Abstract
Stromal cell-derived factor-1 (SDF-1), also known as a homing factor, is a potent chemokine that activates and directs mobilization, migration, and retention of certain cell species via systemic circulation. The responding homing cells largely consist of activated stem cells, so that, in case of tissue lesions, such SDF-1-induced cell migration may execute recruitment of endogenous stem cells to perform autoreparation and compensatory regeneration in situ. In this study, a recombinant adenoviral vector carrying SDF-1 transgene was constructed and applied to transduce a novel scaffold-free living hyaline cartilage graft (SDF-t-LhCG). As an engineered transgenic living tissue, SDF-t-LhCG is capable of continuously producing and releasing SDF-1 in vitro and in vivo. The in vitro trials were examined with ELISA, while the in vivo trials were subsequently performed via a subcutaneous implantation of SDF-t-LhCG in a nude mouse model, followed by series of biochemical and biological analyses. The results indicate that transgenic SDF-1 enhanced the presence of this chemokine in mouse's circulation system; in consequence, SDF-1-induced activation and recruitment of endogenous stem cells were also augmented in both peripheral blood and SDF-t-LhCG implant per se. These results were obtained via flow cytometry analyses on mouse blood samples and implanted SDF-t-LhCG samples, indicating an upregulation of the CXCR4+(SDF-1 receptor) cell population, accompanied by upregulation of the CD34+, CD44+, and Sca-1+ cell populations as well as a downregulation of the CD11b+ cell population. With the supply of SDF-1-recruited endogenous stem cells, enhanced chondrogenesis was observed in SDF-t-LhCG implants in situ.
Introduction
Stromal cell-derived factor-1 (SDF-1) has long been known as a potent chemokine that induces homing of hematopoietic stem cells (HSCs, CD34+) to the site of SDF-1 secretion.1 Bone marrow serves as a major pool of such CD34+ stem/progenitor cells. In case of lesions, such as exposure to fluorouracil, the level of SDF-1 would elevate in the plasma, bone marrow, and liver.2–4 CD34+ cells in bone marrow and peripheral blood would sense the elevation of the SDF-1 level and respond with alterations in their gene expression patterns.5 Actions are initiated by CXCR4, a cell surface receptor responsible for specific interaction and integration with SDF-1, followed by activation of various signaling pathways resulting in secretion of various functional biomolecules such as matrix metalloproteinases, nitric oxide, and vascular endothelial growth factor.6 The expression pattern of cell adhesion receptors, such as integrin family members, would also be changed. These serial changes in cells would enable and guide a chemotactic migration along the ascending concentration gradient of SDF-1, so that the migration would be oriented toward the locations with higher SDF-1 concentration, that is, being directed toward the source of SDF-1 release.7 Cell adhesion molecules also aid in transmembrane migration of SDF-1-activated cells penetrating the basal lamina of vascular endothelium, so that the cells can eventually reach the destination of homing—the source of SDF-1 release, usually the site of injured organs or tissues. As the motivated homing cells largely consist of activated stem cells, this SDF-1-induced cell migration is actually executing recruitment of endogenous therapeutic progenitors for autoreparation and compensatory regeneration in situ. Besides HSCs, CD34+ mesenchymal stem cells (MSCs), and some CD117+ cells have also been found to respond to SDF-1 and home to the injured site for regeneration.8–11 From all the findings on SDF-1, it is clear that SDF-1 is a good biochemical attractant for the homing of endogenous stem/progenitor cells to enhance compensatory tissue regeneration in situ.
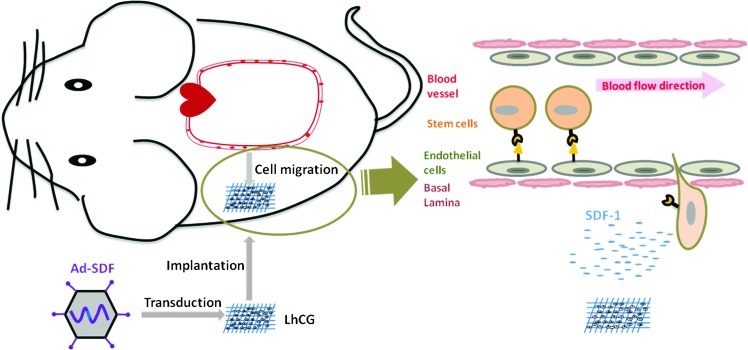
Hyaline cartilage, a tissue-articulating skeleton at joints, is highly prone to damages caused by trauma, diseases, and aging; while once injured, its self-regeneration is difficult and slow due to the avascular nature.12 To regenerate damaged cartilage, a scaffold-free living hyaline cartilage graft (LhCG) has been designed and developed in our previous work, which, as an engineered cartilage graft, only consists of living chondrocytes and cartilaginous extracellular matrices (ECMs) in a porous sponge-like form.13 Given the living cells in this living graft, LhCG system per se can be pretransduced with vectors expressing the SDF-1 transgene. Further, upon implantation of this SDF-1 transgene endowed LhCG (SDF-t-LhCG) in an animal model, it may serve as an in vivo platform of transgenic SDF-1 release as well as an in situ niche of chondrogenesis by SDF-1-recruited endogenous stem cells. In this study, a recombinant adenoviral vector carrying the SDF-1 transgene expression cassette (Ad-SDF) was constructed and introduced into the LhCG system, so that a working model of SDF-t-LhCG was produced for the study of in situ SDF-1 release and its therapeutic potential. By implanting SDF-t-LhCG into nude mice, the release profile of SDF-1 was established; SDF-1-induced recruitment of endogenous stem/progenitor cells and a consequent augmentation of in situ chondrogenesis were witnessed in vivo. A graphical demonstration of the experimental design is presented in Figure 1.
FIG. 1.
Graphical review of the experimental design. LhCG is transduced with Ad-SDF and implanted subcutaneously into nude mice. Released SDF-1 circulates in the blood and attracts the CD34+ stem cells against the concentration gradient. The stem cells will trespass the endothelium and basal lamina and home to the graft site for therapeutic purpose. SDF-1, stromal cell-derived factor-1; LhCG, living hyaline cartilage graft. Color images available online at www.liebertpub.com/tea
Materials and Methods
Construction of Ad-SDF
Mouse SDF-1 cDNA was isolated using reverse transcription and polymerase chain reaction (PCR) from extracted RNA of murine fibroblast cells with the following primers: forward: ACGCGTCGACATGGACGCCAAGGTCGTC; reverse: TGCACTGCAGTTACTTGTTTAAAGCTTTCTC. The amplified SDF-1 cDNA was double-digested with restriction endonucleases SalI and PstI (New England Biolabs, Ipswich, MA) and cloned into a pDNR shuttle vector, which was then recombined with pLP-Adeno-X ViraTrak acceptor vector using Crerecombinase (Clontech, Mountain View, CA). The constructed adenoviral vector was linearized by the restriction endonuclease PacI before being transfected to HEK 293 cells (ATCC, Manassas, VA) by Lipofectamine (Invitrogen, Carlsbad, CA). Ad-SDF was collected from the cells after three freeze–thaw cycles and titrated. The collected virus was kept at −20°C and stored for future amplification using HEK 293 cells.
In vitro Ad-SDF transduction of chondrocytes
Chondrocytes were isolated according to procedures elaborated elsewhere.13 Cells at passage 2 were plated in the wells of 24-well plates, with 10,000 cells in each well. On the second day postplating, cells were transduced with Ad-SDF at multiplicities of infection (MOI) of 100 and 500, respectively. Cells were also transduced with a null adenoviral vector (Ad-N, MOI 500) as a control. Another group of cells without adenoviral transduction was used as a negative control (Neg). Three hours post-transduction, a fresh medium was replaced. The medium was collected every 3 days till day 9 and assayed for SDF-1 concentration with ELISA (R&D Systems, MN). RNA was extracted at day 9 for SDF-1 gene expression analysis.
Quantitative real-time polymerase chain reaction analysis
At day 9, chondrocyte RNA was extracted using TRIZOL® Reagents (Invitogen), 0.5 μg of which was converted to cDNA by reverse transcription. qPCR was carried out for 40 DNA replication cycles using the iQ™ qPCR system (Bio-Rad). For normalization purposes, the RPL4 gene was used as reference gene. The primer sequences used are as follows: SDF-1 forward: 5′-ACGCGTCGACATGGACGCCAAGGTCGTC-3′; SDF-1 reverse: 5′-TGCACTGCAGTTACTTGTTTAAAGCTTTCTC-3′. RPL4 forward: 5′-CAAGAGTAACTACAACCTTC-3′; RPL4 reverse: 5′-GAACTCTAC GATGAATCTTC-3′.
Construction of LhCG
The protocol was as established previously by our laboratory.13,14 Briefly, 30 mL 10% gelatin solution at 70°C was first mixed with 10 mL ethyl acetate. After stirring for 1 min, the mixture was mixed with 60 mL soya oil. The mixed solution was then cooled to −40°C using an ethanol bath, and gelatin microspheres were formed in response to the drop in temperature. Ten minutes later, dioxane thrice was added to remove the soya oil. Lastly, the solution was transferred to a 70°C oven for microsphere drying. The resulting gelatin microspheres were further treated with N-ethyl-N′-(3-dimethylaminopropyl) carbodiimide for surface crosslinking stabilization. The gelatin microspheres were preswelled by immersion into 4°C 1× sterile Dulbecco's phosphate-buffered saline (DPBS) (Invitrogen). About 0.45 g of gelatin microspheres was next mixed with 1.5 mL 1.5% alginate solution and passage 1 porcine chondrocytes (1×107/mL density). The mixed solution was then injected onto a 35-mm Petri dish coated with 15% (w/v) gelatin and CaCl2 (102 mM). Four minutes later, a further 1 mL of 102 mM CaCl2 was added to facilitate gelation. When the gelation is completed after 4 min, the construct can be sliced to 16 pieces (0.7×0.7×0.1 cm) of gel and cultured in a chondrocyte culture medium for 35 days (37°C, 5% CO2). The chondrocyte culture medium comprises of the DMEM, 20% (v/v) fetal bovine serum (Invitrogen), 0.1 mM nonessential amino acids, 0.01 M 4-(2-hydroxyethyl)-piperazine-1-ethanesulfonic acid (HEPES), 0.4 mM proline, 0.05 mg/mL vitamin C, as well as 100 units/mL penicillin and 100 mg/mL streptomycin. Finally, at the end of the 35 days, the construct was treated with 55 mM sodium citrate (in 0.15 M sodium chloride), and a full material-free LhCG was successfully constructed.13
LhCG transduction with Ad-N or Ad-SDF
Before transducing LhCG with Ad-SDF, it is essential to know the cell number in LhCG to know the amount of viral particles to be used for transduction. Therefore, the relationship between the LhCG construct's cell number and wet weight was established. Four different LhCGs were weighed and freeze-dried overnight. Each piece was then digested with papain.15 To test the DNA content, the Hoechst 33258 dye assay was adopted (7.7 pg DNA/cell).16 The cell number–wet weight relationship was plotted, followed by linear regression treatment. An example was established such that 0.0774 g of LhCG corresponds to 3.2×105 cells, and 1.6×108 infectious unit (ifu) of Ad-N or Ad-SDF (MOI 500) was used to transduce this piece of LhCG. For transduction, a certain amount of Ad-N or Ad-SDF in 50 μL of DMEM was added dropwise to each well containing one piece of LhCG in a 96-well plate. After 3 h of transduction, LhCGs were transferred to a 24-well plate and cultured in a chondrocyte culture medium. LhCG without adenoviral transduction was used as negative control, denoted as LhCG, while LhCGs transduced with Ad-N or Ad-SDF were denoted as Null-t-LhCG and SDF-t-LhCG, respectively. Within the subsequent 5-week culture, the medium from all the three groups was changed every 4 days, and the SDF-1 concentration was monitored using the collected medium with ELISA.
The transduction efficiency was determined by transducing 3 pieces of LhCGs of similar wet weight with Ad-SDFat MOI 500. The transduction was allowed to proceed for 3 h before titrating the medium containing Ad-SDF using the Adenovirus titration kit (Clontech). Remaining Ad-SDF in the medium was thus determined, and the transduction efficiency was calculated using the function below:
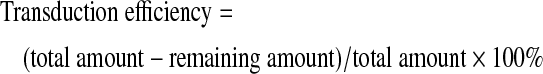 |
In vivo implantation of LhCG
LhCGs that weighed around 0.07 g were selected and transduced with Ad-N or Ad-SDF at MOI 500. One day post-transduction, LhCGs were subcutaneously implanted in 4-week-old severe combined immunodeficiency (SCID) nude mice (mutant BALB/C; i-DNA Biotechnology Singapore) and denoted as day 0 of in vivo experiments. There were two mice in each group (LhCG, Null-t-LhCG and SDF-t-LhCG), and each mouse received subcutaneous transplantation of two implants, each on one side of its back. At day 30, the heart blood sample was collected from each mouse, and the implanted LhCGs were retrieved and washed with PBS after euthanizing each mouse. In total, six SCID mice have been used for the experiment, which had been performed in accordance with regulations of the Institutional Animal Care and Use Committees (IACUC), Nanyang Technological University (NTU), Singapore.
Characterization of blood
Blood serum and cells were separated by centrifugation at 500 g for 5 min. The serum was used in ELISA for the measurement of SDF-1 levels in circulating blood. For CXCR4, MSC marker (CD29, Sca-1, CD73, CD106, CD45, and CD11b), and CD34 marker identifications, blood cells were collected for flow cytometry. Cells were washed twice with 500 μL of PBS containing 0.5% bovine serum albumin and blocked with 500 μL of goat serum (10%, v/v) for 15 min. Each sample was separated into aliquots of 50 μL and mixed with respective rat anti-mouse antibodies for incubation (30 min, 4°C) according to standard manuals. The groups include negative control for flow cytometry without antibody staining, CXCR4, CD29, Sca-1, CD73, CD106, CD45, CD11b, and CD34 (R&D Systems). After the primary blotting, samples were washed twice and incubated with 5 μL of goat anti-rat IgG conjugated with allophycocyanin (R&D Systems) for 30 min at 4°C in dark. One exception is the CXCR4 group, whose primary antibody already possesses allophycocyanin fluorephore, and therefore the secondary antibody incubation step was not required. The samples were further washed twice with PBS, and 500 μL red blood cell (RBC) lysis buffer (11.25% NH4Cl [w/v], 1.25% KHCO3 [w/v], and 0.025% 0.5 M EDTA [v/v]) was added for RBC lysis. The final sample was washed and resuspended in 400 μL PBS for flow cytometry test.
Characterization of retrieved LhCGs
For subcutaneous implants retrieved directly, the samples were digested with 1 mL of 1 mg/mL collagenase II for 5 h. The collected cells were washed, incubated with rat anti-mouse CXCR4 antibody, and resuspended in PBS for flow cytometry test of CXCR4+ cells. The cells retrieved from the pure LhCG group were used as a control in the flow cytometry assay; therefore, results were expressed as a relative incremental percentage of CXCR4+ cells against the pure LhCG group.
The implanted construct was used for histochemical and immunofluorescent staining. Each sample was fixed in 4% (w/v) neutral buffered paraformaldehyde. After that, the samples were embedded in paraffin and sectioned (5-μm thick). All sectioned samples were deparaffinized for hematoxylin and eosin (H&E) staining and type II collagen (Col II) staining. First, 2.5% glutaraldehyde was added for a 30-min treatment. One percent goat serum (w/v, in PBS) was then added to block the sample for 1 h. At 4°C, the primary antibody for Col II (Chemicon) was applied overnight to the sample after washing. After incubating the samples (1 h, 37°C) in the secondary antibody Alexa-Fluor-546-conjugated rabbit anti-mouse IgG (1:500; Invitrogen) the next day, the cells were washed with PBS and observed under a fluorescence microscope.
Biochemical assays were also carried out for the determination of cell number and total glycosaminoglycans (GAG) in LhCGs. Each LhCG was freeze-dried (36 h) and digested with 0.5 mL of papain. DNA content was assessed by applying the Hoechst 33258 dye assay (7.7 pg DNA/cell).16,17 GAG content was determined by adding dimethylmethylene blue dye for absorption reading at 525 nm.18,19
Statistical analysis
Student's t-test was used in the analysis. Graphic results were indicated as mean±standard deviation.
Results
Ad-SDF functioning in chondrocytes cultured in 2D plates
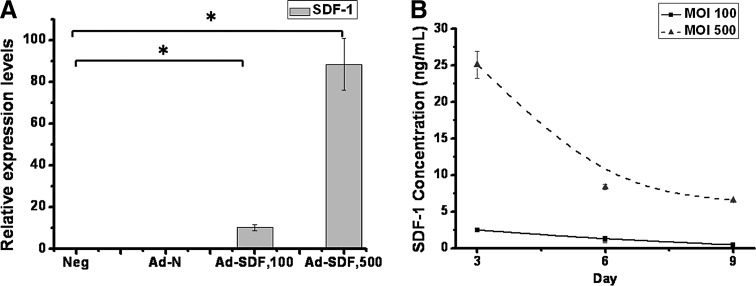
To test if the constructed Ad-SDF functions to express SDF-1, qPCR was performed to analyze the SDF-1 mRNA levels in chondrocytes transduced with Ad-SDF at MOI 100 and 500, respectively, cultured in 2D plates. From Figure 2A, it was observed that SDF-1 was expressed at the mRNA level when the chondrocytes were transduced with Ad-SDF at MOI of either 100 or 500, while mRNA levels in Neg and Ad-N were undetected. Transduction at MOI 500 witnessed an 8-fold higher SDF-1 mRNA level than MOI 100.
FIG. 2.
SDF-1 gene and protein expression in Ad-SDF-transduced chondrocytes cultured in 2D 24-well plates. Ten thousand cells were plated in each well and transduced with Ad-N at MOI 500 or Ad-SDF at MOI 100 and 500, respectively. (A) SDF-1 mRNA levels at MOI 100 and 500. At day 9, cells were lysed, and RNA was isolated and reverse transcribed for quantitative polymerase chain reaction analysis; (B) SDF-1 protein levels at MOI 100 and 500. The medium was collected every 3 days and used in ELISA for SDF-1 protein level determination. *p<0.05. MOI, multiplicities of infection.
ELISA was carried out using the medium collected every 3 days from 2D chondrocyte culture over a period of 9 days to further test the expression of SDF-1 at the protein level, with the expression curves shown in Figure 2B. Curves for Neg and Ad-N were not shown, as the protein levels in these two groups were undetected. For Ad-SDF transduction at MOI 100 and 500, there was a decreasing trend in the expression over the 9 days of culture, which was more apparent at MOI 500. This could be attributed to the episomal manner of adenoviral vectors, which did not integrate the transgene into the host cell genome, and therefore were gradually lost along cell replications. Also, the protein expression level at MOI 500 was around 10-fold higher than MOI 100. Based on the preliminary qPCR and ELISA results with Ad-SDF in 2D chondrocyte culture, it was concluded that the constructed Ad-SDF successfully functioned to express the SDF-1 chemokine. Besides, due to the high expression of SDF-1 at MOI 500, MOI 500 was selected for subsequent transduction protocols.
In vitro LhCG transduction with adenoviral vectors
To establish the relationship between the cell number and LhCG wet weight before transducing LhCG, the cell number was calculated according to DNA content measured by Hoechst dye staining for LhCGs of various wet weights after their digestion with papain. By applying a linear fit for the four sets of data (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea), a relationship between cell number (y) and wet weight (x) was obtained
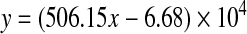 |
Therefore, an LhCG weighing 0.07 g corresponds to a cell number of 2.87×105, and a total of 1.4×108 infectious units of Ad-SDF were applied for LhCG transduction at MOI 500.
The efficiency of Ad-SDF was confirmed by measuring proportion of the added adenoviral particles that were actually transduced into the chondrocytes embedded in LhCG.
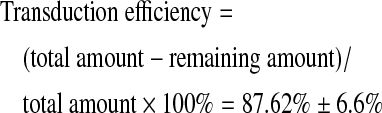 |
A high transduction efficiency of 87.62%±6.6% was achieved, indicating that the majority of Ad-SDF has been transduced into chondrocytes.
In vitro SDF-1 protein quantification secreted from SDF-t-LhCG
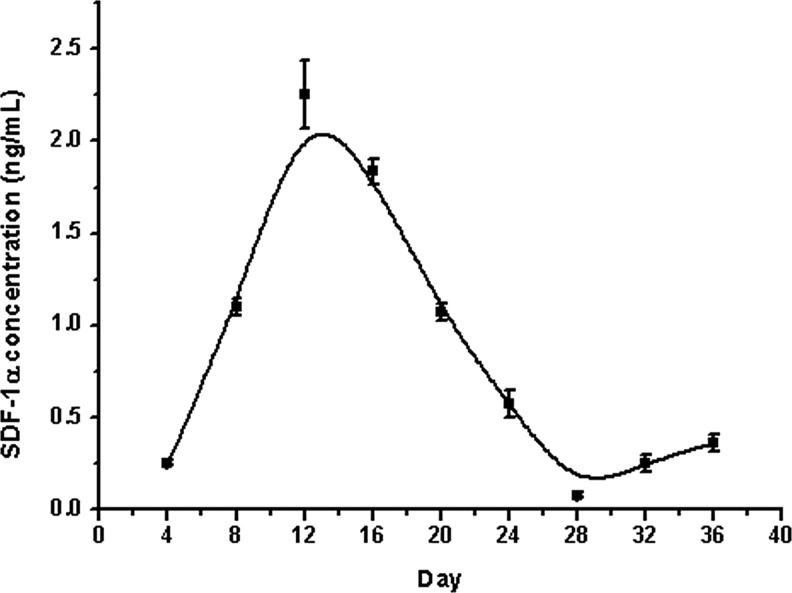
LhCG, Null-t-LhCG (MOI 500) and SDF-t-LhCG (∼0.07 g, MOI 500) were cultured in vitro for 36 days with a medium collected every 4 days for the measurement of SDF-1 protein levels secreted from the LhCGs with ELISA. The amount of SDF-1 in pure LhCG and Null-t-LhCG were under the detection limit. These results indicate that either (nontransduced) pure LhCG or Null-t-LhCG indeed cannot release any transgenic SDF-1 due to the absence of the SDF-1 transgene. Contrastively, from the expression curve for SDF-t-LhCG as shown in Figure 3, it was observed that the secreted SDF-1 amount increased in the initial 12 days of culture, peaking at around 2 ng/mL at day 12. Then, it gradually declined until the released SDF concentration stabilized at a platform of around 0.25 ng/mL.
FIG. 3.
SDF-1 protein expression profiles in SDF-t-LhCG cultured in vitro. LhCG was transduced with Ad-SDF and maintained for in vitro culture for 36 days. The medium was collected every 4 days and used for the determination of SDF-1 protein secretion level by ELISA. SDF-1 expression peaked at around day 12 and decreased afterward until stabilization.
In vivo SDF-1 protein quantification secreted from SDF-t-LhCG
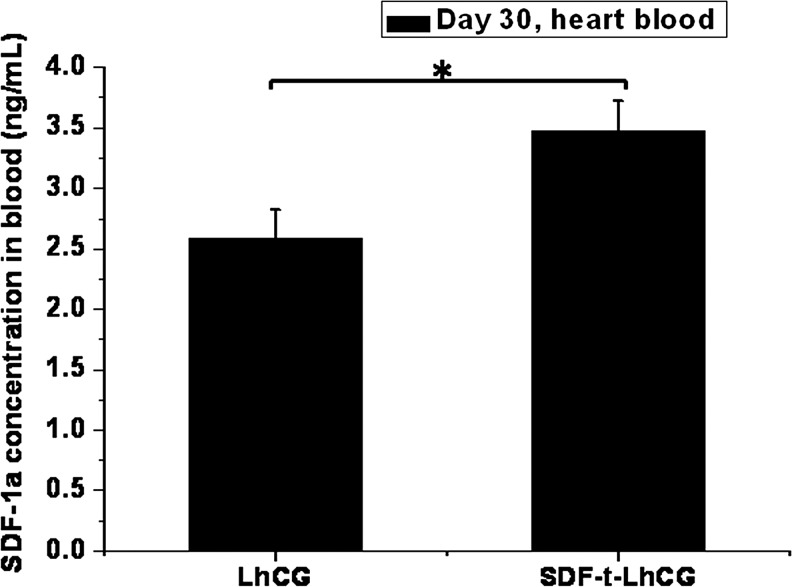
Thirty days after the implantation of SDF-t-LhCG subcutaneously into the nude mice, the amount of total SDF-1 protein from the heart blood was measured, as an indicator of SDF-1 protein level in the circulatory blood system, which contributed to the overall therapeutic cell attraction. In Figure 4, a basal level of SDF-1 expression was observed in the pure LhCG group, which was implanted with LhCG without adenoviral transduction. However, the SDF-1 protein level in the mouse blood implanted with SDF-t-LhCG was significantly higher, reaching around 3.5 ng/mL of blood. In contrast to the (nontransduced) pure LhCG, the extra amount of SDF-1 expression in the blood of SDF-t-LhCG-implanted animals is contributed from the expression of transgenic SDF-1.
FIG. 4.
SDF-1 protein levels in the heart blood of nude mice. Thirty days after implantation with pure LhCG or SDF-t-LhCG, nude mice were sacrificed, and blood was collected from the heart of each nude mouse for the determination of SDF-1 in the circulation blood by ELISA. *p<0.05.
Characterization of CXCR4+ cells in blood system
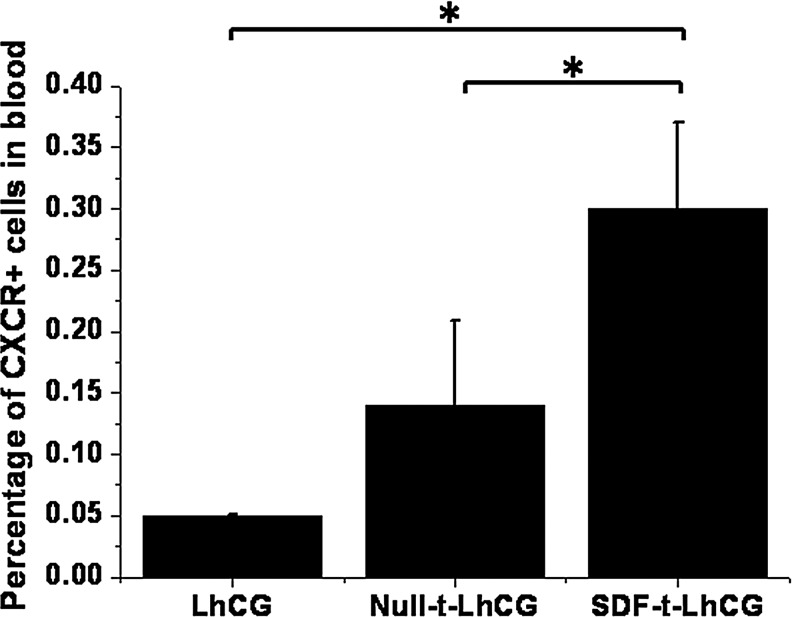
As CXCR4 is an important receptor of SDF-1, the percentage of CXCR4+ cells present in the blood was measured using flow cytometry at day 30 to show the chemoattractive effect of SDF-1 (Fig. 5). Again, there was a basal presence of CXCR4+ cells in the blood in the pure LhCG and Null-t-LhCG groups. However, implantation of SDF-t-LhCG led to a higher presence of CXCR4+ cells, demonstrating the positive effect of secreted SDF-1 on the recruitment of CXCR4+ cells for potential therapeutic purposes.
FIG. 5.
The percentage of CXCR4+ cells in the heart blood of nude mice. Thirty days after implantation with pure LhCG, Null-t-LhCG, or SDF-t-LhCG, heart blood was collected and used for the determination of CXCR+ cells by flow cytometry. *p<0.05.
Characterization of therapeutic stem/progenitor cells in the blood
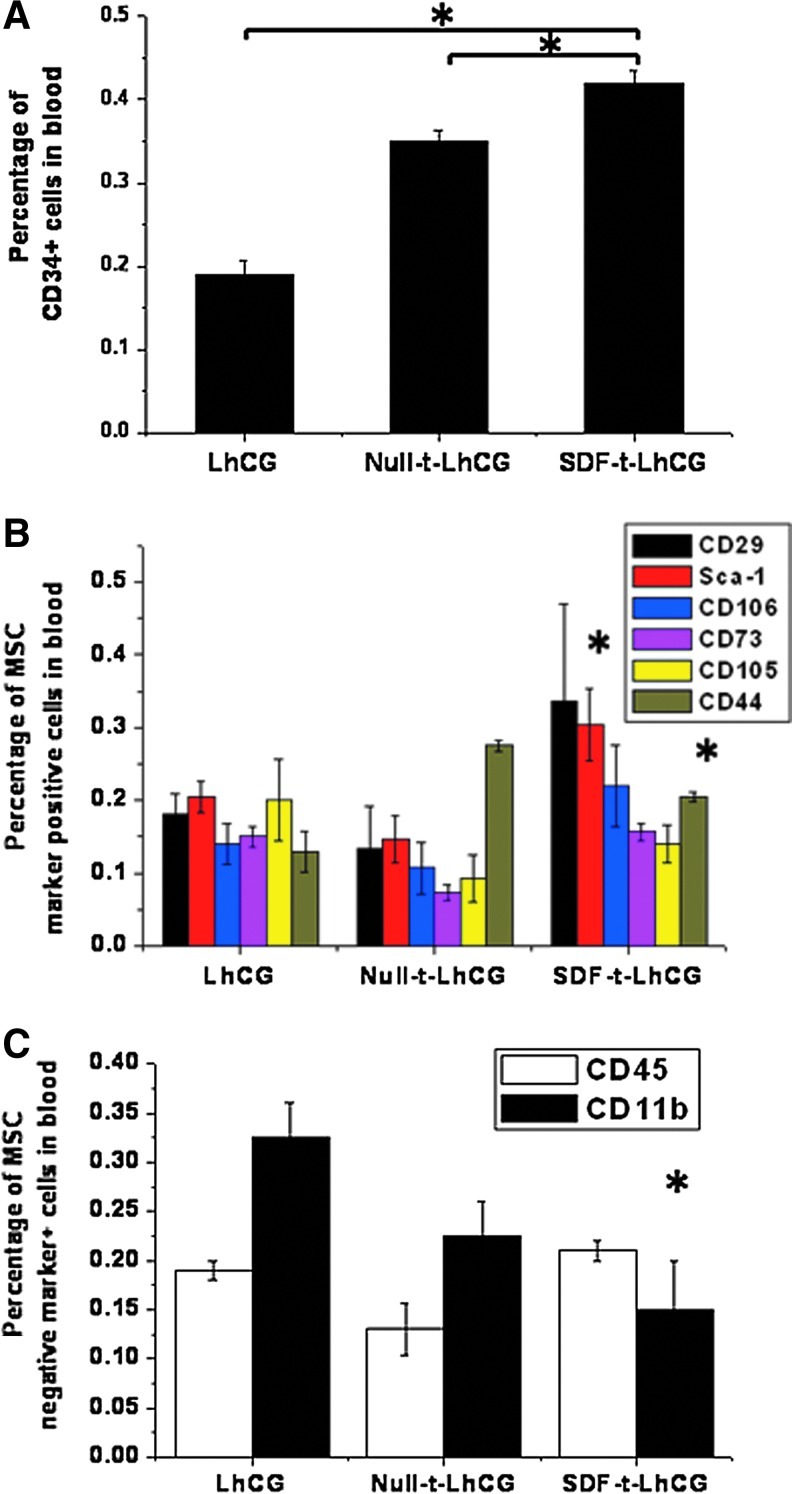
Since HSCs and MSCs are two important stem cell populations that possess therapeutic potentials, the markers of these two cell populations were characterized in the blood. For CD34 marker shown in Figure 6A, there was a basal presence of CD34+ cells in the blood of the pure LhCG group, and implantation of Null-t-LhCG increased the percentage to around 0.35%. Implantation of SDF-t-LhCG further elevated the percentage to 0.4%, providing evidence that the percentage of CD34+ cells increased due to the chemoattractant SDF-1 released from the implanted SDF-t-LhCG. Besides CD34, there was an increase in the percentages of cells with some of the MSC-positive markers, that is, Sca-1 and CD44, in the SDF-t-LhCG group compared with pure LhCG, while there were no significant changes in other positive markers (Fig. 6B). On the contrary, the percentages of MSC-negative markers (CD45 and CD11b in Fig. 6C) maintained or even decreased in the SDF-t-LhCG group compared with pure LhCG. These results have suggested an elevation of the MSC population in the blood attributed to the implantation of SDF-t-LhCG.
FIG. 6.
The percentage of cells positive for CD34 and MSC markers in heart blood of nude mice. Thirty days after implantation with pure LhCG, Null-t-LhCG, or SDF-t-LhCG, heart blood was collected and used for the determination of the percentage of cells positive for the following markers by flow cytometry. (A) CD34; (B) MSC-positive markers (CD29, Sca-1, CD106, CD73, CD105, and CD44); (C) MSC-negative markers (CD45 and CD11b). *p<0.05; in (B) and (C), * only indicates significant difference between LhCG and SDF-t-LhCG. MSC, mesenchymal stem cell. Color images available online at www.liebertpub.com/tea
Characterization of CXCR4+ cells in implanted LhCGs
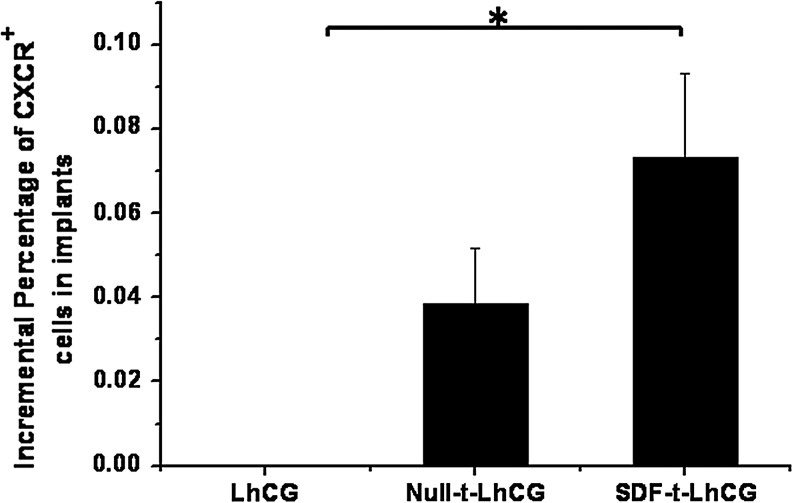
The percentage of CXCR4+ cells in LhCG implants retrieved at day 30 was also measured with flow cytometry (Fig. 7). It should be noted that, as the cells in the pure LhCG group were used as a control in the flow cytometry as background, the relative value for this group was 0; however, it does not necessarily mean that the absolute number of CXCR4+ cells in the pure LhCG group is 0. An increase in the positive cells was observable in both the Null-t-LhCG and SDF-t-LhCG groups, whereas the percentage in the SDF-t-LhCG was even higher than the Null-t-LhCG.
FIG. 7.
The relative incremental percentages of CXCR+ cells in the implants. Thirty days after implantation with pure LhCG, Null-t-LhCG, or SDF-t-the LhCG, implants were retrieved from the nude mice and digested with collagenase. The cell suspension was used to determine the percentage of CXCR+ cells by flow cytometry. Pure LhCG sample was used as negative control in flow cytometry test and therefore was set as 0. *p<0.05.
Total cell number and GAG content in LhCG at day 30
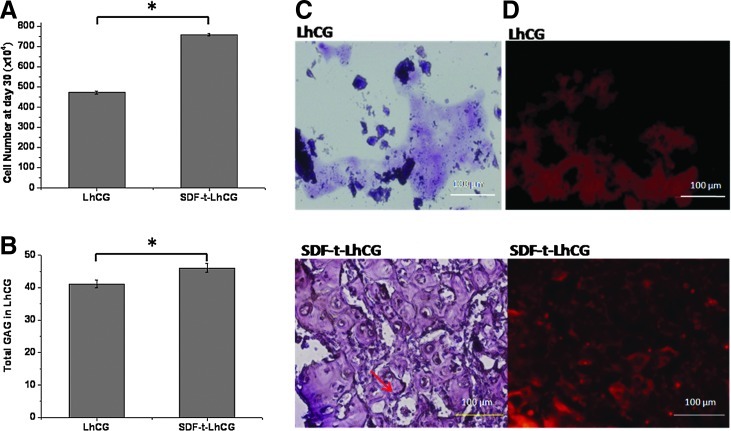
Using biochemical assays, the total cell number was determined in the LhCGs retrieved from the nude mice at day 30. From Figure 8A, it could be seen that a significantly higher cell number was in the SDF-t-LhCG compared with pure LhCG. This could be explained by either cell recruitment or higher cell replication, or a combination of both. Similarly, a higher GAG content (using dimethylmethylene blue) was seen in the SDF-t-LhCG group than the pure LhCG (Fig. 8B).
FIG. 8.
Biochemical and histological analysis of LhCG implants retrieved at day 30 after implantation. (A) Cell number in implants. Each LhCG was freeze-dried (36 h) and digested with 0.5 mL of papain. DNA content was assessed by applying the Hoechst 33258 dye assay (7.7 pg DNA/cell). (B) Total glycosaminoglycan (GAG) content in implants, determined by adding dimethylmethylene blue dye for absorption reading at 525 nm. (C) H&E staining. Lacuna structure was indicated with an arrowhead. Each sample was fixed in 4% (w/v) neutral buffered paraformaldehyde and embedded in paraffin and sectioned (5-μm thick). The sections were stained with H&E and observed under a microscope. (D) Immunofluorescent staining of Col II. Sectioned samples were deparaffinized for immunofluorescent staining of Col II. *p<0.05. Scale bar represents 100 μm. H&E, hematoxylin and eosin. Color images available online at www.liebertpub.com/tea
Histological staining of retrieved LhCG at day 30
H&E stain was used to visualize the cells and the ECM of the LhCG implant at the end of the in vivo experiment (Fig. 8C). The tissue for pure LhCG was mechanically weaker than the SDF-t-LhCG, such that the tissue fell apart easily in the embedding and sectioning process. On the contrary, the SDF-t-LhCG group showed a more uniform structure, with better mechanical strength. The cells were uniformly embedded in the ECM, showing apparent lacuna structures (indicated with arrow).
In immunohistochemistry staining for Col II (Fig. 8D), the fluorescence signal of Col II was detected in both LhCG and SDF-t-LhCG; however, SDF-t-LhCG showed a higher intensity and also a more uniform distribution.
Discussion
SDF-1 is a chemokine that can induce the homing of stem/progenitor cells to the site of release and therefore has therapeutic potential. SDF-1 has been demonstrated to recruit various cell types, including Jurkat T-lymphocytic cells8 and human umbilical vein endothelial cells,20 in an in vitro migration assay using transwells. This cell homing action helps in various tissue regeneration processes inclusive of promoting angiogenesis, improving heart functions during recovery from myocardial infarction, as well as accelerating diabetic wound healing.21 All the previous investigations have shown that SDF-1 is a promising therapeutic molecule that may make significant contribution in regenerative medicine.
In this study, we constructed a recombinant adenoviral vector carrying the SDF-1 transgene. The vector was used to pretransduce LhCG, which is a cartilage graft composed of only living chondrocytes and their secreted cartilaginous ECM-free of any synthetic or natural noncartilaginous compositions. After implantation, SDF-t-LhCG is capable of releasing the transgenic SDF-1 chemokine from the implantation site in an animal model, whereby it promotes the migration of responding stem/progenitor cells via blood circulation, across the basal lamina of vascular endothelium and finally homing to the SDF-t-LhCG implants. As a living graft-based transgenic drug-release platform, once established, it can be translated to release other therapeutic molecules for other functions beyond cartilage regeneration.
The in vitro release profile of SDF-1 from SDF-t-LhCG witnessed an initial elevation in the first 12 days. This could be explained by the recovery of cell viability and cell number after the adenoviral transduction. However, due to the intrinsic episomal features of adenovirus, the transgene is not integrated into the host cell genome and therefore would be gradually lost after each cell cycle, which resulted in the gradual decline of transgene expression in the following days.
Despite the distinct release patterns at various stages, the system was able to maintain an elevated level of SDF-1 protein in the blood, which was responsible for the recruitment of stem/progenitor cells to the implantation site. In a study done by Wright et al., in vitro migration of CXCR4+ stem cells toward a higher gradient of SDF concentration was observed.22 We further proved that CXCR4+ cells also migrate through blood circulation and home to the SDF-t-LhCG implant site in vivo, as evidenced by the increase of CXCR4+ cells in both blood and SDF-t-LhCG observed in Figures 6 and 8.
To further identify the responding cell populations circulating in the blood system, stem cell markers, particularly those for MSCs and HSCs, were employed. CD34 is a transmembrane cell adhesion molecule responsible for the adhesion of cells to stroma.23 CD29 is a subunit of integrin beta-1 and is responsible for cell attachment.24 CD106 is also an adhesion molecule that promotes the adhesion of blood cells to the endothelium, which facilitates the migration of the cells across the endothelium to the injured tissues.25 Sca-1 is a surface antigen of immature hematopoietic progenitor cells.26 CD44 is also a cell surface glycoprotein involved in cell adhesion, migration, and cell–cell interaction.27 The increment in the cell population with positivity of these MSC markers, together with the maintenance or decrease of MSC-negative markers CD45 and CD11b, jointly indicates an elevation of the CD34+ MSC population circulating in the blood system, stemming from the released SDF-1 from SDF-t-LhCG. Besides, as Sca-1 is an important marker of mouse HSCs, the increase of its level evidenced the chemoattracting effect of SDF-t-LhCG on HSCs. However, it should be noted that there is always a basal expression of SDF-1 or basal presence of the various cell populations in the blood system or LhCG in the pure LhCG group. This is not a surprise in consideration that the host may react to the invasive implanting procedures and therefore start to express SDF-1 and promote stem cell migration spontaneously. Also, Null-t-LhCG presented a higher percentage of the CXCR4+ and CD34+ cell population than that of the pure LhCG group, though still lower than that of SDF-t-LhCG. This is believed due to cytoimmune reactions provoked by transduction of adenoviral vectors in LhCG chondrocytes on top of the host reaction to implanting invasion. Despite the background and all the variations, SDF-t-LhCG did contribute to the most obvious effect in SDF-1 release as well as cell recruitment. The net outstanding portion of contribution is undoubtedly attributed to the release of transgenic SDF-1. These recruited stem/progenitor cells, although in a small proportion, may replicate faster than other cells and participate in the regenerative process, thereby amplifying the regenerative effect.
As the LhCG system is mostly suitable for cartilage regeneration, additional assays were carried out to characterize chondrogenesis, including the cell number and the amount of GAG and Col II, which are important components of the cartilage ECM. All of these indices were elevated in SDF-t-LhCG, evidenced by biochemical assays and immunofluorescent staining analysis. This suggests that the release of transgenic SDF-1 did promote cartilage regeneration by recruiting more endogenous therapeutic stem/progenitor cells of the recipient mice. Moreover, due to the presence of more cells and ECM induced by SDF-1, the tissue became more robust, and hence more suitable for reconstruction of this hard tissue.
In conclusion, the SDF-t-LhCG system is capable of releasing transgenic SDF-1 chemokine to the blood system, recruit endogenous therapeutic cells, and promote their homing to the implant. The release of transgenic SDF-1 elevated the production of key ECM components of cartilage tissues. Therefore, this system is a suitable candidate for improved cartilage tissue regeneration.
Supplementary Material
Acknowledgments
This research was financially supported by the Grant NMRC/EDG/1001/2010, National Medical Research Council, Singapore.
Disclosure Statement
No competing financial interests exist.
References
- 1.Grunewald M. Avraham I. Dor Y. Bachar-Lustig E. Itin A. Jung S., et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 2.Kucia M. Ratajczak J. Reca R. Janowska-Wieczorek A. Ratajczak M.Z. Tissue-specific muscle, neural and liver stem/progenitor cells reside in the bone marrow, respond to an SDF-1 gradient and are mobilized into peripheral blood during stress and tissue injury. Blood Cells Mol Dis. 2004;32:52. doi: 10.1016/j.bcmd.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 3.Ponomaryov T. Peled A. Petit I. Taichman R.S. Habler L. Sandbank J., et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106:1331. doi: 10.1172/JCI10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kollet O. Shivtiel S. Chen Y.Q. Suriawinata J. Thung S.N. Dabeva M.D., et al. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112:160. doi: 10.1172/JCI17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aiuti A. Webb I.J. Bleul C. Springer T. Gutierrez-Ramos J.C. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185:111. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding Z. Issekutz T.B. Downey G.P. Waddell T.K. L-selectin stimulation enhances functional expression of surface CXCR4 in lymphocytes: implications for cellular activation during adhesion and migration. Blood. 2003;101:4245. doi: 10.1182/blood-2002-06-1782. [DOI] [PubMed] [Google Scholar]
- 7.Peled A. Kollet O. Ponomaryov T. Petit I. Franitza S. Grabovsky V., et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95:3289. [PubMed] [Google Scholar]
- 8.Wang W. Li W. Ong L.L. Lutzow K. Lendlein A. Furlani D., et al. Localized and sustained SDF-1 gene release mediated by fibronectin films: A potential method for recruiting stem cells. Int J Artif Organs. 2009;32:141. doi: 10.1177/039139880903200304. [DOI] [PubMed] [Google Scholar]
- 9.Bhakta S. Hong P. Koc O. The surface adhesion molecule CXCR4 stimulates mesenchymal stem cell migration to stromal cell-derived factor-1 in vitro but does not decrease apoptosis under serum deprivation. Cardiovasc Revasc Med. 2006;7:19. doi: 10.1016/j.carrev.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Son B.R. Marquez-Curtis L.A. Kucia M. Wysoczynski M. Turner A.R. Ratajczak J., et al. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24:1254. doi: 10.1634/stemcells.2005-0271. [DOI] [PubMed] [Google Scholar]
- 11.Wynn R.F. Hart C.A. Corradi-Perini C. O'Neill L. Evans C.A. Wraith J.E., et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643. doi: 10.1182/blood-2004-02-0526. [DOI] [PubMed] [Google Scholar]
- 12.Somers T.J. Keefe F.J. Godiwala N. Hoyler G.H. Psychosocial factors and the pain experience of osteoarthritis patients: new findings and new directions. Curr Opin Rheumatol. 2009;21:501. doi: 10.1097/BOR.0b013e32832ed704. [DOI] [PubMed] [Google Scholar]
- 13.Su K. Lau T. T. Leong W.Y. Gong Y.H. Wang D. A. Creating a living hyaline cartilage graft free of non-cartilaginous constituents: an intermediate role of biomaterial scaffold. Adv Funct Mater. 2012;22:972. [Google Scholar]
- 14.Gong Y. Su K. Lau T.T. Zhou R. Wang D.A. Microcavitary hydrogel-mediating phase transfer cell culture for cartilage tissue engineering. Tissue Eng Part A. 2010;16:3611. doi: 10.1089/ten.tea.2010.0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ameer G.A. Mahmood T.A. Langer R. A biodegradable composite scaffold for cell transplantation. J Orthop Res. 2002;20:16. doi: 10.1016/S0736-0266(01)00074-2. [DOI] [PubMed] [Google Scholar]
- 16.Kim Y.J. Sah R.L. Doong J.Y. Grodzinsky A.J. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988;174:168. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- 17.Freed L.E. Hollander A.P. Martin I. Barry J.R. Langer R. Vunjak-Novakovic G. Chondrogenesis in a cell-polymer-bioreactor system. Exp Cell Res. 1998;240:58. doi: 10.1006/excr.1998.4010. [DOI] [PubMed] [Google Scholar]
- 18.Farndale R.W. Buttle D.J. Barrett A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 19.Freed L.E. Marquis J.C. Nohria A. Emmanual J. Mikos A.G. Langer R. Neocartilage formation in vitro and in vivo using cells cultured on synthetic biodegradable polymers. J Biomed Mater Res. 1993;27:11. doi: 10.1002/jbm.820270104. [DOI] [PubMed] [Google Scholar]
- 20.Tang J. Wang J. Yang J. Kong X. Zheng F. Guo L., et al. Mesenchymal stem cells over-expressing SDF-1 promote angiogenesis and improve heart function in experimental myocardial infarction in rats. Eur J Cardiothorac Surg. 2009;36:644. doi: 10.1016/j.ejcts.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 21.Badillo A.T. Chung S. Zhang L. Zoltick P. Liechty K.W. Lentiviral gene transfer of SDF-1 alpha to wounds improves diabetic wound healing. J Surg Res. 2007;143:35. doi: 10.1016/j.jss.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 22.Wright D.E. Bowman E.P. Wagers A.J. Butcher E.C. Weissman I.L. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J Exp Med. 2002;195:1145. doi: 10.1084/jem.20011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin G. Finger E. Gutierrez-Ramos J.C. Expression of CD34 in endothelial cells, hematopoietic progenitors and nervous cells in fetal and adult mouse tissues. Eur J Immunol. 1995;25:1508. doi: 10.1002/eji.1830250606. [DOI] [PubMed] [Google Scholar]
- 24.Aldridge V. Garg A. Davies N. Bartlett D.C. Youster J. Beard H., et al. Human mesenchymal stem cells are recruited to injured liver in a beta1 integrin and CD44 dependent manner. Hepatology. 2012;56:1063. doi: 10.1002/hep.25716. [DOI] [PubMed] [Google Scholar]
- 25.Levesque J.P. Liu F. Simmons P.J. Betsuyaku T. Senior R.M. Pham C., et al. Characterization of hematopoietic progenitor mobilization in protease-deficient mice. Blood. 2004;104:65. doi: 10.1182/blood-2003-05-1589. [DOI] [PubMed] [Google Scholar]
- 26.Spangrude G.J. Brooks D.M. Mouse strain variability in the expression of the hematopoietic stem cell antigen Ly-6A/E by bone marrow cells. Blood. 1993;82:3327. [PubMed] [Google Scholar]
- 27.Goodison S. Urquidi V. Tarin D. CD44 cell adhesion molecules. Mol Pathol. 1999;52:189. doi: 10.1136/mp.52.4.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.