Abstract
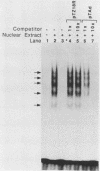
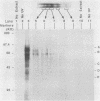
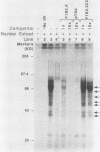
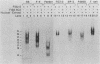
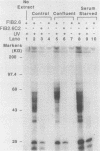
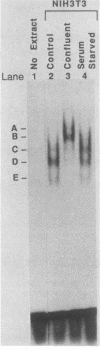
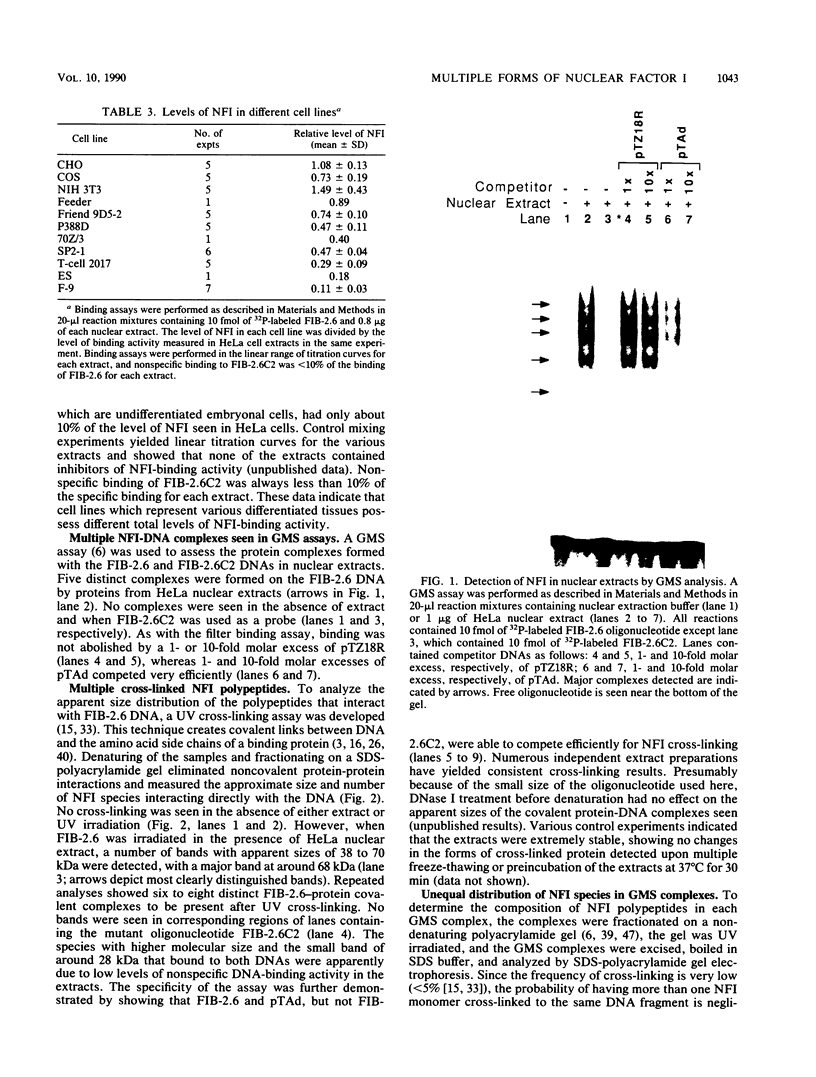
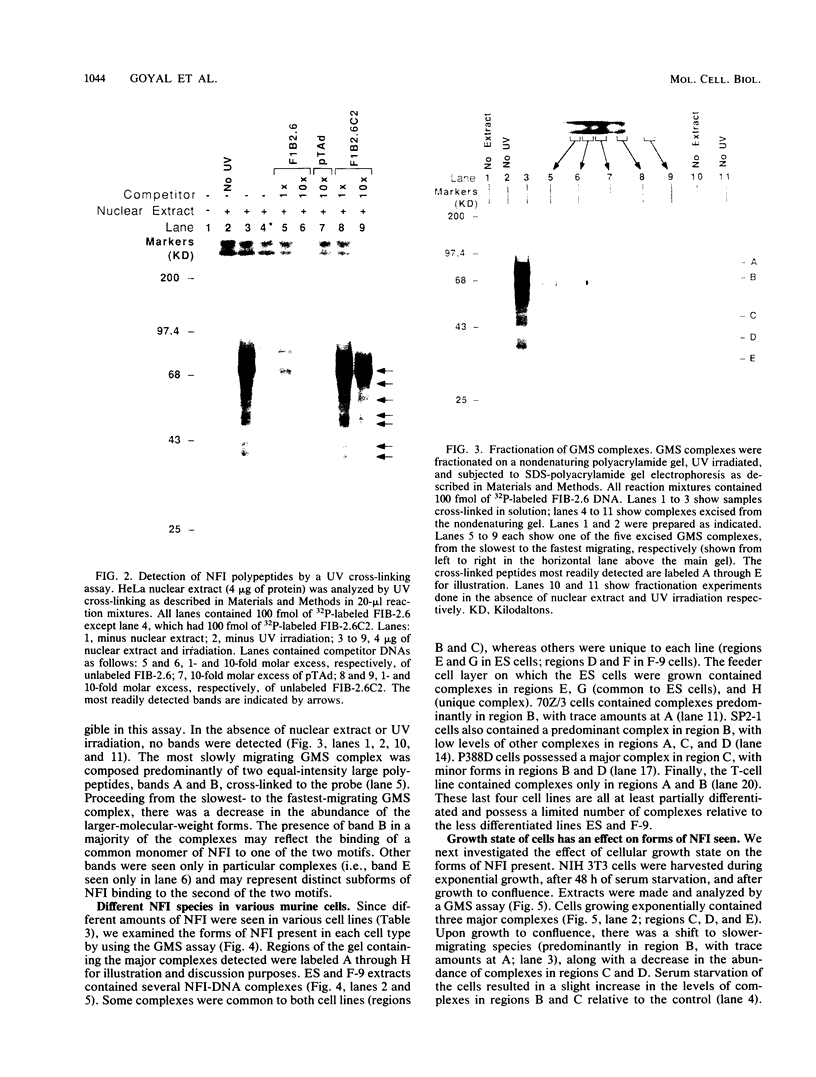
Nuclear factor I (NFI) is a group of related site-specific DNA-binding proteins that function in adenovirus DNA replication and cellular RNA metabolism. We have measured both the levels and forms of NFI that interact with a well-characterized 26-base-pair NFI-binding site. Five different NFI-DNA complexes were seen in HeLa nuclear extracts by using a gel mobility shift (GMS) assay. In addition, at least six forms of NFI were shown to cross-link directly to DNA by using a UV cross-linking assay. The distinct GMS complexes detected were composed of different subspecies of NFI polypeptides as assayed by UV cross-linking. Different murine cell lines possessed varying levels and forms of NFI binding activity, as judged by nitrocellulose filter binding and GMS assays. The growth state of NIH 3T3 cells affected both the types of NFI-DNA complexes seen in a GMS assay and the forms of the protein detected by UV cross-linking.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chodosh L. A., Baldwin A. S., Carthew R. W., Sharp P. A. Human CCAAT-binding proteins have heterologous subunits. Cell. 1988 Apr 8;53(1):11–24. doi: 10.1016/0092-8674(88)90483-7. [DOI] [PubMed] [Google Scholar]
- Cohen R. B., Yang L., Thompson J. A., Safer B. Identification of a downstream sequence and binding protein that regulate adenovirus major late promoter transcription in vitro. J Biol Chem. 1988 Jul 25;263(21):10377–10385. [PubMed] [Google Scholar]
- Dorn A., Bollekens J., Staub A., Benoist C., Mathis D. A multiplicity of CCAAT box-binding proteins. Cell. 1987 Sep 11;50(6):863–872. doi: 10.1016/0092-8674(87)90513-7. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil G., Smith J. R., Goldstein J. L., Slaughter C. A., Orth K., Brown M. S., Osborne T. F. Multiple genes encode nuclear factor 1-like proteins that bind to the promoter for 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8963–8967. doi: 10.1073/pnas.85.23.8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves B. J., Johnson P. F., McKnight S. L. Homologous recognition of a promoter domain common to the MSV LTR and the HSV tk gene. Cell. 1986 Feb 28;44(4):565–576. doi: 10.1016/0092-8674(86)90266-7. [DOI] [PubMed] [Google Scholar]
- Gronostajski R. M., Adhya S., Nagata K., Guggenheimer R. A., Hurwitz J. Site-specific DNA binding of nuclear factor I: analyses of cellular binding sites. Mol Cell Biol. 1985 May;5(5):964–971. doi: 10.1128/mcb.5.5.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronostajski R. M. Analysis of nuclear factor I binding to DNA using degenerate oligonucleotides. Nucleic Acids Res. 1986 Nov 25;14(22):9117–9132. doi: 10.1093/nar/14.22.9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronostajski R. M., Knox J., Berry D., Miyamoto N. G. Stimulation of transcription in vitro by binding sites for nuclear factor I. Nucleic Acids Res. 1988 Mar 25;16(5):2087–2098. doi: 10.1093/nar/16.5.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronostajski R. M. Site-specific DNA binding of nuclear factor I: effect of the spacer region. Nucleic Acids Res. 1987 Jul 24;15(14):5545–5559. doi: 10.1093/nar/15.14.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggenheimer R. A., Stillman B. W., Nagata K., Tamanoi F., Hurwitz J. DNA sequences required for the in vitro replication of adenovirus DNA. Proc Natl Acad Sci U S A. 1984 May;81(10):3069–3073. doi: 10.1073/pnas.81.10.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennighausen L., Fleckenstein B. Nuclear factor 1 interacts with five DNA elements in the promoter region of the human cytomegalovirus major immediate early gene. EMBO J. 1986 Jun;5(6):1367–1371. doi: 10.1002/j.1460-2075.1986.tb04368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillel Z., Wu C. W. Photochemical cross-linking studies on the interaction of Escherichia coli RNA polymerase with T7 DNA. Biochemistry. 1978 Jul 25;17(15):2954–2961. doi: 10.1021/bi00608a003. [DOI] [PubMed] [Google Scholar]
- Hockensmith J. W., Kubasek W. L., Vorachek W. R., von Hippel P. H. Laser cross-linking of nucleic acids to proteins. Methodology and first applications to the phage T4 DNA replication system. J Biol Chem. 1986 Mar 15;261(8):3512–3518. [PubMed] [Google Scholar]
- Jackson S. P., Tjian R. O-glycosylation of eukaryotic transcription factors: implications for mechanisms of transcriptional regulation. Cell. 1988 Oct 7;55(1):125–133. doi: 10.1016/0092-8674(88)90015-3. [DOI] [PubMed] [Google Scholar]
- Jeang K. T., Rawlins D. R., Rosenfeld P. J., Shero J. H., Kelly T. J., Hayward G. S. Multiple tandemly repeated binding sites for cellular nuclear factor 1 that surround the major immediate-early promoters of simian and human cytomegalovirus. J Virol. 1987 May;61(5):1559–1570. doi: 10.1128/jvi.61.5.1559-1570.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Rosenfeld P. J., Kelly T. J., Tjian R. A cellular DNA-binding protein that activates eukaryotic transcription and DNA replication. Cell. 1987 Jan 16;48(1):79–89. doi: 10.1016/0092-8674(87)90358-8. [DOI] [PubMed] [Google Scholar]
- Koren H. S., Handwerger B. S., Wunderlich J. R. Identification of macrophage-like characteristics in a cultured murine tumor line. J Immunol. 1975 Feb;114(2 Pt 2):894–897. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lichtsteiner S., Wuarin J., Schibler U. The interplay of DNA-binding proteins on the promoter of the mouse albumin gene. Cell. 1987 Dec 24;51(6):963–973. doi: 10.1016/0092-8674(87)90583-6. [DOI] [PubMed] [Google Scholar]
- Lillie J. W., Green M. R. Transcription activation by the adenovirus E1a protein. Nature. 1989 Mar 2;338(6210):39–44. doi: 10.1038/338039a0. [DOI] [PubMed] [Google Scholar]
- Martinson H. G., Shetlar M. D., McCarthy B. J. Histone-histone interactions within chromatin. Crosslinking studies using ultraviolet light. Biochemistry. 1976 May 4;15(9):2002–2007. doi: 10.1021/bi00654a030. [DOI] [PubMed] [Google Scholar]
- Meisterernst M., Rogge L., Foeckler R., Karaghiosoff M., Winnacker E. L. Structural and functional organization of a porcine gene coding for nuclear factor I. Biochemistry. 1989 Oct 3;28(20):8191–8200. doi: 10.1021/bi00446a034. [DOI] [PubMed] [Google Scholar]
- Mowat M., Cheng A., Kimura N., Bernstein A., Benchimol S. Rearrangements of the cellular p53 gene in erythroleukaemic cells transformed by Friend virus. Nature. 1985 Apr 18;314(6012):633–636. doi: 10.1038/314633a0. [DOI] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Enomoto T., Lichy J. H., Hurwitz J. Adenovirus DNA replication in vitro: identification of a host factor that stimulates synthesis of the preterminal protein-dCMP complex. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6438–6442. doi: 10.1073/pnas.79.21.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Hurwitz J. Specific binding of a cellular DNA replication protein to the origin of replication of adenovirus DNA. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6177–6181. doi: 10.1073/pnas.80.20.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige C. J., Kincade P. W., Ralph P. Independent control of immunoglobulin heavy and light chain expression in a murine pre-B-cell line. Nature. 1981 Aug 13;292(5824):631–633. doi: 10.1038/292631a0. [DOI] [PubMed] [Google Scholar]
- Paonessa G., Gounari F., Frank R., Cortese R. Purification of a NF1-like DNA-binding protein from rat liver and cloning of the corresponding cDNA. EMBO J. 1988 Oct;7(10):3115–3123. doi: 10.1002/j.1460-2075.1988.tb03178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C. S., Hillel Z., Wu C. W. DNA strand specificity in promoter recognition by RNA polymerase. Nucleic Acids Res. 1980 Dec 11;8(23):5895–5912. doi: 10.1093/nar/8.23.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins D. R., Rosenfeld P. J., Wides R. J., Challberg M. D., Kelly T. J., Jr Structure and function of the adenovirus origin of replication. Cell. 1984 May;37(1):309–319. doi: 10.1016/0092-8674(84)90327-1. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P. J., Kelly T. J. Purification of nuclear factor I by DNA recognition site affinity chromatography. J Biol Chem. 1986 Jan 25;261(3):1398–1408. [PubMed] [Google Scholar]
- Rosenfeld P. J., O'Neill E. A., Wides R. J., Kelly T. J. Sequence-specific interactions between cellular DNA-binding proteins and the adenovirus origin of DNA replication. Mol Cell Biol. 1987 Feb;7(2):875–886. doi: 10.1128/mcb.7.2.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstraus M. J. Isolation and characterization of an embryonal carcinoma cell line lacking SSEA-1 antigen. Dev Biol. 1983 Oct;99(2):318–323. doi: 10.1016/0012-1606(83)90281-6. [DOI] [PubMed] [Google Scholar]
- Rossi P., Karsenty G., Roberts A. B., Roche N. S., Sporn M. B., de Crombrugghe B. A nuclear factor 1 binding site mediates the transcriptional activation of a type I collagen promoter by transforming growth factor-beta. Cell. 1988 Feb 12;52(3):405–414. doi: 10.1016/s0092-8674(88)80033-3. [DOI] [PubMed] [Google Scholar]
- Rupp R. A., Sippel A. E. Chicken liver TGGCA protein purified by preparative mobility shift electrophoresis (PMSE) shows a 36.8 to 29.8 kd microheterogeneity. Nucleic Acids Res. 1987 Dec 10;15(23):9707–9726. doi: 10.1093/nar/15.23.9707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safer B., Cohen R. B., Garfinkel S., Thompson J. A. DNA affinity labeling of adenovirus type 2 upstream promoter sequence-binding factors identifies two distinct proteins. Mol Cell Biol. 1988 Jan;8(1):105–113. doi: 10.1128/mcb.8.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro C., Mermod N., Andrews P. C., Tjian R. A family of human CCAAT-box-binding proteins active in transcription and DNA replication: cloning and expression of multiple cDNAs. Nature. 1988 Jul 21;334(6179):218–224. doi: 10.1038/334218a0. [DOI] [PubMed] [Google Scholar]
- Shaul Y., Ben-Levy R., De-Medina T. High affinity binding site for nuclear factor I next to the hepatitis B virus S gene promoter. EMBO J. 1986 Aug;5(8):1967–1971. doi: 10.1002/j.1460-2075.1986.tb04451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Spolski R., Miescher G., Erard F., Risser R., MacDonald H. R., Mak T. W. Regulation of expression of T cell gamma chain, L3T4 and Ly-2 messages in Abelson/Moloney virus-transformed T cell lines. Eur J Immunol. 1988 Feb;18(2):295–300. doi: 10.1002/eji.1830180218. [DOI] [PubMed] [Google Scholar]
- Weber K., Platt T., Ganem D., Miller J. H. Altered sequences changing the operator-binding properties of the Lac repressor: colinearity of the repressor protein with the i-gene map. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3624–3628. doi: 10.1073/pnas.69.12.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wides R. J., Challberg M. D., Rawlins D. R., Kelly T. J. Adenovirus origin of DNA replication: sequence requirements for replication in vitro. Mol Cell Biol. 1987 Feb;7(2):864–874. doi: 10.1128/mcb.7.2.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Wilson S., Walker B., Dawid I., Paisley T., Zimarino V., Ueda H. Purification and properties of Drosophila heat shock activator protein. Science. 1987 Nov 27;238(4831):1247–1253. doi: 10.1126/science.3685975. [DOI] [PubMed] [Google Scholar]
- de Vries E., van Driel W., van den Heuvel S. J., van der Vliet P. C. Contactpoint analysis of the HeLa nuclear factor I recognition site reveals symmetrical binding at one side of the DNA helix. EMBO J. 1987 Jan;6(1):161–168. doi: 10.1002/j.1460-2075.1987.tb04734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]