5 expression is associated with increased tumor neovascularization, as well as enhanced cancer growth and metastasis by autocrine and paracrine activation of tumor cells and through the recruitment of stromal cell types to sites of primary tumor growth (Karnoub et al., 2007).
Due to its expression in follicular cell-derived thyroid cancer cells, the sodium iodide symporter (NIS) provides the molecular basis for diagnostic and therapeutic application of radioiodine for the treatment of thyroid cancer patients. Cloning of NIS has therefore allowed the development of a new therapeutic strategy for the treatment of tumors without endogenous NIS expression based on targeted, tumor-selective NIS gene transfer followed by administration of 131I or other radionuclides that are transported by NIS, such as 188Re or 211At (Spitzweg and Morris, 2002; Willhauck et al., 2007, 2008a; Hingorani et al., 2010). 188Re is characterized by a shorter physical half-life and decay properties that are seen as superior to 131I, and thus may provide a powerful tool to enhance the therapeutic efficacy of NIS-mediated radionuclide therapy, in particular because of its enhanced crossfire effect (max path length of up to 10.4 mm) (Willhauck et al., 2007; Klutz et al., 2011). Importantly, the use of NIS as a theranostic gene offers the possibility of direct noninvasive molecular scintigraphy and PET imaging allowing dosimetric calculations as a crucial prerequisite for the exact planning of therapy studies (Spitzweg and Morris, 2002; Dingli et al., 2003; Groot-Wassink et al., 2004; Willhauck et al., 2007; Klutz et al., 2011).
In our previous studies, human MSCs were stably transfected with a sodium iodide symporter (NIS)-expressing plasmid, where NIS was driven by the broadly expressed cytomegalovirus (CMV) promoter. Using NIS as reporter gene, active MSC recruitment into the tumor stroma of HCC xenografts was demonstrated after systemic injection. In addition, repetitive MSC injections followed by 131I administration showed a significant reduction in tumor growth with an improved survival (Knoop et al., 2011). Since MSCs are known to be recruited also in nontumor tissues such as spleen or skin, the use of the unspecific CMV promoter might be disadvantageous due to transgene expression in these nontarget organs with the risk of extratumoral toxicity. Therefore, in the current study, we have made use of the RANTES/CCL5 promoter to biologically target NIS transgene expression in engineered human MSCs to the stroma of HCC xenografts. In parallel, the accumulation and therapeutic efficacy of 131I was examined in direct comparison to 188Re in the context of systemic MSC-mediated NIS gene transfer.
Materials and Methods
Cell culture
Establishment and characterization of MSCs and cultivation of the human HCC cell line Huh7 (JCRB 0403) have been described previously (Knoop et al., 2011).
Plasmid construct
The full-length NIS cDNA was removed from the pcDNA3 expression vector (kindly provided by Dr S.M. Jhiang, Ohio State University, Columbus, OH) by restriction digestion using XbaI and HindIII, agarose gel purified and ligated into the expression vector pcDNA3-CCL5Pro. CCL5Pro-NIS was removed and ligated in the pCMV/Bsd vector (Invitrogen/Life Technologies) using the restriction enzyme HindIII resulting in pCMV/Bsd-RaPro-hNIS. The sequence of the CCL5 promoter used −972 of the upstream region and the complete 5′ untranslated region (Nelson et al., 1993). The vector included a CMV-controlled Bsr2 blasticidin resistance gene to select transfected cells at a blasticidin concentration of 5 μg/ml.
Mesenchymal stem cells
Wild-type MSCs stably transfected with pCMV/Bsd-RaPro-hNIS were established as described previously (Knoop et al., 2011). The clone expressing the highest levels of NIS mRNA was subsequently referred to as RANTES-NIS-MSC and used for all further experiments. To prepare cells for injection into mice, cells were detached from culture flasks, washed three times with 1×phosphate buffered saline (PBS) and resuspended in 1×PBS at a concentration of 500,000 cells per 500 μl.
Establishment of Huh7 xenograft tumors
Huh7 xenograft tumors were established in female CD1 nu/nu mice (Charles River) as described previously (Knoop et al., 2011). The experimental protocol was approved by the regional governmental commission for animals (Regierung von Oberbayern).
MSC application and radionuclide biodistribution analysis in vivo
Experiments were started when a tumor size of 3 to 5 mm was reached and following a 10-day pretreatment with L-T4 as described previously (Knoop et al., 2011). The pretreatment schedule was based on a study by Di Cosmo et al. (2009) using a dose generally accepted as a supraphysiological LT-4 dose (10 μg L-T4/100 g body weight). The mice used in our experiments weighed between 20–25 g, and therefore a dose of 2 μg L-T4/mice per day was chosen. Wild-type (WT)-MSCs or RANTES-NIS-MSCs were injected into the tail vein at a concentration of 5×105 cells/500 μl PBS. Two groups of mice were established with the following treatments: (a) three i.v. applications of RANTES-NIS-MSC in four-day intervals (n=48) and (b) three i.v. applications of WT-MSC in four-day intervals (n=9). As an additional control, in a subset of mice injected with RANTES-NIS-MSC (n=18), the specific NIS inhibitor sodium-perchlorate (NaClO4, 2 mg per mouse) was injected i.p. 30 min prior to radionuclide administration. Seventy-two hr after the last MSC application, 18.5 MBq 123I or 111 MBq 188Re (188ReO4− perrenhate) were injected i.p., and radionuclide biodistribution was assessed using a gamma camera equipped with UXHR collimator (Ecam) as described previously (Willhauck et al., 2007, 2008a; Knoop et al., 2011).
Biodistribution analysis of radionuclides was also performed ex vivo after mice were injected with RANTES-NIS-MSCs (n=16) or WT-MSCs (n=6), as described above, followed by i.p. injection of 18.5 MBq 123I or 111 MBq 188Re, respectively. As an additional control, a subset of RANTES-NIS-MSC-injected mice (n=8) were pretreated with NaClO4. Gamma counter analysis was performed as described previously (Knoop et al., 2011).
NIS mRNA analysis by quantitative real-time PCR
Analysis of NIS mRNA expression by quantitative real-time polymerase chain reaction (qPCR) was performed as described previously (Klutz et al., 2009; Knoop et al., 2011).
Radionuclide therapy studies in vivo
Following a 10-day L-T4 pretreatment as described above, four groups of mice were established receiving 55.5 MBq 131I (sodium iodide; GE Healthcare Buchler GmbH) or 188Re (188ReO4− perrhenate; ITG GmbH) 48 hr after the final of three RANTES-NIS-MSC (RANTES-NIS-MSC+131I, n=15; RANTES-NIS-MSC+188Re, n=15) or WT-MSC (WT-MSC+131I, n=15; WT-MSC+188Re, n=15) applications in two-day-intervals (each 5×105 cells/500 μl PBS), respectively. This cycle was repeated once 24 hr after the last radionuclide application. Twenty-four hr later, one additional MSC (5×105 cells) injection was applied, followed by another radionuclide (55.5 MBq 131I or 188Re, respectively) injection 48 hr later. As a control group, mice were injected with RANTES-NIS-MSC (n=15) followed by application of saline. Another control group received saline only (n=15). The follow-up of mice, including tumor measurements, were performed as outlined previously (Knoop et al., 2011).
Indirect immunofluorescence assay
Immunofluorescence staining of frozen sections was performed as described previously (Knoop et al., 2011) using the following primary antibodies: hNIS (mouse monoclonal, provided by J.C. Morris, Division of Endocrinology, Mayo Clinic and Medical School, Rochester, MN); mouse RANTES/CCL5 (goat polyclonal, AF478, R&D Systems), SV40 large T-antigen (mouse monoclonal, Calbiochem/Merck), CD31 (rat monoclonal, Pharmingen/BD), or Ki67 (rabbit polyclonal, Abcam). Staining and evaluation of proliferation and vessel density were preformed as described previously (Knoop et al., 2011).
Statistical methods
Statistical significance of in vitro experiments was tested using Student's t test. Statistical significance of in vivo experiments has been calculated using Man-Whitney U test.
Results
Radionuclide biodistribution studies after in vivo NIS gene transfer
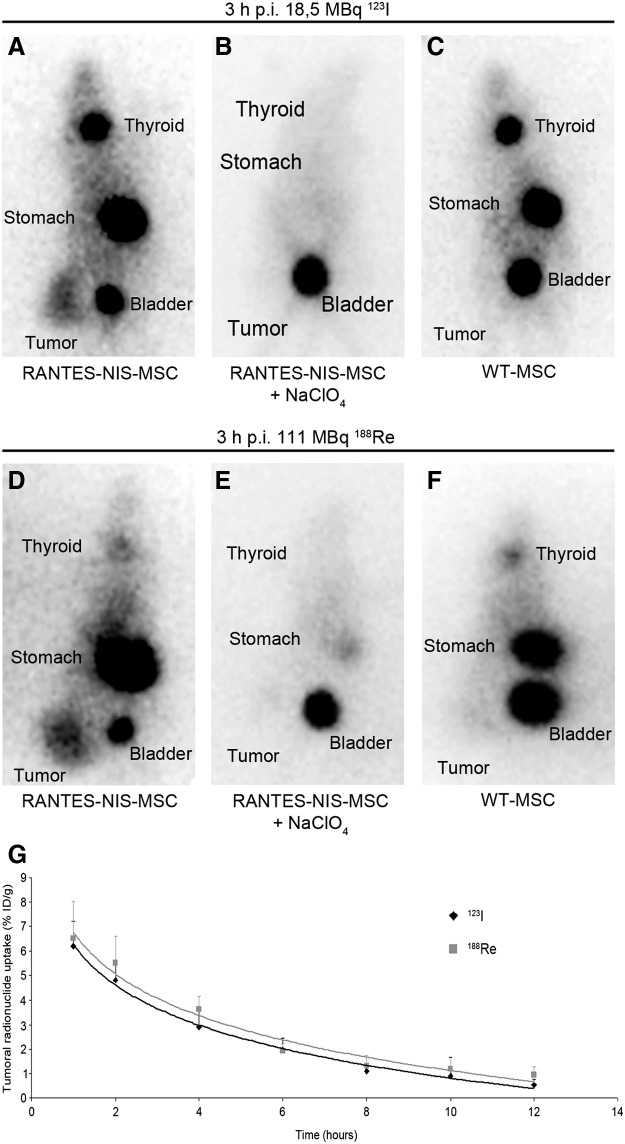
Significant iodide accumulation was observed in 67% of Huh7 tumors following application of RANTES-NIS-MSCs (Fig. 1A). In contrast, after application of WT-MSCs no tumoral iodide accumulation was measured (Fig. 1C). Serial imaging revealed a tumoral uptake of approximately 6.5% ID/g 123I after application of RANTES-NIS-MSCs, with a biological half-life of 3.7 hr (Fig. 1G). Considering a tumor mass of 1 g, and an effective half-life of 3.5 hr for 131I, a tumor-absorbed dose of 44.3±8.6 mGy/MBq 131I was calculated. In comparison, approximately 7% ID/g 188Re was concentrated in 67% of Huh7 tumors, with a biological half life of 4.1 hr (Fig. 1D and G). With an effective half life of 3.5 hr for 188Re, a tumor-absorbed dose of 128.7±28.2 mGy/MBq 188Re was determined. Physiologic accumulation of radionuclides was also observed in thyroid gland and stomach, due to endogenous NIS expression, as well as in the bladder due to renal excretion of radionuclides (Fig. 1A, C, D, F). In a subset of mice injected with RANTES-NIS-MSCs, pretreatment with perchlorate (NaClO4; 2 mg), 30 min prior to injection of the respective radionuclide, completely abolished radionuclide uptake in the tumor as well as in the thyroid gland and stomach, confirming that the observed radionuclide accumulation is indeed NIS-mediated (Fig. 1B and E). As expected, physiologic radionuclide accumulation in nontarget organs (thyroid gland, stomach, and bladder) was also seen after WT-MSC injection (Fig. 1C and F). As outlined above, in 1/3 of the animals no specific radionuclide accumulation after RANTES-NIS-MSCs injection was observed, however, by immunohistochemistry it was shown that MSCs were recruited into the tumor stroma to a lower extent (Supplementary Fig. 1; Supplementary Data available online at www.liebertonline.com/hum), resulting in a radionuclide accumulation that was obviously below the detection limit.
FIG. 1.
Radionuclide biodistribution studies in vivo. Gamma-camera imaging of mice harboring Huh7 tumors after mesenchymal stem cell (MSC)-mediated sodium iodide symporter (NIS) gene delivery 3 hr following 123I or 188Re administration. After three intravenous i.v. applications of RANTES-NIS-MSCs significant tumor-specific iodide (A) and rhenium (D) accumulation was induced, which was completely abolished upon pretreatment with NaClO4 (B) and (E). In contrast, mice injected with wild-type (WT)-MSCs showed no tumoral iodide (C) or rhenium (F) uptake. Radionuclides were also accumulated physiologically in thyroid, stomach, and bladder. (G) Time course of 123I and 188Re accumulation in Huh7 tumors after three i.v. RANTES-NIS-MSC applications followed by injection of 18.5 MBq 123I or 111 MBq 188Re, respectively, as determined by serial scanning. Maximum tumoral radioiodine uptake was 6.5% ID/g tumor and 7% ID/g tumor for 188Re, respectively. RANTES, regulated on activation, normal T-cell expressed and presumably secreted.
Analysis of NIS protein expression by indirect immunofluorescence
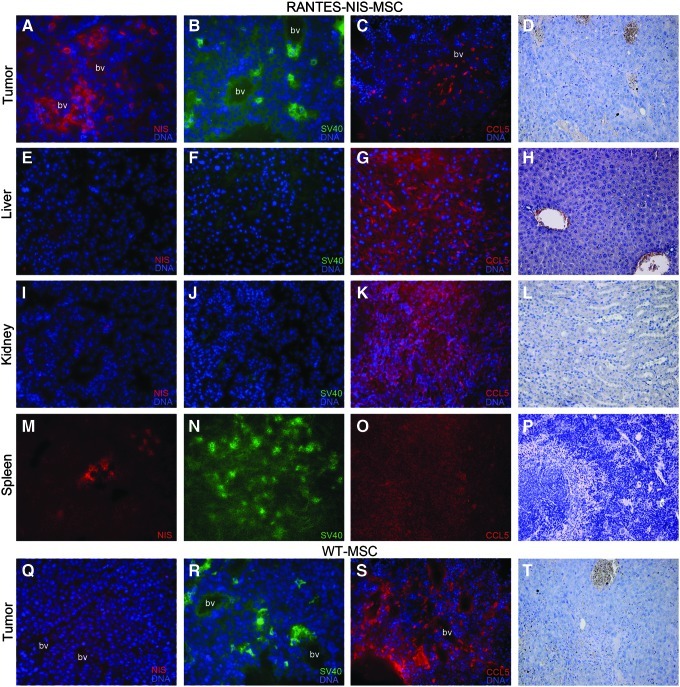
The distribution of MSCs in tumors and nontarget organs such as liver, kidney, and spleen was determined in more detail by immunofluorescence staining using NIS-, SV40 large T Ag- and RANTES/CCL5-specific antibodies. Hematoxylin and eosin (H&E stainings) of all organs or tumors are provided (Fig. 2D, H, L, P, T). Immortalization of MSCs via SV40 large T Ag provides a useful marker for the ex vivo detection of adoptively transferred MSCs. NIS-specific immunoreactivity based on RANTES/CCL5 promoter activity was detected throughout the tumor stroma, predominantly in the vicinity of blood vessels, which paralleled the localization of SV40 large T Ag and the distribution of RANTES/CCL5 expression (Fig. 2A–C). Liver and kidneys showed no detectable NIS, SV40 large T Ag, or RANTES/CCL5 immunoreactivity (Fig. 2E–G, I–K). In contrast, a high density of SV40 large T Ag–expressing cells was detected in the spleen after RANTES-NIS-MSC application (Fig. 2N), while no NIS- and RANTES/CCL5-specific immunofluorescence staining was observed (Fig. 2M and O), demonstrating restricted transgene expression. Injection of WT-MSCs resulted in SV40 large T Ag– and RANTES/CCL5-specific immunofluorescence staining in tumors (Fig. 2R and S), demonstrating active tumoral recruitment of MSCs after i.v. application. In contrast, no NIS-specific immunostaining was observed after WT-MSC gene transfer (Fig. 2Q).
FIG. 2.
Immunohistochemical staining of Huh7 tumors and nontarget organs after application of RANTES-NIS-MSCs or WT-MSCs. After application of RANTES-NIS-MSCs, Huh7 tumors revealed NIS-specific immunoreactivity throughout the tumor stroma (A) with a similar distribution of SV40 large T Ag- (B) and RANTES/CCL5-positive cells (C). Other organs, like liver or kidney, showed no detectable NIS, SV40 large T Ag, or RANTES/CCL5 protein expression (E–G; I–K). In contrast, strong accumulation of SV40 large T Ag-expressing cells was detected in the spleen of mice that were injected with RANTES-NIS-MSCs (N), while no NIS- (M) or RANTES/CCL5-specific immunoreactivity (O) was detected. After application of WT-MSCs, no NIS-specific immunoreactivity was detected in Huh7 tumors (Q), while strong cytoplasmatic SV40 large T Ag (R) and RANTES/CCL5 (S) staining was detected. Slides were counterstained with Hoechst nuclear stain. Hematoxylin and eosin (H&E) stainings of Huh7 tumors (D), liver (H), kidney (L), and spleen (P) after injection of RANTES-NIS-MSC and tumors of mice injected with WT-MSCs (T) are provided. Magnification: NIS, SV40 large T AG and H&E staining:×200; RANTES/CCL5 staining:×100. Bv, blood vessel.
Ex vivo radionuclide biodistribution studies
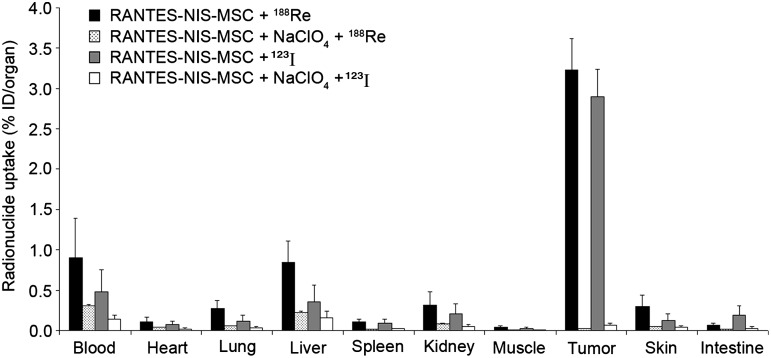
Significant levels of tumoral iodide and rhenium uptake were confirmed after application of RANTES-NIS-MSCs by ex vivo biodistribution studies revealing a radionuclide uptake of 3 – 3.5% ID/organ 123I or 188Re four hours after radionuclide injection. In nontarget organs (lung, liver, spleen, kidney) no specific radionuclide accumulation was detected. The competitive NIS inhibitor perchlorate significantly blocked radionuclide uptake in tumors of mice injected with RANTES-NIS-MSCs (Fig. 3). Significant radionuclide accumulation was also detected in tissues that physiologically express NIS (stomach, thyroid gland) and in the bladder due to renal elimination of radionuclides (Spitzweg et al., 2001, 2002). The thyroid gland and the stomach accumulated approximately 40% and 39% ID/organ, respectively, for 123I and 15% ID/organ and 40% ID/organ, respectively, for 188Re (data not shown). The effective half-life in the thyroid gland was approx. 38 hr for 131I and only 6.5 hr for 188Re. In this regard, it is important to outline that NIS expression is exclusively regulated by thyroid stimulating hormone (TSH) in the thyroid gland, which allows effective downregulation of radionuclide accumulation in the thyroid gland by thyroid hormone pretreatment as shown by Wapnir et al. (2004). Moreover, during prolonged anaesthesia for imaging purposes, gastric juices are pooled in the stomach, which results in significantly higher gastric radionuclide accumulation than routinely observed in humans. In the bladder, radionuclide accumulation and retention time can be minimized by stimulation of diuresis, thereby lowering the delivered dose and side effects to bladder and adjacent tissues.
FIG. 3.
Evaluation of radionuclide biodistribution ex vivo 4 hr following injection of 18.5 MBq 123I or 111 MBq 188Re. Tumors in RANTES-NIS-MSC-injected mice showed high perchlorate-sensitive radionuclide uptake activity (∼3–3.5% ID/organ), while no significant radionuclide accumulation was measured in nontarget organs. Results are reported as percent of injected dose per organ±SD.
NIS mRNA analysis by qPCR
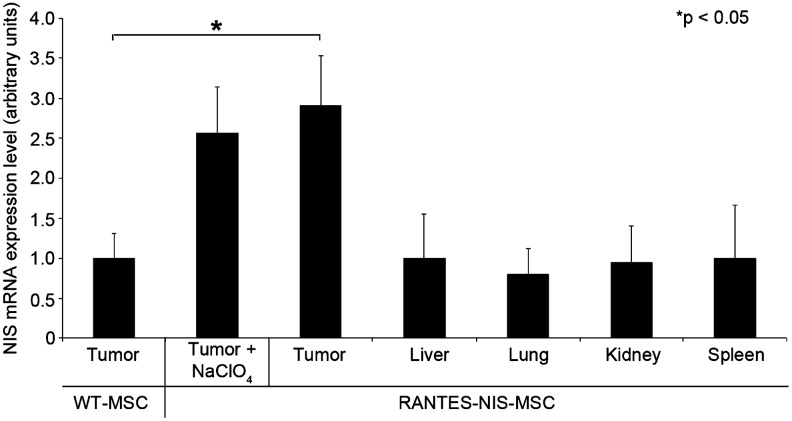
mRNA was isolated from tumors and nontarget organs (liver, lung, kidney, and spleen) after systemic MSC injection and analyzed by qPCR using NIS-specific oligonucleotide primers. Systemic injection of RANTES-NIS-MSCs in tumor-bearing mice resulted in significantly increased levels of NIS mRNA in tumors, whereas no significant NIS mRNA expression was detected in nontarget organs (Fig. 4).
FIG. 4.
Analysis of NIS mRNA expression in Huh7 tumors and nontarget organs by quantitative real-time polymerase chain reaction (qPCR). While only a low background level of NIS mRNA expression was detected in tumors injected with WT-MSCs, significant levels of NIS mRNA expression were induced in Huh7 tumors after three applications of RANTES-NIS-MSCs with or without NaClO4 pretreatment. In nontarget organs like liver, lung, kidney, or spleen, no NIS mRNA expression was detected.
Radionuclide therapy studies in vivo after MSC-mediated systemic NIS gene transfer
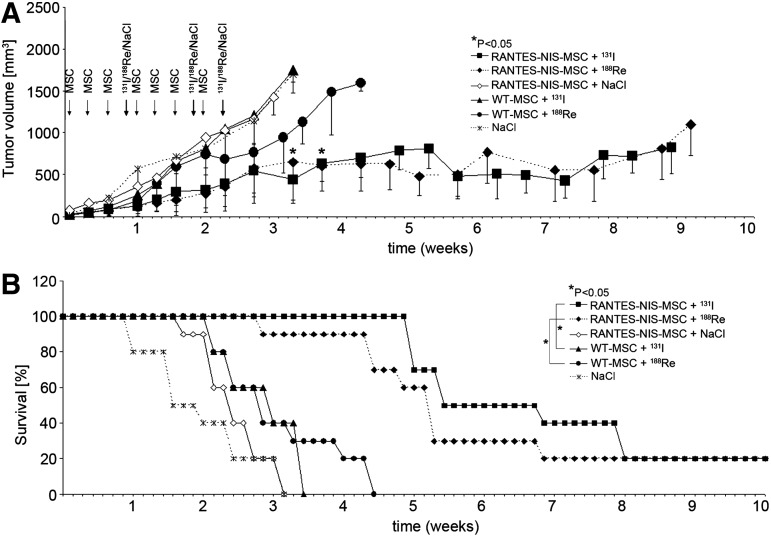
The effect of therapeutic radionuclides (131I and 188Re) was then compared and contrasted in the context of RANTES-NIS-MSC treatment. Control mice treated with RANTES-NIS-MSCs followed by saline application, mice treated with WT-MSCs followed by application of 131I or 188Re, or mice treated with saline only showed an exponential tumor growth and had to be killed after 3–4 weeks (Fig. 5A). In contrast, RANTES-NIS-MSC and 131I- or 188Re-treated tumors showed a dramatic control of growth (day 24: p=0.02 [NaCl] and p=0.05 [WT-MSC]) that extended through the end of the experiment (nine weeks) (Fig. 5A) and that resulted in an improved survival (Fig. 5B). The application of 188Re did not result in an obvious increase in therapeutic efficacy as compared with 131I, but enhanced therapeutic effects would be difficult due to the dramatic effects of 131I treatment in this experimental setting. No major adverse effects of radionuclide or MSC treatment were observed in terms of lethargy or respiratory failure.
FIG. 5.
Radionuclide therapy studies in vivo after MSC-mediated systemic NIS gene transfer. Four groups of mice were established receiving 55.5 MBq 131I or 188Re 48 hr after the final of three RANTES-NIS-MSC or WT-MSC applications in two-day-intervals. This cycle was repeated once 24 hr after the last radionuclide application. Twenty-four hours after these two treatment cycles, one additional MSC injection was administered followed by a third 131I or 188Re (55.5 MBq) injection 48 hr later. 131I and 188Re therapy after RANTES-NIS-MSC application resulted in a significant delay in tumor growth as compared with the control groups, which were injected with WT-MSCs followed by 131I or 188Re or with RANTES-NIS-MSCs followed by saline or with saline only (p<0.05) (A). The significantly reduced tumor growth was associated with markedly improved survival (Kaplan-Meier-Plot, p<0.05) (B).
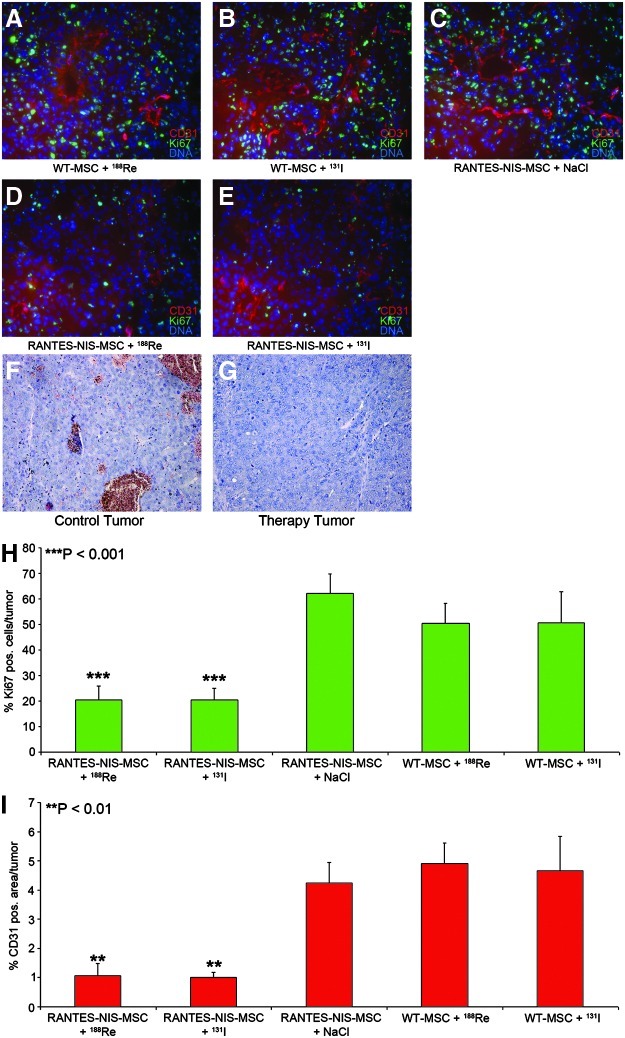
At the end of the observation period, mice were sacrificed and tumors dissected, followed by H&E staining (Fig. 6F and G) and immunofluorescence staining using a Ki67-specific antibody (green, labeling proliferating cells) and a CD31-specific antibody (red, labeling blood vessels). Striking differences were observed between mice treated with WT-MSC (Fig. 6A and B) or mice treated with saline (Fig. 6C) as compared to mice treated with RANTES-NIS-MSC followed by application of 188Re or 131I (Fig. 6D and E). Tumors of mice injected with RANTES-NIS-MSC followed by NaCl showed a Ki67-index of approximately 62±8% and a mean vessel density of 4.25±0.7% (Fig. 6H and I), whereas tumors treated with RANTES-NIS-MSC and 188Re or 131I showed significantly reduced levels of intratumoral proliferation index (20.3±5%) and blood vessel density (1±0.2%) (Fig. 6H and I).
FIG. 6.
Immunofluorescence analyses of radionuclide therapy studies. Immunofluorescence analysis of tumors using a Ki67-specific antibody (green, labeling proliferating cells) and an antibody against CD31 (red, labeling blood vessels). As compared to control tumors (A–C), RANTES-NIS-MSC and 188Re- or 131I-treated tumors showed visible differences in tumor cell proliferation and blood vessel density (D, E). Quantification of blood vessel density and tumor cell proliferation showed significantly reduced tumor cell proliferation (20.3±5%) (H) and blood vessel density (1±0.2%) (I) after systemic RANTES-NIS-MSC application in 188Re- or 131I-treated tumors as compared to control tumors (Ki67: 62±8%, p<0.001; CD31: 4.25±0.7%, p<0.01) (H, I). Slides were counterstained with Hoechst nuclear stain. H&E stainings of control and treated tumors (F, G). Magnification ×200.
Discussion
Several research groups including our own have demonstrated that mesenchymal stem cells (MSCs) are actively recruited into growing tumor stroma where they play a major role in forming the tumor's fibrovascular network (Karnoub et al., 2007; Zischek et al., 2009; Knoop et al., 2011; Niess et al., 2011). MSC recruitment to tumor stroma is thought to be driven by high local concentrations of inflammatory chemokines and growth factors such as MCP-1/CCL2, IL-8/CXCL8, RANTES/CCL5, and SDF-1α/CXCL12 among others (Karnoub et al., 2007; Spaeth et al., 2008). Within the tumor stroma, MSCs can differentiate into carcinoma-associated fibroblasts or pericyte-like cells where they contribute to tumor growth through secretion of inflammatory and pro-angiogenic growth factors like VEGF, PDGF, SDF-1α/CXCL12, EGF, IGF, IL-6, and RANTES/CCL5 (Spaeth et al., 2009). This tropism of MSCs for tumor environments makes them uniquely suited as tumor stroma–selective gene delivery vehicles. In models of pancreatic, breast, and liver cancer, we have applied MSCs transduced with herpes simplex virus type 1 thymidine kinase (HSV-Tk) and demonstrated active tumor stroma–selective recruitment of MSCs that significantly reduced tumor growth and metastasizing potential after treatment with ganciclovir (Zischek et al., 2009; Niess et al., 2011).
MSCs have been used to deliver a diverse array of agents, including interferon-β, cytosine deaminase, tumor necrosis factor–related apoptosis-inducing ligand, the immunostimulatory chemokine CX3CL1, and oncolytic viruses. These approaches have generally yielded positive antitumor effects (Spaeth et al., 2008; Braunersreuther et al., 2012). While effective, we sought to evaluate a potentially more flexible and potent therapy gene approach using the sodium iodide symporter (NIS). In a previous study using a liver cancer mouse model, MSCs were transfected with NIS under the control of the broadly unspecific CMV promoter. The engineered cells were actively recruited to the tumor and induced a significant antitumor effect after application of 131I (Knoop et al., 2011). A similar approach was subsequently confirmed in a breast cancer model by Dwyer et al. (2011).
With its flexibility regarding diagnostics, imaging, and potent therapeutic actions, the NIS gene represents an important new dimension in MSC-mediated tumor therapy. Because of the potential side effects of MSC recruitment to nontumor tissues, we examined the potential linkage of restricted tissue expression delivered through the use of the RANTES/CCL5 promoter to NIS-mediated MSC therapy in a model of HCC.
Upregulation of RANTES/CCL5 by MSCs in tumor stroma is associated with their differentiation into cancer-associated fibroblasts. In a murine model of breast cancer, MSCs were shown to increase the number of lung metastases. These effects were mediated in part by the RANTES/CCL5 produced by the MSCs in the presence of breast cancer cells that acted in a paracrine fashion on cancer cells to enhance their motility, invasion, and metastasis (Karnoub et al., 2007). Pinilla et al. (2009) demonstrated that MSCs derived from human adipose tissue (hASCs) produce RANTES/CCL5 in coculture with breast cancer cells or in breast cancer cell conditioned medium, thereby increasing invasion of cancer cells, and conclude that RANTES/CCL5 plays a crucial role for tumor invasion in the interplay of tissue resident stem cells from fat tissue and breast cancer cells.
In our study, human MSCs were engineered to express NIS under control of the RANTES/CCL5 promoter to more specifically target NIS expression to the tumor stroma and reduce potential side effects linked to MSC recruitment to nontumor tissues. Initial demonstration of tumor stroma–selective RANTES/CCL5 promoter-driven expression of reporter or therapy genes in engineered MSCs was provided by our own work (Zischek et al., 2009), where murine MSCs stably transfected with either reporter genes (red fluorescent protein [RFP], enhanced green fluorescent protein [eGFP]), or a therapy gene (HSV-Tk) driven by the RANTES/CCL5 promoter was used to evaluate the dynamics of expression in mice carrying orthotopic, syngeneic pancreatic tumors (Zischek et al., 2009). Application of ganciclovir in these animals resulted in a significant reduction in primary tumor growth, as well as reduced incidence of metastases (Zischek et al., 2009). The CCL5-based transgenic approach was later directly compared to a Tie2-tumor angiogenesis–targeting approach in an orthotopic HCC xenograft model (Niess et al., 2011). While both methods showed positive results, the results suggested a better outcome when the RANTES/CCL5 promoter was used to drive therapeutic transgenes in engineered MSC (Zischek et al., 2009; Niess et al., 2011).
In contrast to our experiments, most of the previously published studies analyzed MSC biodistribution and tumor-specific recruitment by ex vivo analysis of reporter gene expression. However, using NIS as a reporter gene allows efficient noninvasive imaging of transgene expression by 99mTc-scintigraphy, 123I-scintigraphy or SPECT, and 124I-PET imaging, as demonstrated in our study where tumor homing and engraftment of MSCs was noninvasively demonstrated by routine 123I- or 188Re-scintigraphy. This would allow an important new dimension in future patient studies, as exact planning of clinical gene therapy trials requires a thorough understanding of MSC biodistribution as well as level, duration, and distribution of transgene expression. Feasibility and efficacy of the NIS gene therapy approach using NIS as a theranostic gene has been shown in several former studies by different research groups including our own (Dwyer et al., 2005a, 2005b; Herve et al., 2008; Kakinuma et al., 2003; Klutz et al., 2009; Li et al., 2010; Peerlinck et al., 2009; Scholz et al., 2005; Spitzweg et al., 1999, 2000, 2001, 2007; Willhauck et al., 2007, 2008a, 2008b, 2008c).
Following systemic application of RANTES-NIS-MSCs, 67% of implanted HCC tumors showed a tumor-specific 123I and 188Re accumulation as shown by gamma camera imaging with a radionuclide accumulation of approximately 6–7% ID/g and a biological half-life of 3.7 hr, which is comparable to the data we had obtained in our previous study using the CMV promoter to drive NIS expression in MSCs (7–9% ID/g, biological half-life 4 hr) (Knoop et al., 2011). Perchlorate injection prior to radionuclide application and the use of control MSCs confirmed NIS-specific radionuclide accumulation. The in vivo imaging data were confirmed by ex vivo biodistribution and immunofluorescence analysis, which demonstrated tumor stroma–specific accumulation of MSCs in addition to RANTES/CCL5 promoter activation as shown by immunohistochemical staining. In contrast, nontarget organs like liver or kidney showed no NIS, SV40 large T-Ag, or RANTES/CCL5 expression. Immunofluorescence analysis did reveal an accumulation of MSCs in the spleen, however, these cells did not show NIS-specific immunoreactivity. The presence of MSCs in the spleen may result either from direct recruitment of the cells (via CCR7) (Von Luttichau et al., 2005) or from filtration of the exogenously applied MSCs from the peripheral circulation. Importantly, the lack of RANTES/CCL5 promoter-driven transgene expression demonstrates enhanced selectivity of the approach.
After three cycles of repetitive RANTES-NIS-MSC injection followed by the application of 131I or 188Re, an effective control of tumor growth was seen. This was associated with a dramatically improved survival of tumor-bearing animals to the end of the nine-week experiment. By contrast, control animals showed a maximum survival of 4 weeks. These results show a significant improvement over those we previously reported using the CMV promoter to drive NIS expression in the same model system where the mice lived 7 weeks after NIS-mediated radioiodine therapy (Knoop et al., 2011).
In the current study, we also examined the potential use of 188Re as an alternative therapeutic radionuclide to 131I. 188Re is also transported by NIS, but in contrast to 131I, offers the possibility of higher energy deposition in the tumor in a shorter period of time due to its shorter physical half-life and higher energy. Another advantage is that 188Re is less harmful to the thyroid, which is primarily caused by the lack of 188Re organification and therefore significantly shorter effective half-life of 188Re in the thyroid gland, thereby not only reducing radiation damage to the thyroid gland but also increasing tumoral 188Re uptake by the elimination of the thyroid “sink” effect (Dadachova et al., 2002). In consideration of the scattered MSC biodistribution in the tumor stroma, an enhanced therapeutic effect of 188Re based on an increase in crossfire effect, due to the longer path length, was expected. In a breast cancer mouse model, Dadachova and colleagues showed a radiation dose 4.5 times higher for 188Re than for 131I, resulting in an improved therapeutic efficacy in mice (Dadachova et al., 2005). Similarly, in one of our previous studies we have convincingly demonstrated the superior therapeutic effect of 188Re as compared to 131I in a prostate cancer xenograft mouse model based on a 4.7-fold higher tumor-absorbed dose after application of 188Re as compared to 131I (Willhauck et al., 2007). In the current study, however, therapeutic efficacy of 188Re compared to 131I was similar in direct comparison, although, the tumor-absorbed dose for 188Re was calculated to be three times higher than for 131I (128.7 mGy/MBq vs. 44.3 mGy/MBq). Based on the survival curve obtained for both radionuclides, it is possible that a maximal therapeutic effect was already achieved with 131I treatment. A more detailed titration of MSC and radionuclide application in future studies may allow a more precise contrasting of the therapeutic consequences of 188Re vs. 131I.
In addition, since dosimetric calculations are optimized for a homogenous tumoral radionuclide uptake and standard dosimetry models, not taking into account microdosimetry aspects, calculation of tumor-absorbed doses after MSC-mediated NIS gene transfer might be inaccurate due to inhomogeneous NIS expression and therefore inhomogenous radionuclide accumulation in the tumor stroma.
The mechanism underlying RANTES/CCL5 induction by MSC in tumor milieus is not well understood. Osteopontin (OPN), a secreted phosphoprotein that signals through αvβ3 integrin and CD44 (Denhardt et al., 1995; McAllister et al., 2008; Mi et al., 2011) has been shown to induce expression of RANTES/CCL5 in MSCs (Mi et al., 2011). EGFR and insulin-like growth factor 1 (IGF-1) signaling have also been linked to RANTES/CCL5 upregulation by MSCs (Mascia et al., 2003; Karar and Maity, 2009). A better understanding of this biology may allow the reengineering of the RANTES/CCL5 promoter to optimize tumor specificity and reduce potential expression in other tissues (Grone et al., 1999; Edelmann et al., 2011).
Taken together, our data demonstrate high tumor selectivity of MSC recruitment and improved NIS expression driven by the RANTES/CCL5 promoter after systemic MSC application in an HCC xenograft model. The resulting biologically targeted, tumor-selective radionuclide accumulation was high enough for a therapeutic effect of 131I and 188Re opening the exciting prospect of NIS-mediated radionuclide therapy of metastatic cancer using engineered MSCs as gene delivery vehicles.
Supplementary Material
Acknowledgments
We are grateful to J.C. Morris, Division of Endocrinology, Mayo Clinic and Medical School, Rochester, Minnesota, for providing the NIS-specific antibody, as well as to S.M. Jhiang, Ohio State University, Columbus, Ohio, for supplying the full-length human NIS cDNA. We also thank W. Münzing, Julia Schlichtiger, and Heidrun Zankl, Department of Nuclear Medicine, Ludwig-Maximilians-University, Munich, Germany, for their assistance with the imaging and therapy studies. This study was supported by grant SFB 824 (Sonderforschungsbereich 824) from the Deutsche Forschungsgemeinschaft, Bonn, Germany, to C. Spitzweg and by a grant from the Wilhelm-Sander-Stiftung (2008.037.1) to C. Spitzweg and P.J. Nelson.
Author Disclosure Statement
No competing financial interests exist.
References
- Aquino J.B. Bolontrade M.F. Garcia M.G., et al. Mesenchymal stem cells as therapeutic tools and gene carriers in liver fibrosis and hepatocellular carcinoma. Gene Ther. 2010;17:692–708. doi: 10.1038/gt.2010.10. [DOI] [PubMed] [Google Scholar]
- Braunersreuther V. Viviani G.L. Mach F. Montecucco F. Role of cytokines and chemokines in non-alcoholic fatty liver disease. World J Gastroenterol. 2012;18:727–735. doi: 10.3748/wjg.v18.i8.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad C. Gupta R. Mohan H., et al. Genetically engineered stem cells for therapeutic gene delivery. Curr Gene Ther. 2007;7:249–260. doi: 10.2174/156652307781369119. [DOI] [PubMed] [Google Scholar]
- Conrad C. Huesemann Y. Niess H., et al. Linking Transgene Expression of Engineerd Mesenchymal Stem Cells and Angiopoietin-1-induced Differentiation to Target Cancer Angiogenesis. Ann Surg. 2011;253:566–571. doi: 10.1097/SLA.0b013e3181fcb5d8. [DOI] [PubMed] [Google Scholar]
- Dadachova E. Bouzahzah B. Zuckier L.S. Pestell R.G. Rhenium-188 as an alternative to iodine-131 for treatment of breast tumors expressing the sodium/iodide symporter (NIS) Nucl Med Biol. 2002;29:13–18. doi: 10.1016/s0969-8051(01)00279-7. [DOI] [PubMed] [Google Scholar]
- Dadachova E. Nguyen A. Lin E.Y., et al. Treatment with rhenium-188-perrhenate and iodine-131 of NIS-expressing mammary cancer in a mouse model remarkably inhibited tumor growth. Nucl Med Biol. 2005;32:695–700. doi: 10.1016/j.nucmedbio.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Denhardt D.T. Lopez C.A. Rollo E.E., et al. Osteopontin-induced modifications of cellular functions. Ann N Y Acad Sci. 1995;760:127–142. doi: 10.1111/j.1749-6632.1995.tb44625.x. [DOI] [PubMed] [Google Scholar]
- Di Cosmo C. Liao X.H. Dumitrescu A.M., et al. A thyroid hormone analog with reduced dependence on the monocarboxylate transporter 8 for tissue transport. Endocrinology. 2009;150:4450–4458. doi: 10.1210/en.2009-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingli D. Russell S.J. Morris J.C. In vivo imaging and tumor therapy with the sodium iodide symporter. J Cell Biochem. 2003;90:1079–1086. doi: 10.1002/jcb.10714. [DOI] [PubMed] [Google Scholar]
- Dwyer R.M. Bergert E.R. O'Connor M, K., et al. In vivo radioiodide imaging and treatment of breast cancer xenografts after MUC1-driven expression of the sodium iodide symporter. Clin Cancer Res. 2005a;11:1483–1489. doi: 10.1158/1078-0432.CCR-04-1636. [DOI] [PubMed] [Google Scholar]
- Dwyer R.M. Schatz S.M. Bergert E.R., et al. A preclinical large animal model of adenovirus-mediated expression of the sodium-iodide symporter for radioiodide imaging and therapy of locally recurrent prostate cancer. Mol Ther. 2005b;12:835–841. doi: 10.1016/j.ymthe.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Dwyer R.M. Khan S. Barry F.P., et al. Advances in mesenchymal stem cell-mediated gene therapy for cancer. Stem Cell Res Ther. 2010;1:25. doi: 10.1186/scrt25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer R.M. Ryan J. Havelin R.J., et al. Mesenchymal Stem Cell-mediated delivery of the sodium iodide symporter supports radionuclide imaging and treatment of breast cancer. Stem Cells. 2011;29:1149–1157. doi: 10.1002/stem.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann S.L. Nelson P.J. Brocker T. Comparative promoter analysis in vivo: identification of a dendritic cell-specific promoter module. Blood. 2011;118:e40–e49. doi: 10.1182/blood-2011-03-342261. [DOI] [PubMed] [Google Scholar]
- Grone H.J. Weber C. Weber K.S., et al. Met-RANTES reduces vascular and tubular damage during acute renal transplant rejection: blocking monocyte arrest and recruitment. FASEB J. 1999;13:1371–1383. [PubMed] [Google Scholar]
- Groot-Wassink T. Aboagye E.O. Wang Y., et al. Quantitative imaging of Na/I symporter transgene expression using positron emission tomography in the living animal. Mol. Therapy. 2004;9:436–442. doi: 10.1016/j.ymthe.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Herve J. Cunha A.S. Liu B., et al. Internal radiotherapy of liver cancer with rat hepatocarcinoma-intestine-pancreas gene as a liver tumor-specific promoter. Hum Gene Ther. 2008;19:915–926. doi: 10.1089/hum.2007.153. [DOI] [PubMed] [Google Scholar]
- Hingorani M. Spitzweg C. Vassaux G., et al. The biology of the sodium iodide symporter and its potential for targeted gene delivery. Curr. Cancer Drug Targets. 2010;10:242–267. doi: 10.2174/156800910791054194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakinuma H. Bergert E.R. Spitzweg C., et al. Probasin promoter (ARR(2)PB)-driven, prostate-specific expression of the human sodium iodide symporter (h-NIS) for targeted radioiodine therapy of prostate cancer. Cancer Res. 2003;63:7840–7844. [PubMed] [Google Scholar]
- Karar J. Maity A. Modulating the tumor microenvironment to increase radiation responsiveness. Cancer Biol Ther. 2009;8:1994–2001. doi: 10.4161/cbt.8.21.9988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnoub A.E. Dash A.B. Vo A.P., et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- Klopp A.H. Spaeth E.L. Dembinski J.L., et al. Tumor irradiation increases the recruitment of circulating mesenchymal stem cells into the tumor microenvironment. Cancer Res. 2007;67:11687–11695. doi: 10.1158/0008-5472.CAN-07-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klutz K. Russ V. Willhauck M.J., et al. Targeted radioiodine therapy of neuroblastoma tumors following systemic nonviral delivery of the sodium iodide symporter gene. Clin Cancer Res. 2009;15:6079–6086. doi: 10.1158/1078-0432.CCR-09-0851. [DOI] [PubMed] [Google Scholar]
- Klutz K. Willhauck M.J. Wunderlich N., et al. Sodium iodide symporter (NIS)-mediated radionuclide ((131)I, (188)Re) therapy of liver cancer after transcriptionally targeted intratumoral in vivo NIS gene delivery. Hum Gene Ther. 2011;22:1403–1412. doi: 10.1089/hum.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoop K. Kolokythas M. Klutz K., et al. Image-guided, tumor stroma-targeted 131I therapy of hepatocellular cancer after systemic mesenchymal stem cell-mediated NIS gene delivery. Mol Ther. 2011;19:1704–1713. doi: 10.1038/mt.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. Peng K.W. Dingli D., et al. Oncolytic measles viruses encoding interferon beta and the thyroidal sodium iodide symporter gene for mesothelioma virotherapy. Cancer Gene Ther. 2010;17:550–558. doi: 10.1038/cgt.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascia F. Mariani V. Girolomoni G. Pastore S. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am. J. Pathol. 2003;163:303–312. doi: 10.1016/S0002-9440(10)63654-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister S.S. Gifford A.M. Greiner A.L., et al. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell. 2008;133:994–1005. doi: 10.1016/j.cell.2008.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi Z. Bhattacharya S.D. Kim V.M., et al. Osteopontin promotes CCL5-mesenchymal stromal cell-mediated breast cancer metastasis. Carcinogenesis. 2011;32:477–487. doi: 10.1093/carcin/bgr009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P.J. Kim H.T. Manning W.C., et al. Genomic organization and transcriptional regulation of the RANTES chemokine gene. J Immunol. 1993;151:2601–2612. [PubMed] [Google Scholar]
- Niess H. Bao Q. Conrad C., et al. Selective targeting of genetically engineered mesenchymal stem cells to tumor stroma microenvironments using tissue-specific suicide gene expression suppresses growth of hepatocellular carcinoma. Ann Surg. 2011;254:767–774. doi: 10.1097/SLA.0b013e3182368c4f. [DOI] [PubMed] [Google Scholar]
- Parkin D.M. Bray F. Ferlay J. Pisani P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Peerlinck I. Merron A. Baril P., et al. Targeted radionuclide therapy using a Wnt-targeted replicating adenovirus encoding the Na/I symporter. Clin Cancer Res. 2009;15:6595–6601. doi: 10.1158/1078-0432.CCR-09-0262. [DOI] [PubMed] [Google Scholar]
- Pinilla S. Alt E. Abdul Khalek F.J., et al. Tissue resident stem cells produce CCL5 under the influence of cancer cells and thereby promote breast cancer cell invasion. Cancer Lett. 2009;284:80–85. doi: 10.1016/j.canlet.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Pinter M. Hucke F. Graziadei I., et al. Advanced-Stage Hepatocellular Carcinoma: Transarterial Chemoembolization versus Sorafenib. Radiology. 2012;263:590–599. doi: 10.1148/radiol.12111550. [DOI] [PubMed] [Google Scholar]
- Scholz I.V. Cengic N. Baker C.H., et al. Radioiodine therapy of colon cancer following tissue-specific sodium iodide symporter gene transfer. Gene Ther. 2005;12:272–280. doi: 10.1038/sj.gt.3302410. [DOI] [PubMed] [Google Scholar]
- Spaeth E. Klopp A. Dembinski J., et al. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 2008;15:730–738. doi: 10.1038/gt.2008.39. [DOI] [PubMed] [Google Scholar]
- Spaeth E.L. Dembinski J.L. Sasser A.K., et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One. 2009;4:e4992. doi: 10.1371/journal.pone.0004992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzweg C. Zhang S. Bergert E.R., et al. Prostate-specific antigen (PSA) promoter-driven androgen-inducible expression of sodium iodide symporter in prostate cancer cell lines. Cancer Res. 1999;59:2136–2141. [PubMed] [Google Scholar]
- Spitzweg C. O'Connor M.K. Bergert E.R., et al. Treatment of prostate cancer by radioiodine therapy after tissue-specific expression of the sodium iodide symporter. Cancer Res. 2000;60:6526–6530. [PubMed] [Google Scholar]
- Spitzweg C. Dietz A.B. O'Connor M.K., et al. In vivo sodium iodide symporter gene therapy of prostate cancer. Gene Ther. 2001;8:1524–1531. doi: 10.1038/sj.gt.3301558. [DOI] [PubMed] [Google Scholar]
- Spitzweg C. Morris J.C. The sodium iodide symporter: its pathophysiological and therapeutic implications. Clin. Endocrinol. (Oxf) 2002;57:559–574. doi: 10.1046/j.1365-2265.2002.01640.x. [DOI] [PubMed] [Google Scholar]
- Spitzweg C. Baker C.H. Bergert E.R., et al. Image-guided radioiodide therapy of medullary thyroid cancer after carcinoembryonic antigen promoter-targeted sodium iodide symporter gene expression. Hum. Gene Ther. 2007;18:916–924. doi: 10.1089/hum.2007.081. [DOI] [PubMed] [Google Scholar]
- Van Sande J. Massart C. Beauwens R., et al. Anion selectivity by the sodium iodide symporter. Endocrinology. 2003;144:247–252. doi: 10.1210/en.2002-220744. [DOI] [PubMed] [Google Scholar]
- Von Luttichau I. Notohamiprodjo M. Wechselberger A., et al. Human adult CD34- progenitor cells functionally express the chemokine receptors CCR1, CCR4, CCR7, CXCR5, and CCR10 but not CXCR4. Stem Cells Dev. 2005;14:329–336. doi: 10.1089/scd.2005.14.329. [DOI] [PubMed] [Google Scholar]
- Wapnir I.L. Goris M. Yudd A., et al. The Na+/I- symporter mediates iodide uptake in breast cancer metastases and can be selectively down-regulated in the thyroid. Clin Cancer Res. 2004;10:4294–4302. doi: 10.1158/1078-0432.CCR-04-0074. [DOI] [PubMed] [Google Scholar]
- Willhauck M.J. Sharif-Samani B.R. Gildehaus F.J., et al. Application of 188Re as an Alternative Radionuclide for Treatment of Prostate Cancer after Tumor-Specific Sodium Iodide Symporter Gene Expression. J. Clin. Endocrinol Metab. 2007;92:4451–4458. doi: 10.1210/jc.2007-0402. [DOI] [PubMed] [Google Scholar]
- Willhauck M.J. Samani B.R. Wolf I., et al. The potential of 211Astatine for NIS-mediated radionuclide therapy in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging. 2008a;35:1272–1281. doi: 10.1007/s00259-008-0775-4. [DOI] [PubMed] [Google Scholar]
- Willhauck M.J. Sharif-Samani B. Senekowitsch-Schmidtke R., et al. Functional sodium iodide symporter expression in breast cancer xenografts in vivo after systemic treatment with retinoic acid and dexamethasone. Breast Cancer Res. Treat. 2008b;109:263–272. doi: 10.1007/s10549-007-9646-0. [DOI] [PubMed] [Google Scholar]
- Willhauck M.J. Sharif Samani B.R. Klutz K., et al. Alpha-fetoprotein promoter-targeted sodium iodide symporter gene therapy of hepatocellular carcinoma. Gene Ther. 2008c;15:214–223. doi: 10.1038/sj.gt.3303057. [DOI] [PubMed] [Google Scholar]
- Zischek C. Niess H. Ischenko I., et al. Targeting tumor stroma using engineered mesenchymal stem cells reduces the growth of pancreatic carcinoma. Ann. Surg. 2009;250:747–753. doi: 10.1097/SLA.0b013e3181bd62d0. [DOI] [PubMed] [Google Scholar]
- Zlotnik A. Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.