Abstract
A simple procedure for recovering biodegradable polymer from bacterial cells has been developed using economical and environmentally friendly solvent or chemicals. Recombinant bacterium, Cupriavidus necator harboring pBBR1MCS-C2 plasmid polyhydroxyalkanoate (PHA) synthase gene was used for the production of copolymer P(3HB-co-3HHx) from crude palm kernel oil (CPKO). NaOH was chosen in this study as it could give high purity and recovery yield. Increase of NaOH concentration had resulted in an increase of the PHA purity, but the recovery yield had decreased. The greater improvement of PHA purity and recovery were achieved by incubating the freeze-dried cells (10–30 g/L) in NaOH (0.1 M) for 1–3 h at 30°C and polishing using 20% (v/v) of ethanol. The treatment caused negligible degradation of the molecular weight of PHA recovered from the bacterial cells. The present review also highlights other extraction methods to provide greater insights into economical and sustainable recovery of PHA from bacterial cells.
Keywords: PHA, recovery, Cupriavidus necator, alkaline digestion, P(3HB-co-3HHx)
The biodegradable polymer is not highly competitive to petrochemical-based plastics due to its high production and recovery cost. Particularly, the cost of PHA recovery from bacteria cells is more than 50% of the total PHA production cost as revealed by economic evaluation.1 For large scale production, PHA extraction cost should be as low as possible with high PHA recovery yield at the end of this product. The efficient recovery process for PHA production depends on several factors. The main factor is the chemicals or solvents used for recovery process which should be a green chemical or green solvent. It will be a more economical process if the green chemical or green solvent is an inexpensive material. Besides that, a simple process during operation of the PHA extraction which reduces the energy, time and cost should also be taken into consideration especially in the large scale recovery process.
PHA is an intracellular product, thus the methods adopted for its recovery focus either on its solubilization or on the solubilization of non-polymer cellular materials (NPCM). The NPCM which consist of nucleic acids, lipids, phospholipids, peptidoglycan and proteinaceous materials will be separated out from the polymer during recovery process.2,3
There are many different strategies for the extraction and recovery of the polymer or PHA from bacterial cells. Numerous recovery processes have been proposed to recover PHA from C. necator. These involve solvent extraction, enzymatic digestion method, sodium hypochlorite digestion and the use of mechanical process method.4-6 However, these methods are generally complex, expensive, highly toxic, cause severe degradation of molecular weight of polymer and require large quantities of non-environmental friendly solvents.4 Due to this, a simple, efficient and more economical recovery method using green chemicals or solvents is developed in this research (Fig. 1). The green chemicals or solvents are environmentally friendly and their usage can result in reduced waste, safer outputs and eliminated pollution.
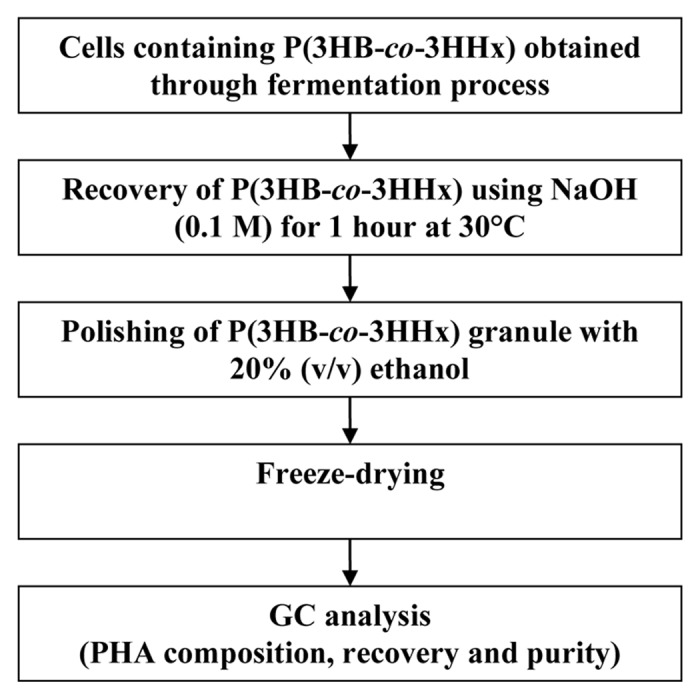
Figure 1. The work flow for the recovery of P(3HB-co-3HHx) produced by recombinant Cupriavidus necator.
Currently, there are a lot of improved methods to avoid the application of non-environmental friendly solvent and chemical that can reduce molecular weight of polymer. Some improvement methods include enzymatic digestion, mechanical cell disruption and the use of supercritical carbon dioxide. Nevertheless, these methods are still not in consideration for the recovery process because they are economically unattractive in comparison with other methods.6-8 New methods like spontaneous liberation, dissolved air flotation and air classification are still under investigation.6,9,10 Table 1 compares various PHA extraction methods.
Table 1. Comparison of polyhydroxyalkanoates extraction methods6.
| Extraction method | Advantages | Disadvantages |
|---|---|---|
| |
|
|
|
Solvent extraction |
Elimination of endotoxin/high purity |
Break PHA granules morphology |
| |
No polymer degradation |
Hazards connected with halogenated solvents |
| |
|
High price/Low recovery |
|
Digestion by surfactants |
Treatment of high cell densities |
Low purity/Water waste |
| |
No polymer degradation |
treatment needed |
|
Digestion by NaOCl |
High purity |
Degradation of the polymer |
|
Digestion by NaOCl and chloroform |
Low polymer degradation/ high purity |
High quantity of solvent needed |
|
Digestion by NaOCl and surfactants |
Limited degradation/low operating cost |
|
|
Digestion by chelate and surfactants |
High purity/low environmental pollution |
Large volume of wastewater |
| |
|
Low degradation of the polymer |
|
Selective dissolution of NPCM |
High recovery and purity, low operating costs |
|
|
Enzymatic digestion |
Good recovery |
High costs of enzymes |
|
Bead mill disruption |
No chemicals used |
Require several passes |
|
High pressure homogenization |
No chemicals used |
Poor disruption rate for low biomass levels |
| |
|
Low micronization |
|
Supercritical CO2 |
Low cost and toxicity |
Low recovery |
|
Using cell fragility |
Use of weak extracting conditions |
|
|
Air classification |
High purity |
Low recovery |
|
Dissolved air flotation |
No chemicals used |
Require several consecutive flotation steps |
| Spontaneous liberation | No extracting chemicals needed | Low recovery |
We had studied different alkalines (NaOH, KOH, NH4OH and NaOCl) because hydrolysis and saponification of protein and lipopolysaccharides would occur in the alkaline condition. This action would make the cell membrane partially permeable which enable separation and purification of the polymers inside this bacterium.11 The use of acids (HCl and H2SO4) was considered as they acted as digester of bacterial cell walls. The NPCM and peptidoglycan of cell walls were vulnerable to acidic solution which led to the release of protein and other biological macromolecules into the aqueous solution.5 Salt would also disrupt and lyse the bacterial cell walls as the water rapidly have a function entered the cells due to the osmotic pressure condition.6 Moreover, various surfactants (Tween 20, Tween 80 and Triton-x-100) were also studied because surfactants monomer could incorporate into bilayers cell envelope and cause disruption and solubilization of NPCM.4
Aqueous solutions of NaOH, KOH, and NaOCl were found to have high potential of dissolving the NPCM of bacterial cells and to produce high PHA purity and recovery yield. NaOH was selected as a good candidate to recover copolymer P(3HB-co-3HHx) from recombinant bacterium, C. necator. The solubilisation of NPCM by NaOH was the key phase in this recovery process. Protein released was determined to ensure that the protein in the cell was completely digested (Fig. 2). Protein released reached their maximum dry biomass of 0.129 g/g at 0.10 M NaOH. Released of higher protein indicated that more NPCM were degraded, resulting in the increase of PHA purity. NaOH at the concentration of 0.10 M demonstrated the appropriate concentration for the recovery of copolymer P(3HB-co-3HHx) from bacterial cells.
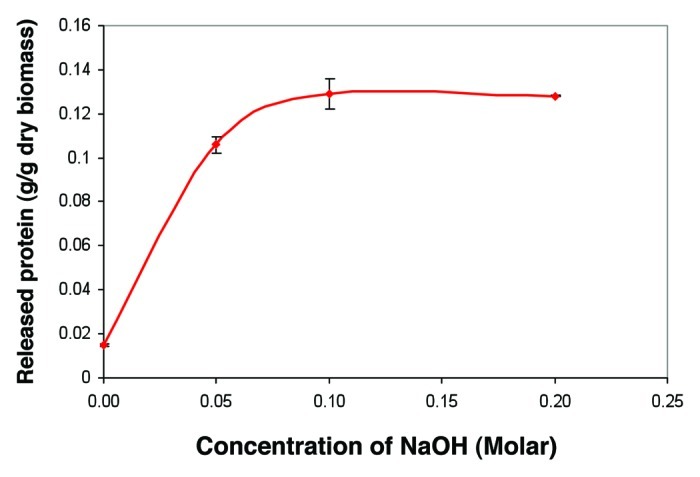
Figure 2. Effect of different concentrations of NaOH on the release of protein from bacterial cells. Values are means of three replications.
The purity of recovered polymers increased as the temperature was increased above 50°C.12 However, we had reported drastic decrease of recovery yield from 84.9 wt% to 57.7 wt% as the temperature was increased from 50°C to 80°C. It could be explained that when temperature was raised, acceleration of the chemical reaction would occur which speeded up the cell disruption and PHA degradation.4 As a result, the purity of polymer would increase but the yield of recovery might decrease. Temperature of 30°C was considered as effective in this study (particularly in tropical climate) as less energy was required (i.e., heating or chilling) during the recovery process. It is favorable to limit the incubation time as to lessen the degradation of polymer.12 Based on our study, exposure of the bacterial cells to the NaOH must be not more than 3 h in order to limit the degradation of the PHA. In addition, the recovery yields increased gradually with the increment of cell concentration from 5 g/L to 30 g/L and were relatively constants above 30 g/L. Decrease in the PHA purity with the increment of cell concentration was mainly due to the increase in the viscosity of the reaction mixture caused by the release of large amount of nucleic acids.13 The recovery yields were relatively constant because high cell concentration reduced the efficiency of NaOH digestion of NPCM.
Organic solvent such as methanol, acetone and ethanol were selected to polish the recovered polymers. Basically, these mild polar compounds or short chain alcohols would solubilize the NPCM, leaving the polymer granules remain intact in the solvent.14 Methanol could remove the cell components and more impurities except polymer while acetone was common solvent used in the mcl-PHA extraction.15 Ethanol also preferable to polish the recovered polymer as it could extract grease and lipid from the cell debris.14 Ethanol was chosen as the most suitable solvents for polishing the recovered polymer because it is environmentally benign, cost effective and can be produced by renewable source.16 However, the recovery yield of recovered polymer decreased after polishing process.
This method is applicable regardless of bacteria and types of PHA. The improvement recovery condition of the process; 10–30 g/L of cells incubated in NaOH (0.1 M) for 60–180 min at 30°C and polished using 20% (v/v) of ethanol. Under such conditions, the recovered copolymer P(3HB-co-3HHx) could reach 80 to 90 wt% of purity and recovery yield.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/bioe/article/22350
References
- 1.Chen GQ, Zhang G, Park SJ, Lee SY. Industrial scale production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) Appl Microbiol Biotechnol. 2001;57:50–5. doi: 10.1007/s002530100755. [DOI] [PubMed] [Google Scholar]
- 2.Doi Y. (1990) Microbial Polyesters New York: VCH Publishers, Inc., Yokohama, Japan. [Google Scholar]
- 3.Braunegg G, Lefebvre G, Genser KF. Polyhydroxyalkanoates, biopolyesters from renewable resources: physiological and engineering aspects. J Biotechnol. 1998;65:127–61. doi: 10.1016/S0168-1656(98)00126-6. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Chen J, Yu C, Du G, Lun S. Recovery of poly-3-hydroxybutyrate from Alcaligenes eutrophus by surfactant-chelate aqueous system. Process Biochem. 1999;34:153–7. doi: 10.1016/S0032-9592(98)00082-X. [DOI] [Google Scholar]
- 5.Yu J, Chen LXL. Cost-effective recovery and purification of polyhydroxyalkanoates by selective dissolution of cell mass. Biotechnol Prog. 2006;22:547–53. doi: 10.1021/bp050362g. [DOI] [PubMed] [Google Scholar]
- 6.Jacquel N, Lo CW, Wei YH, Wu HS, Wang SS. Isolation and purification of bacterial poly(3-hydroxyalkanoates) Biochem Eng J. 2008;39:15–27. doi: 10.1016/j.bej.2007.11.029. [DOI] [Google Scholar]
- 7.Holmes PA, Lim GB. (1990) Separation process. USA Patent No: 4910145.
- 8.Tamer IM, Moo-Young M, Chisti Y. Optimization of poly(β-hydroxybutyric acid) recovery from Alcaligenes latus: combined mechanical and chemical treatments. Bioprocess Eng. 1998;19:459–68. doi: 10.1007/PL00009030. [DOI] [Google Scholar]
- 9.Jung IL, Phyo KH, Kim KC, Park HK, Kim IG. Spontaneous liberation of intracellular polyhydroxybutyrate granules in Escherichia coli. Res Microbiol. 2005;156:865–73. doi: 10.1016/j.resmic.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 10.van Hee P, Elumbaring ACMR, van der Lans RGJM, Van der Wielen LAM. Selective recovery of polyhydroxyalkanoate inclusion bodies from fermentation broth by dissolved-air flotation. J Colloid Interface Sci. 2006;297:595–606. doi: 10.1016/j.jcis.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Yang H, Zhou Q, Chen J, Gu G. Cleaner recovery of poly(3-hydroxybutyric acid) synthesized in Alcaligenes eutrophus. Process Biochem. 2001;36:501–6. doi: 10.1016/S0032-9592(00)00225-9. [DOI] [Google Scholar]
- 12.Choi JI, Lee SY. Efficient and economical recovery of poly(3-hydroxybutyrate) from recombinant Escherichia coli by simple digestion with chemicals. Biotechnol Bioeng. 1999;62:546–53. doi: 10.1002/(SICI)1097-0290(19990305)62:5<546::AID-BIT6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Hahn SK, Ryu HW, Chang YK. Comparison and optimization of poly(3-hydroxybutyrate) recovery from Alcaligenes eutrophus and recombinant Escherichia coli. Korean Journal of Chemical Engineering. 1998;15:51–5. doi: 10.1007/BF02705305. [DOI] [Google Scholar]
- 14.Manangan T, Shawaphun S. Quantitative extraction and determination of polyhydroxyalkanoate accumulated in Alcaligenes latus dry cells. Sci Asia. 2010;36:199–203. doi: 10.2306/scienceasia1513-1874.2010.36.199. [DOI] [Google Scholar]
- 15.Jiang X, Ramsay JA, Ramsay BA. Acetone extraction of mcl-PHA from Pseudomonas putida KT2440. J Microbiol Methods. 2006;67:212–9. doi: 10.1016/j.mimet.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 16.Morris, M. & Hill, A. (2006) Ethanol Opportunities and Questions. A Publication of ATTRA - National Sustainable Agriculture Information Service.


