Abstract
Biomolecular environments encountered in vivo are complex and dynamic, with combinations of biomolecules presented in both freely diffusible (liquid-phase) and sequestered (bound to the extracellular matrix) states. Strategies for integrating multiple biomolecular signals into a biomimetic scaffold provide a platform to simultaneously control multiple cell activities, such as motility, proliferation, phenotype, and regenerative potential. Here we describe an investigation elucidating the influence of the dose and mode of presentation (soluble, sequestered) of five biomolecules (stromal cell-derived factor 1α [SDF-1α], platelet-derived growth factor BB [PDGF-BB], insulin-like growth factor 1 [IGF-1], basic fibroblast growth factor [bFGF], and growth/differentiation factor 5 [GDF-5]) on the recruitment, proliferation, collagen synthesis, and genomic stability of equine tenocytes within an anisotropic collagen-GAG scaffold for tendon regeneration applications. Critically, we found that single factors led to a dose-dependent trade-off between driving tenocyte proliferation (PDGF-BB, IGF-1) versus maintenance of a tenocyte phenotype (GDF-5, bFGF). We identified supplementation schemes using factor pairs (IGF-1, GDF-5) to rescue the tenocyte phenotype and gene expression profiles while simultaneously driving proliferation. These results suggest coincident application of multi-biomolecule cocktails has a significant value in regenerative medicine applications where control of cell proliferation and phenotype are required. Finally, we demonstrated an immobilization strategy that allows efficient sequestration of bioactive levels of these factors within the scaffold network. We showed sequestration can lead to a greater sustained bioactivity than soluble supplementation, making this approach particularly amenable to in vivo translation where diffusive loss is a concern and continuous biomolecule supplementation is not feasible.
Introduction
Tendons are highly organized connective tissues composed of a hierarchy of collagen fiber bundles that join muscle to bone. Tendons can be injured through a variety of acute and chronic modes1–4 with some 35 million injuries occurring annually in the United States.5 Of these injuries, severe tendon traumas account for more than 1 million physician visits and 250,000 surgical procedures annually in the United States.6 While tendon homeostasis is maintained by fibroblast-like cells called tenocytes interspersed within collagen fibrils, these cells have limited intrinsic regenerative capacity. As a result, tendon defects undergo a repair-mediated wound-healing process that results in the formation of a mechanically and histomorphologically inferior extracellular matrix (ECM).2–4 The current clinical gold standard for many types of ligament and tendon repair is an autograft, often taken from the middle section of the patellar ligament or a piece of hamstring tendon. Like all autograft procedures, this approach is not ideal due to the creation of a secondary injury site,7 necessitating the development of novel treatments to drive functional regeneration.
Biomaterials such as tissue engineering scaffolds may offer a pathway to functional regeneration of tendon injuries, including rotator cuff lesions8 as well as quadriceps, patellar, and Achilles tendon ruptures. Collagen–glycosaminoglycan (CG) scaffolds are regulatory compliant ECM analogs that have been applied to a wide range of regenerative medicine applications, both in vivo for soft tissue applications such as dermis, peripheral nerves, conjunctiva, brain, and cartilage9–12 as well as in vitro as three-dimensional (3D) templates to probe various cellular activities, including adhesion, proliferation, migration, and differentiation.13–17 CG scaffolds have also been adapted for a variety of orthopedic applications covering osteochondral, bone, and tendon tissue engineering.18–21 We have recently demonstrated a directional freeze-drying method to create geometrically anisotropic CG scaffolds with aligned tracks of ellipsoidal pores that mimic elements of native tendon anisotropy.21 While we showed that the platelet-derived growth factor BB (PDGF-BB) and the insulin-like growth factor 1 (IGF-1) promoted increased tenocyte proliferation and migration within these anisotropic scaffolds,21 a major concern remains the trade-off between increased proliferation and retention of the tenocyte-associated phenotype.22
Soluble factor supplementation is a widely applied scaffold enhancement strategy and has shown considerable functional value for tendon tissue engineering.23,24 PDGF-BB and IGF-1 have previously been shown to promote dose-dependent increases in tenocyte proliferation and collagen synthesis, both individually and in concert.21,22,25–27 Additionally, both factors have been noted as chemoattractants of tenocytes21 as well as mesenchymal stem cells (MSCs).28–30 This study aimed to assess the individual and combined effects of a wider range of soluble factors on cell viability, protein synthesis, and gene expression in the context of tendon tissue engineering. While largely unexplored, optimal supplementation strategies should not only support increased cell migration and proliferation, but also matrix protein synthesis and upregulation of tenocyte phenotypic markers. Along with PDGF-BB and IGF-1, we investigated three additional factors: the basic fibroblast growth factor (bFGF), the stromal cell-derived factor 1α (SDF-1α), and the growth/differentiation factor 5 (GDF-5). The growth factor bFGF has shown the capacity to improve tenocyte proliferation and collagen synthesis,22 MSC chemotaxis,31 and the tenogenic differentiation of MSCs both in two-dimensional (2D)32 and 3D33 culture systems. SDF-1α has been implicated as a potent chemoattractant of MSCs during wound healing,29,34 but recently has shown the ability to improve ligament stem cell proliferation and migration35 as well as functional tendon regeneration in a rat Achilles model.36 GDF-5 has been shown to increase both tenocyte and adipose-derived stem cell proliferation and expression of tendon markers37–39 as well as the formation of neo-tendinous tissue in a rat ectopic model.40 Together, these factors have been implicated in tenocyte recruitment, proliferation, and functional action, although their individual (dose-dependent) and combinatorial effects on tenocyte genomic stability and phenotype within an engineered scaffold remain largely unexplored. Additionally, many growth factors are sequestered, as opposed to freely soluble, in the native ECM.41 Therefore, while important to probe dose effects of soluble factors on cell activity, it is important to consider strategies to immobilize and pattern proteins, such as growth factors and other cytokines, to develop long-term tissue engineering solutions.32,42–45 Factor immobilization may offer numerous advantages, including improved protein stability and reduced diffusion, increasing the localization of the therapeutic effects within the material.42
This manuscript describes the evaluation of the effects of five biomolecules (PDGF-BB, IGF-1, bFGF, SDF-1α, and GDF-5) on tenocyte chemotaxis, proliferation, soluble collagen synthesis, and gene expression within anisotropic CG scaffolds. While previous studies have evaluated the roles of one or several of these factors, often in 2D culture settings, the comprehensive evaluation of the dose-dependent, combinatorial, and matrix-bound versus soluble effects of these factors on the tenocyte phenotype and proliferative potential within a 3D anisotropic scaffold has not been undertaken.
Materials and Methods
CG suspension preparation
A 1.5 w/v% CG suspension was prepared by homogenizing type I microfibrillar collagen from bovine tendon (Sigma-Aldrich) and chondroitin sulfate from shark cartilage (Sigma-Aldrich) in 0.05 M acetic acid.9,21,46
Anisotropic CG scaffold fabrication via freeze-drying
Scaffolds were fabricated via directional solidification as previously described.21 Briefly, degassed CG suspension was added to a polytetrafluoroethylene (PTFE)-copper mold and placed on a precooled (−10°C) freeze-dryer shelf (VirTis). The CG suspension was frozen at −10°C for 2 h, and then sublimated at 0°C and 200mTorr to remove ice crystals. The thermal conductivity mismatch between copper and PTFE promoted unidirectional heat transfer, resulting in a dry, highly porous CG scaffold with aligned tracks of ellipsoidal pores.21 The anisotropic scaffold variant used throughout these studies was previously shown to support a high tenocyte bioactivity, while resisting a tenocyte-mediated contraction.21,47
Scaffold crosslinking
Dry scaffolds were dehydrothermally crosslinked at 105°C for 24 h under vacuum (<25 torr) in a vacuum oven (Welch) following lyophilization. Chemical crosslinking of scaffolds was achieved using carbodiimide chemistry with a solution of 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC; Sigma-Aldrich) and N-hydroxysulfosuccinimide (NHS; Sigma-Aldrich) at a molar ratio of 5:2:1 EDC:NHS:COOH48,49 for 1 h at 37°C.
Tenocyte isolation and culture
Equine tenocytes were isolated via collagenase II digestion of superficial digital flexor tendons from horses aged 2–3 years euthanized for reasons not related to tendon injury using a method approved by the University of Illinois IACUC and previously described in significant detail.50 Tenocytes were cultured in a high-glucose Dulbecco's modified Eagle's medium (DMEM; Fisher) as previously described.50 Cells were fed every 3 days and cultured to confluence at 37°C and 5% CO2. Cells were used at passage 4.
Tenocyte chemotaxis assay
Tenocyte chemotaxis into anisotropic CG scaffolds was analyzed with a modified Transwell membrane experiment.21 Unseeded scaffolds were placed in 24-well plates underneath polycarbonate Transwell membrane inserts (8-μm pore size, 6.5 mm diameter; Fisher Scientific). Scaffolds were cut so that they were in direct contact with the membrane above without being compressed. Serum-free media supplemented with an individual dose of one of the five soluble factors assayed (Table 1) was placed in the lower chamber with the CG scaffold. 5×105 tenocytes were then added to the top side of the Transwell membrane in the serum-free, nonsupplemented DMEM. Directed tenocyte migration through the membrane and into the scaffold was assayed after 24 h by removing the scaffolds and quantifying the amount of DNA to determine the total number of tenocytes in the scaffold.21
Table 1.
Soluble Factor Doses (ng/mL) Used for Tenocyte Chemotaxis, Proliferation, Soluble Collagen Synthesis, and Gene Expression Experiments
| |
Soluble factor dose (ng/mL) |
|
||
|---|---|---|---|---|
| Soluble factor | Low | Medium | High | References |
| PDGF-BB | 10 | 50 | 100 | 22,25,27 |
| IGF-1 | 10 | 50 | 200 | 26,27 |
| bFGF | 0.1 | 5 | 10 | 22,27 |
| SDF-1α | 10 | 50 | 200 | 35 |
| GDF-5 | 10 | 100 | 500 | 37–39 |
Soluble factor doses were informed by the literature and selected based on preliminary screening experiments that assayed at least six dose levels per factor.
PDGF-BB, platelet-derived growth factor BB; IGF-1, insulin-like growth factor 1; bFGF, basic fibroblast growth factor; SDF-1α, stromal cell-derived factor 1α; GDF-5, growth/differentiation factor 5.
Scaffold culture conditions
Geometrically anisotropic CG scaffold cylinders (6.5 mm diameter, ∼5 mm thickness) were set in ultra-low attachment six-well plates (Corning Life Sciences).21 Confluent tenocytes were trypsinized and resuspended at a concentration of 5×105 cells per 20 μL media. 10 μL of cell suspension (2.5×105 cells) was added to each side of the scaffold (5×105 cells/scaffold) using a previously described static seeding method.51 Scaffolds were cultured in serum-free media to eliminate the influence of exogenous serum components with the exception of the positive control (10% fetal bovine serum). Rat recombinant PDGF-BB and human recombinant SDF-1α were purchased from R&D Systems. Human recombinant IGF-1, bFGF, and GDF-5 were acquired from ProSpec. All soluble factors were reconstituted in the manufacturer's recommended solutions, and then diluted to experimental concentrations (Table 1) in serum-free media. Soluble factor doses were selected based on the literature22,25–27,35,37–39 and on preliminary screening experiments that assayed at least six dose levels per factor (data not shown). Immobilization experiments were carried out in serum-free, nonsupplemented media. Scaffolds were incubated at 37°C and 5% CO2 and fed every 3 days.
Protein immobilization
Scaffolds used for immobilization studies were crosslinked using the same 5:1 EDC:COOH ratio as before, but with the NHS amount increased to a mass ratio of 1:2.5 EDC:NHS to promote more efficient immobilization.42 Following 30-min incubation in the crosslinking solution, 10 μL of protein solution was added directly to the top of the scaffold (6.5 mm diameter, ∼5 mm thickness). Scaffolds were then incubated at 37°C for 15 min and turned over. An additional 10 μL of protein solution was added to the scaffold followed by a 45-min incubation period. All scaffolds were then rinsed twice in phosphate-buffered saline (PBS) under shaking for 1.5 h and stored in fresh PBS at 4°C until use.
Protein immobilization efficiency studies were first performed with a bovine serum albumin Alexa Fluor® 594 conjugate (BSA594; Invitrogen) and PDGF-BB (R&D Systems). Scaffolds were digested in a papain buffer at 60°C for 2 h to enable direct measurement of protein immobilization levels from scaffold digest solutions. BSA594 immobilization was determined by reading scaffold digest fluorescence (excitation: 560 nm, emission: 635 nm) on a fluorescent spectrophotometer (Tecan) and interpolating the results on a standard curve created with BSA594 that underwent identical thermal and enzymatic treatments.52 Additionally, PDGF-BB immobilization was subsequently quantified with an ELISA kit (R&D Systems).
Mechanical testing
Tensile testing was performed on scaffolds (6 mm diameter, 20-mm gauge length) crosslinked with two different EDC:NHS:COOH ratios (standard 5:2:1 and an increased NHS content for protein immobilization).20 Scaffolds were pulled to failure at a rate of 1 mm min−1 using an MTS Insight electromechanical load frame. The elastic modulus was calculated from the slope of the stress–strain curve over a strain range of 5%–10%.20,53 Ultimate tensile strength was determined from the peak stress value on the stress–strain curve.
Tenocyte number quantification
The number of tenocytes in each scaffold specimen was determined using a DNA quantification assay.21 Scaffolds were rinsed in PBS to remove any dead or unattached cells, and then digested in the papain buffer solution at 60°C for 24 h. DNA was fluorescently labeled with the Hoechst 33258 dye (Invitrogen).54 Fluorescence was read (excitation: 360 nm, emission: 465 nm) on a fluorescent spectrophotometer (Tecan).
Tenocyte metabolic activity quantification
The mitochondrial metabolic activity of tenocytes seeded within scaffolds was evaluated via the alamarBlue® assay.21 Scaffolds were incubated in the alamarBlue solution (Invitrogen) with gentle shaking for 2 h.55 Viable cells reduce resazurin in the alamarBlue solution to the fluorescent by-product resorufin. Resorufin fluorescence was measured (excitation: 540 nm, emission: 580 nm) on a fluorescent spectrophotometer (Tecan).
Tenocyte soluble collagen synthesis quantification
The total amount of soluble collagen synthesized by tenocytes within scaffolds and released into culture media was quantified using the Sircol soluble collagen kit (Biocolor Ltd.).33 Total soluble collagen synthesis over the 7-day duration of the culture experiments was measured by pooling media samples from each feeding time point. Collagen was labeled with the Sirius Red dye for 30 min under shaking. Solution absorbance was measured at 555 nm; results were interpolated on the standard curve created using a 0.5 mg/mL bovine collagen solution.
RNA isolation
Scaffolds were rinsed in the PBS to remove dead/unattached cells, and then immersed in the lysis buffer supplemented with 10 μM β-mercaptoethanol (Sigma-Aldrich) for 5 min on ice. Following lysis, RNA was extracted from scaffolds with an RNeasy Plant Mini kit (Qiagen).15 RNA was quantified via spectrophotometry.
Reverse transcription and real-time PCR
Reverse transcription and real-time PCR were performed on tenocytes postculture using the QuantiTect Reverse Transcription and SYBR Green PCR kits, respectively, (Qiagen) as previously described.47 RNA was reverse transcribed to cDNA using a Bio-Rad S1000 thermal cycler. Real-time PCR reactions were performed in triplicate with 10 ng of cDNA per reaction in an Applied Biosystems 7900HT Fast Real-Time PCR System. Expression profiles of the following genes were evaluated: collagen type I alpha 2 (COL1A2), collagen type III alpha I (COL3A1), cartilage oligomeric matrix protein (COMP), decorin (DCN), scleraxis (SCXB), and tenascin-C (TNC). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene. Primer sequences were taken from literature56 and synthesized by Integrated DNA Technologies. Data were analyzed using Sequence Detection Systems software v2.4 (Applied Biosystems). Results were generated using the delta–delta Ct method and all results were expressed as fold changes normalized to the expression levels of tenocytes cultured in nonsupplemented control scaffolds.
Statistical analysis
One-way analysis of variance was performed on the tenocyte number, metabolic activity, soluble collagen, and gene expression data sets followed by the Tukey-HSD post hoc tests. Significance was set at p<0.05. Chemotaxis experiments used n=6 scaffolds per group, while the 7-day culture experiments used n=9 scaffolds per group. The tenocyte metabolic activity was evaluated for all nine scaffolds at days 1, 4, and 7. At day 7, n=6 scaffolds were used to evaluate tenocyte number, while n=3 scaffolds were applied to gene expression analyses. Soluble collagen synthesis was quantified for n=3 media samples per experimental group. Error is reported in figures as the standard error of the mean unless otherwise noted.
Results
Tenocyte chemotaxis
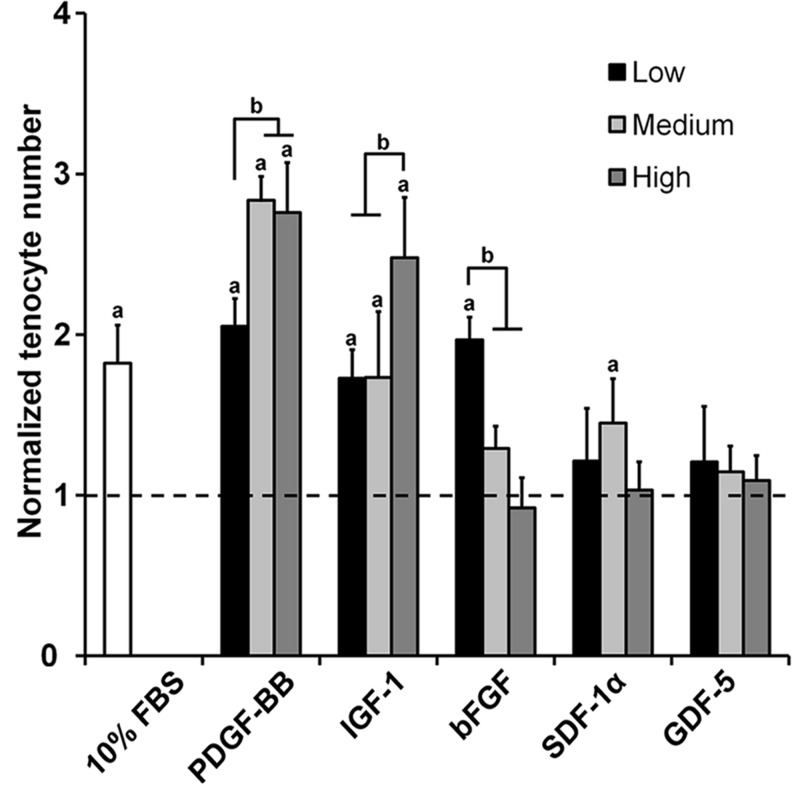
Tenocyte chemotaxis toward soluble factor gradients into anisotropic CG scaffolds was evaluated after a 24-h migration period (Fig. 1). PDGF-BB and IGF-1 induced significant increases in tenocyte migration compared to the nonsupplemented, serum-free media control for all dose levels (p<0.002). Dose-dependent trends were observed with the higher PDGF-BB doses as well as the highest IGF-1 dose (p<0.0007). While the low dose of bFGF induced a significant increase in tenocyte chemotaxis (p<0.0001), chemotaxis for the higher doses decreased significantly in a dose-dependent manner (p<0.001). No significant differences were observed between the control group and the SDF-1α/GDF-5 groups except for the medium dose of SDF-1α (p<0.05).
FIG. 1.
Tenocyte migration into anisotropic CG scaffolds in response to soluble factor gradients. Tenocyte chemotaxis over 24 h was measured for three different doses of PDGF-BB, IGF-1, SDF-1α, bFGF, and GDF-5 (n=6). Black dashed line: level of tenocyte chemotaxis for nonsupplemented media control. aSignificant increase compared to nonsupplemented media control. bDose-dependent increase. CG, collagen–glycosaminoglycan; FBS, fetal bovine serum; PDGF-BB, platelet-derived growth factor BB; IGF-1, insulin-like growth factor 1; bFGF, basic fibroblast growth factor; SDF-1α, stromal cell-derived factor 1α; GDF-5, growth/differentiation factor 5.
Tenocyte proliferation, metabolic activity, and soluble collagen synthesis
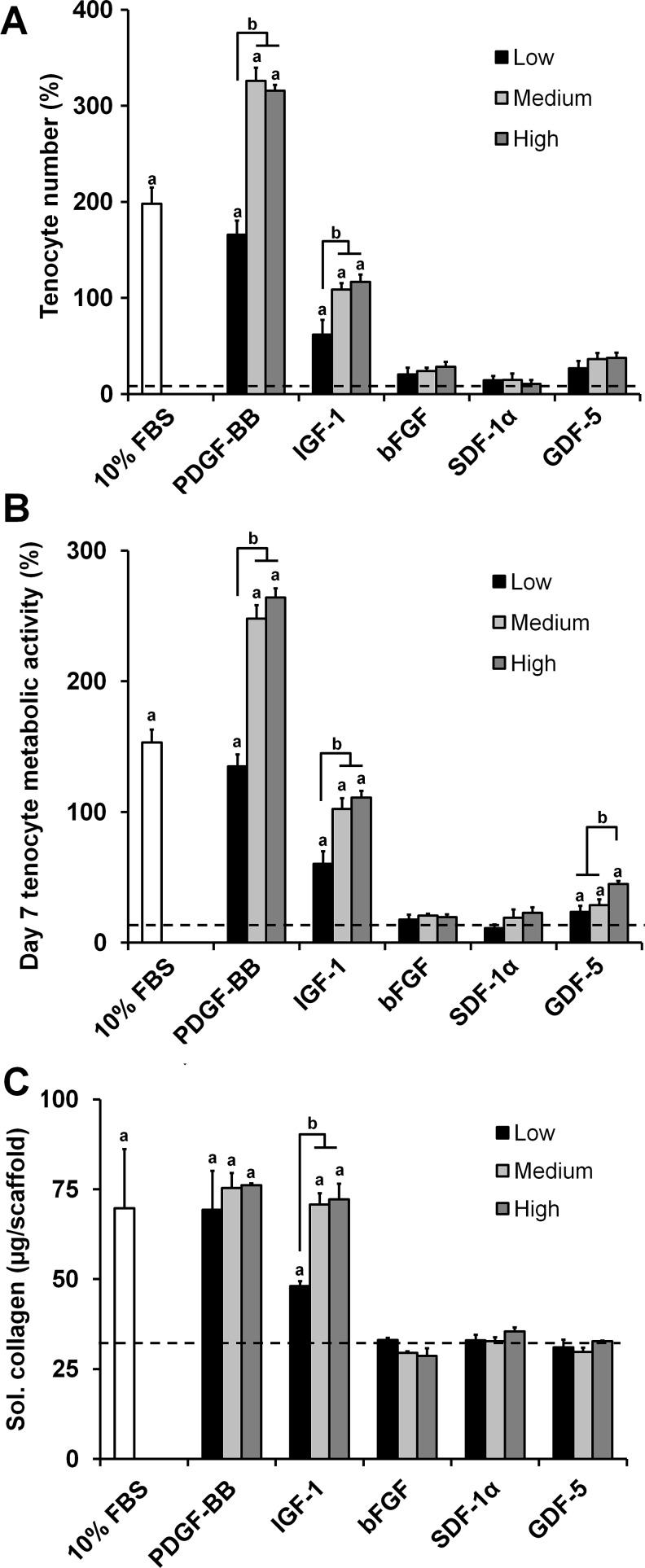
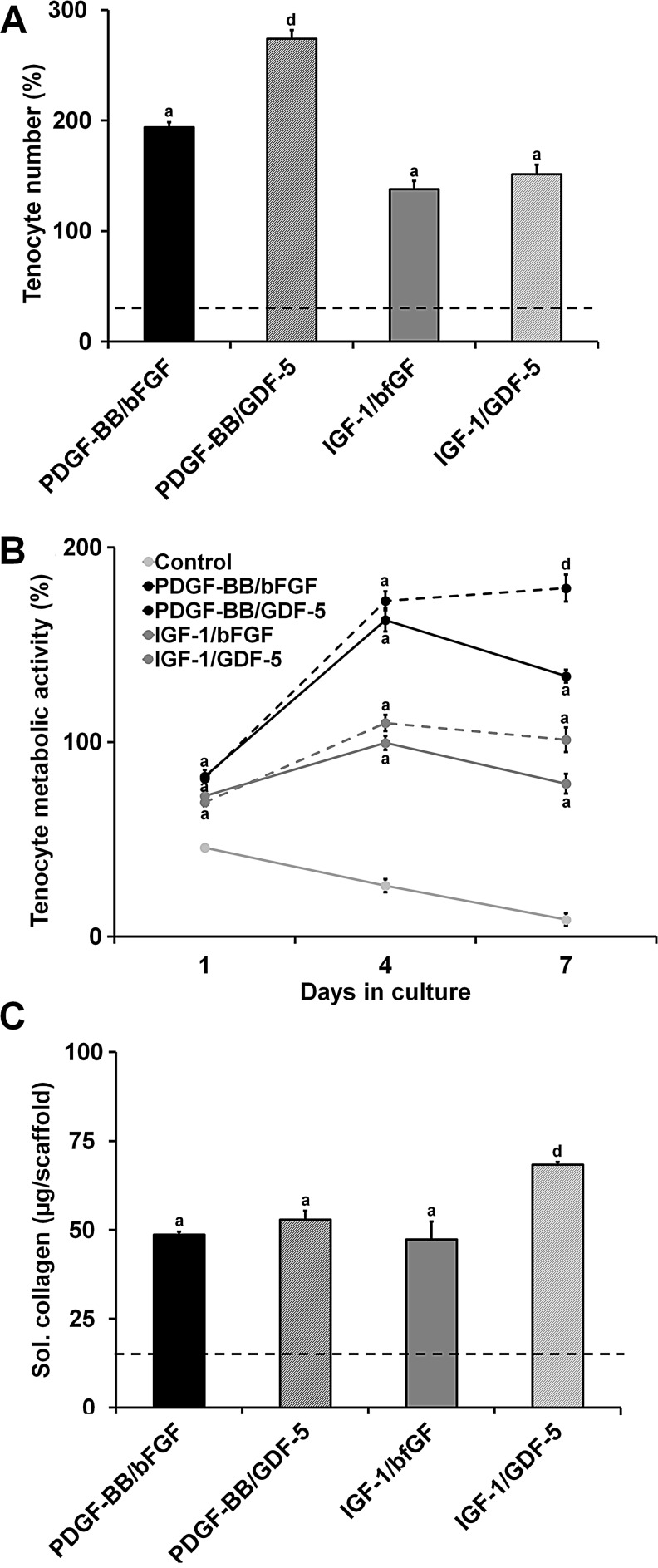
Tenocyte number, metabolic activity, and soluble collagen synthesis were evaluated following a 7-day in vitro culture period in media containing the same soluble factor doses as the chemotaxis experiment (Fig. 2). We chose to assess both number and metabolic activity to ensure that tenocytes were proliferating, while remaining healthy and active. The tenocyte number and metabolic activity results largely followed the trends established in the chemotaxis results with the PDGF-BB and IGF-1 groups eliciting the greatest response. The PDGF-BB and IGF-1 groups showed dose-dependent significant increases in the tenocyte number (p<0.0001) and metabolic activity (p<0.0001) (Fig. 2A, B). There were no significant differences in the tenocyte number between the negative control and the bFGF, SDF-1α, and GDF-5 groups (Fig. 2A). However, the GDF-5 experimental groups showed a significantly greater metabolic activity than the negative control (p<0.04) with the high-dose group having a significantly higher metabolic activity than the other two doses (p<0.02) (Fig. 2B).
FIG. 2.
Tenocyte viability and collagen synthesis in anisotropic CG scaffolds in response to soluble factor supplementation. Tenocyte (A) number (n=6), (B) metabolic activity (n=9), and (C) soluble collagen synthesis (n=3) after 7 days culture in anisotropic CG scaffolds were measured for three different doses of PDGF-BB, IGF-1, SDF-1α, bFGF, and GDF-5. Black dashed line: level of nonsupplemented media control. aSignificant increase compared to nonsupplemented media control. bDose-dependent increase.
Cumulative soluble collagen synthesis results over the 7-day culture period showed that PDGF-BB and IGF-1 supplemented groups exhibited the greatest total soluble collagen synthesis (Fig. 2C). However, these increases were not as pronounced as those observed for the cell number and metabolic activity results, suggesting a significantly decreased collagen synthesis on a per cell basis. PDGF-BB and IGF-1 supported significant increases over the control for all dose levels (p<0.003). There was no dose dependence observed for the PDGF-BB groups; however, the low dose of IGF-1 supported a significantly lower collagen synthesis than the medium and high doses (p<0.0003). No significant differences were observed between the control and the bFGF, SDF-1α, and GDF-5 groups.
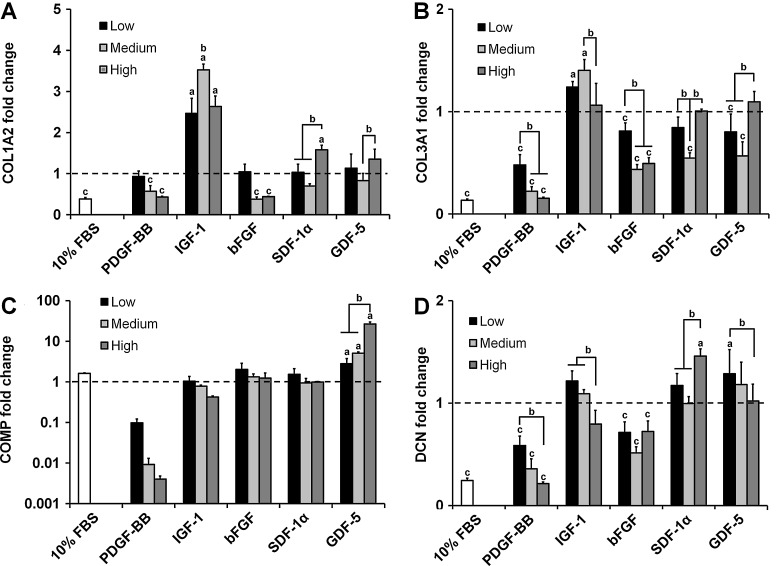
Structural protein gene expression
The expression levels of tendon-associated structural protein genes COL1A2, COL3A1, COMP, and DCN were evaluated at the end of the 7-day culture time (Fig. 3). COL1A2 expression was significantly decreased in a dose-dependent manner with PDGF-BB supplementation (p<0.03), while expression in the IGF-1 groups was significantly upregulated (p<0.0001) (Fig. 3A). Expression in the higher doses of bFGF was significantly downregulated (p<0.006), while the high dose for both the SDF-1α and GDF-5 groups was significantly higher than their respective lower doses (p<0.03).
FIG. 3.
Expression levels for tenocyte structural protein genes within anisotropic CG scaffolds. Expression of (A) COL1A2, (B) COL3A1, (C) COMP, and (D) DCN after 7 days culture in anisotropic CG scaffolds was measured for three different doses of PDGF-BB, IGF-1, SDF-1α, bFGF, and GDF-5 (n=3). Black dashed line: expression level of nonsupplemented media control. aSignificant upregulation compared to nonsupplemented media control. bDose-dependent increase. cSignificant downregulation compared to nonsupplemented media control. COL1A2, collagen type I alpha 2; COL3A1, collagen type III alpha I; COMP, cartilage oligomeric matrix protein; DCN, decorin.
COL3A1 expression profiles followed similar trends to COL1A2 (Fig. 3B). PDGF-BB exhibited dose-dependent downregulation (p<0.0001), while the low and medium IGF-1 groups were significantly upregulated (p<0.02) with peak levels at the medium dose. Downregulation was observed for all three bFGF groups (p<0.05) with the medium and high doses displaying a significantly lower expression than the low dose (p<0.002). As with COL1A2, the highest expression levels for the SDF-1α and GDF-5 groups were achieved for the high dose.
COMP expression decreased in a dose-dependent fashion for all soluble factors tested except GDF-5 (Fig. 3C). In the case of PDGF-BB, COMP was drastically downregulated (∼10-, 100-, and 250-fold for the low, medium, and high doses, respectively). Conversely, COMP expression in the GDF-5 groups increased significantly with dose (p<0.05).
The PDGF-BB supplemented groups again showed dose-dependent downregulation of the DCN gene (p<0.002). DCN was also downregulated for all bFGF groups (p<0.02). DCN expression remained relatively unchanged for the IGF-1, SDF-1α, and GDF-5 groups with only the high SDF-1α and low GDF-5 doses showing significant differences compared to the nonsupplemented control (p<0.02).
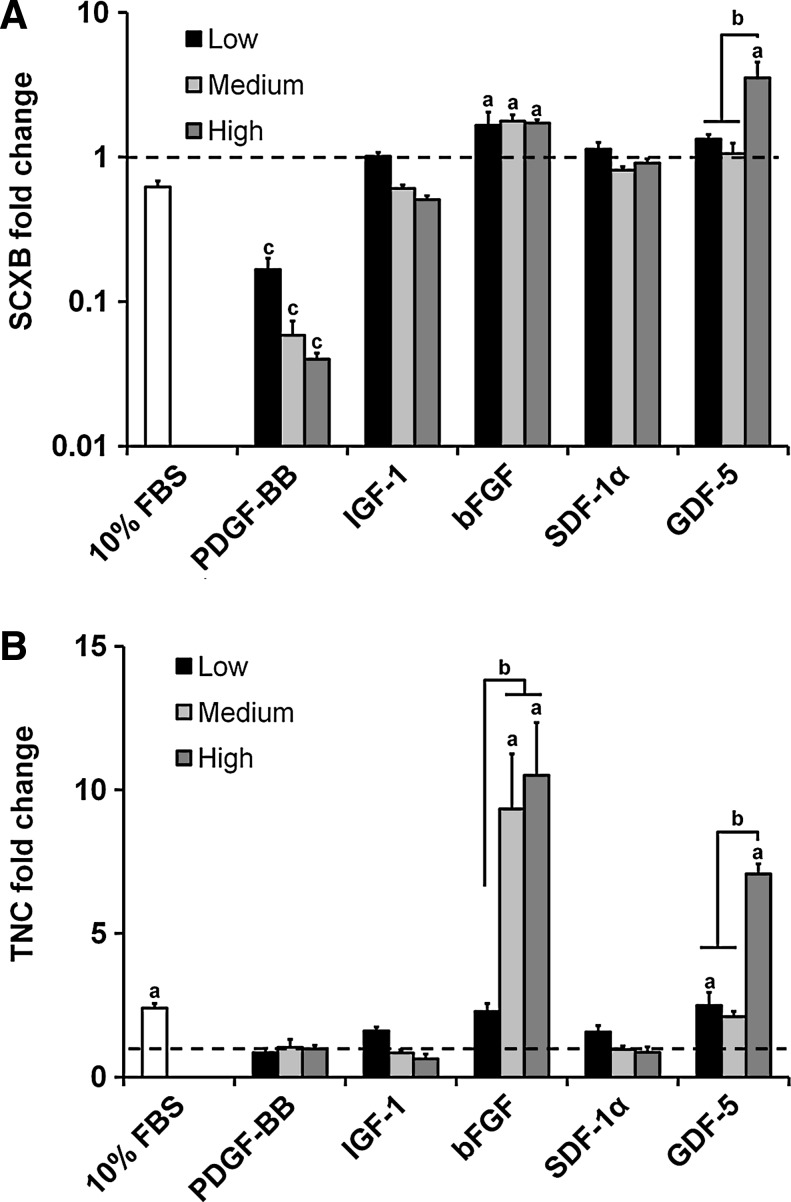
Tendon phenotype gene expression
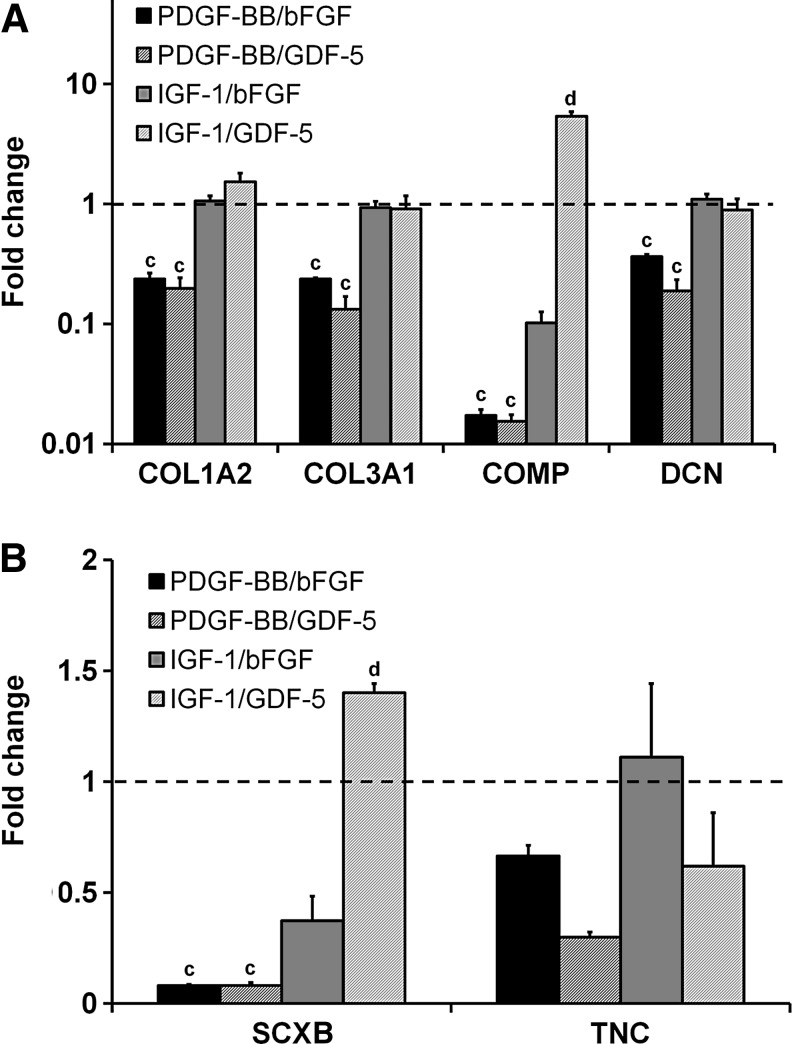
As with the structural protein genes, PDGF-BB induced dose-dependent downregulation of SCXB (p<0.004) (Fig. 4A). SCXB expression was upregulated for all three doses of bFGF (p<0.02) as well as the high dose of GDF-5 (p<0.0001). There were no significant differences between the nonsupplemented control and the IGF-1/SDF-1α experimental groups.
FIG. 4.
Expression levels for tenocyte phenotype genes within anisotropic CG scaffolds. Expression of (A) SCXB and (B) TNC after 7 days culture in anisotropic CG scaffolds was measured for three different doses of PDGF-BB, IGF-1, SDF-1α, bFGF, and GDF-5 (n=3). Black dashed line: expression level of nonsupplemented media control. aSignificant upregulation compared to nonsupplemented media control. bDose-dependent increase. cSignificant downregulation compared to nonsupplemented media control. SCXB, scleraxis; TNC, tenascin-C.
TNC expression stayed near the baseline for the PDGF-BB, IGF-1, and SDF-1α groups (Fig. 4B). TNC was upregulated for the bFGF (medium and high doses, p<0.0001) and the GDF-5 (low and high doses, p<0.04) groups. High doses for both bFGF and GDF-5 elicited a significantly higher expression of TNC compared to their respective low doses (p<0.0001).
Soluble factor pairs: tenocyte proliferation, metabolic activity, and soluble collagen synthesis
The synergistic effects of pairing PDGF-BB or IGF-1 with bFGF or GDF-5 were then evaluated over a 7-day culture period in an attempt to simultaneously drive proliferation and phenotypic stability. The medium-dose level for all factors was used (PDGF-BB: 50 ng/mL, IGF-1: 50 ng/mL, and bFGF: 5 ng/mL) except for GDF-5 (high dose, 500 ng/mL). The tenocyte number after 7 days was significantly greater for all four pairings tested compared to the nonsupplemented control (p<0.0001) (Fig. 5A). Additionally, the pairings with PDGF-BB had a significantly higher cell number than the IGF-1 pairings (p<0.004) with the PDGF-BB/GDF-5 pair showing the greatest tenocyte proliferation of all the groups (p<0.0001). The tenocyte metabolic activity showed similar trends to the cell number results (Fig. 5B). All four soluble factor pairings demonstrated a significantly greater metabolic activity than the control at all time points (days 1, 4, and 7; p<0.0001). As with the cell number, the PDGF-BB groups showed a significantly higher metabolic activity than the IGF-1 groups at all points (p<0.02). By day 7, the pairings with GDF-5 had a higher metabolic activity than their bFGF counterparts (p<0.006) with the PDGF-BB/GDF-5 pairing eliciting the greatest overall response (p<0.0001).
FIG. 5.
Influence of soluble factor combinations on tenocyte viability and collagen synthesis. Tenocyte (A) number (n=6), (B) metabolic activity (n=9), and (C) soluble collagen synthesis (n=3) after 7 days culture in anisotropic CG scaffolds were measured for four different soluble factor pairs: PDGF-BB/bFGF, PDGF-BB/GDF-5, IGF-1/bFGF, and IGF-1/GDF-5. Black dashed line: level of nonsupplemented media control. aSignificant increase compared to nonsupplemented media control. dSignificantly greater than all other experimental groups.
Soluble collagen synthesis over the course of the 7-day experiment was significantly increased for all four soluble factor pairings compared to the control (p<0.003) (Fig. 5C). Despite supporting less proliferation than the PDGF-BB pairings, the IGF-1/GDF-5 pairing induced a significantly greater amount of soluble collagen synthesis than all of the other experimental groups (p<0.03).
Soluble factor pairs: tenocyte gene expression
Neither bFGF nor GDF-5 was able to rescue the tenocyte phenotype when paired with PDGF-BB as expression of COL1A2, COL3A1, COMP, DCN, and TNC was significantly downregulated (p<0.05) (Fig. 6). Both IGF-1 supplemented groups showed no significant differences with the nonsupplemented control for expression of COL1A2, COL3A1, DCN, and TNC. However, the IGF-1/GDF-5 pairing elicited a nonsignificant increase in COL1A2 expression as well as significant increases in COMP and SCXB expression (p<0.04).
FIG. 6.
Influence of soluble factor combinations on tenocyte gene expression. Tenocyte expression of (A) structural protein genes (COL1A2, COL3A1, COMP, and DCN) and (B) phenotype genes (SCXB and TNC) were measured for four different soluble factor pairs: PDGF-BB/bFGF, PDGF-BB/GDF-5, IGF-1/bFGF, and IGF-1/GDF-5 after 7 days culture in anisotropic CG scaffolds (n=3). Black dashed line: expression level of nonsupplemented media control. cSignificant downregulation compared to nonsupplemented media control. Significantly greater than all other experimental groups.
Soluble factor immobilization validation
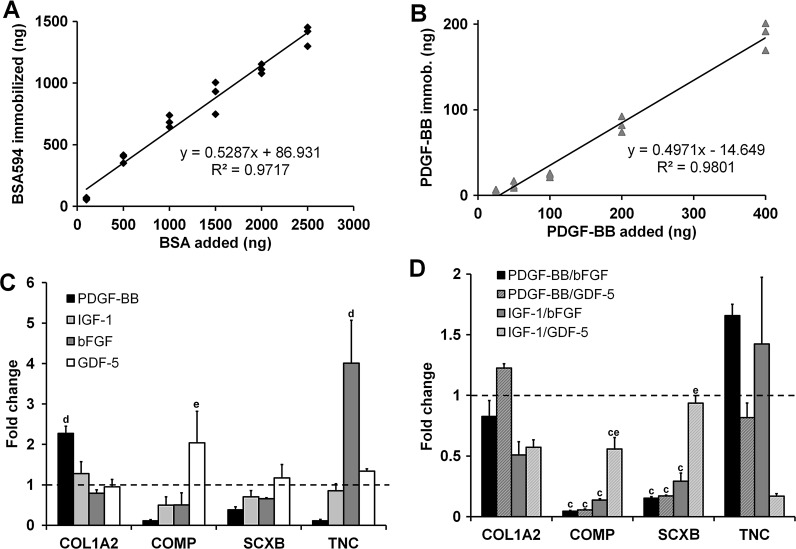
Efficient protein immobilization in the range of ∼50% was achieved for both BSA594 and PDGF-BB (Fig. 7A, B). Consistent immobilization efficiency over the range of tested protein loadings was shown for both proteins (R2>0.95). As much as 1 μg of BSA594 could be immobilized per scaffold without any observed detrimental effects on tenocyte proliferation after 7 days in culture (p>0.05). The increased NHS content used during crosslinking for immobilization had minimal effects on scaffold mechanical properties, eliciting a slightly significant decrease in tensile elastic modulus (520.9±106.6 kPa vs. 414.0±66.2 kPa, p=0.047) but a nonsignificant increase in ultimate tensile strength (52.8±16.4 kPa vs. 55.4±10.2 kPa, p=0.588).
FIG. 7.
Immobilization of biomolecules to the CG scaffold via carbodiimide chemistry. Characteristic calibration curves for immobilization of (A) bovine serum albumin labeled with Alexa Fluor® 594 (BSA594) and (B) PDGF-BB. (C) Expression of COL1A2, COMP, SCXB, and TNC for tenocytes seeded in scaffolds in response to immobilized PDGF-BB, IGF-1, bFGF, and GDF-5(n=3). (D) Expression of COL1A2, COMP, SCXB, and TNC for tenocytes seeded in scaffolds in response to immobilized factor pairs PDGF-BB/bFGF, PDGF-BB/GDF-5, IGF-1/bFGF, and IGF-1/GDF-5 (n=3). Black dashed line: expression level of nonsupplemented media control. cSignificant downregulation compared to nonsupplemented media control. dSignificantly greater than all other experimental groups. eSignificantly greater than other factor pair groups.
Immobilized factor tenocyte gene expression
The effect of covalently sequestering PDGF-BB, IGF-1, bFGF, or GDF-5 within scaffolds on the expression of tenocyte-associated markers COL1A2, COMP, SCXB, and TNC was measured after 7 days of culture (Fig. 7C). Candidate factors were immobilized at equivalent levels (estimated based on 50% immobilization efficiency as seen with BSA594 and PDGF-BB) to the medium soluble dose for each factor with the exception of GDF-5 (high dose). The PDGF-BB group showed a significant increase in COL1A2 expression compared to the other experimental groups (p<0.02), while the bFGF elicited a significant upregulation of TNC over all other groups (p<0.02).
We also compared the expression levels of COL1A2, COMP, SCXB, and TNC transcripts for immobilized factor pairs (PDGF-BB/bFGF, PDGF-BB/GDF-5, IGF-1/bFGF, and IGF-1/GDF-5) following 7 days of culture (Fig. 7D). All groups showed significantly downregulated levels of COMP (p<0.02) and SCXB (p<0.008) compared to the control except for IGF-1/GDF-5 SCXB expression. However, the IGF-1/GDF-5 group had significantly higher levels of both COMP (p<0.03) and SCXB (p<0.02) expression compared to the other immobilized factor pair groups. Groups with the bFGF trended toward elevated TNC expression as seen with both the soluble and immobilized bFGF alone.
Comparison of equivalent soluble and immobilized PDGF-BB doses
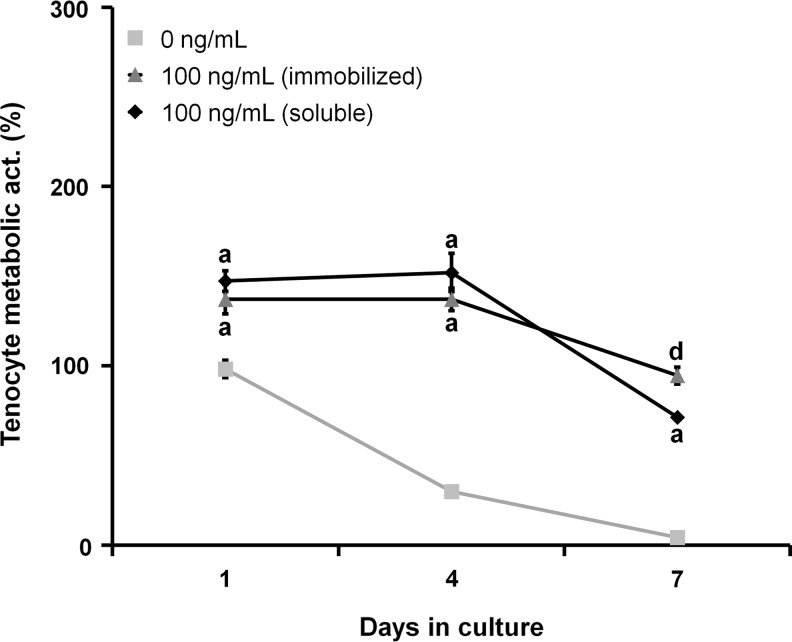
We then evaluated the effect of a single equivalent soluble versus immobilized PDGF-BB dose (100 ng/mL) on the tenocyte metabolic activity (Fig. 8). The soluble group was fed a single dose of PDGF-BB at the beginning of the experiment (replaced with nonsupplemented media at day 2), while the immobilized group was fed with nonsupplemented media throughout (same total PDGF-BB dose presented over the life of the experiment). Both PDGF-BB groups had a significantly higher metabolic activity than the control at all time points (p<0.01). While the soluble group initially had a higher metabolic activity at days 1 and 4 (although these differences were nonsignificant), by day 7, the immobilized group showed a significantly higher metabolic activity than the soluble group (p=0.036).
FIG. 8.
Comparison of equivalent soluble and immobilized doses of PDGF-BB on tenocyte metabolic activity. Tenocyte metabolic activity over 7 days of culture was compared for an equivalent single dose of soluble or immobilized PDGF-BB (n=3). By day 7, the immobilized PDGF-BB group had significantly higher metabolic activity than both the soluble PDGF-BB and control groups. aSignificant increase compared to nonsupplemented media control. dSignificant influence of soluble versus immobilized dose at given time point.
Discussion
This work establishes relationships between biomolecule supplementation and functional tenocyte behaviors, including proliferation, migration, protein synthesis, and transcript expression in a model anisotropic CG scaffold system for tendon tissue engineering. We hypothesized that the five soluble factors tested would differentially affect metrics of tenocyte bioactivity. Additionally, we hypothesized that combinations of the factors would lead to more optimal tenocyte proliferation, matrix synthesis, and transcriptomic stability, and that insoluble factor presentation would enable a pathway to more efficient in vivo translation of these scaffold systems. As others have suggested that the tenocyte isolation method may impact proliferation and phenotypic behavior,57 we used a single isolation method previously established to support retrieval of tenocytes capable of alignment and functional matrix biosynthesis.50
CG scaffolds have previously been deployed as acellular constructs in vivo, offering the potential for accelerated regulatory approval and clinical translation.9,10 Therefore, one of our initial design goals was to identify soluble factor agonists of tenocyte chemotaxis to increase the speed of cell recruitment into the scaffold. We measured the capacity of each biomolecule (in freely soluble form) to recruit tenocytes to the anisotropic CG scaffolds using a modified Transwell membrane assay.29,58 We had previously shown that PDGF-BB and IGF-1 promoted dose-dependent increases in tenocyte chemotaxis into anisotropic CG scaffolds.21 For the studies described here, we tested a wider range of PDGF-BB and IGF-1 doses in addition to bFGF, SDF-1α, and GDF-5. For this work, we used a new anisotropic scaffold variant with a higher relative density (1.5%) than those used earlier with PDGF-BB and IGF-1 (0.5%). This variant was chosen for its ability to prevent significant tenocyte-mediated contraction, along with the resultant loss of tenocyte alignment and downregulation of tenocyte-specific gene profiles, during long-term culture.47 However, as they are three times denser than previous scaffolds, it is critical to examine strategies to improve tenocyte recruitment as well as subsequent proliferation and metabolic activity and compare the effectiveness of diffusion-mediated biomolecule transport versus sequestered biomolecules for enhancing the tenocyte response.
As expected based on our previous results,21 PDGF-BB and IGF-1 both induced dose-dependent significant increases in tenocyte chemotaxis (Fig. 1) and proliferation (Fig. 2A, B). Although some significant differences were observed between certain bFGF, SDF-1α, and GDF-5 groups and the nonsupplemented control for these metrics, no overall trends were found. In addition to measures of cell motility and viability, it was critical to evaluate functional outputs as they related to soluble factor supplementation. To this end, we evaluated the amount of collagen synthesized by tenocytes and secreted into the culture media over the course of the 7-day experiment (Fig. 2C). We assessed soluble collagen synthesis instead of the total collagen content in the scaffold to more precisely resolve differences between groups (tenocytes produced only μg quantities of collagen over the 7-day culture, while each scaffold specimen had a dry weight of about 3 mg before cell seeding). While some of the collagen measured is likely degraded from the original scaffold, all scaffolds were identical with slow degradation kinetics based on the crosslinking density used.59 Additionally, since soluble factor treatment can affect matrix metalloproteinase activity,60 it is possible that this also contributed to the soluble collagen readout, although this needs to be investigated further. Again, the PDGF-BB and IGF-1 groups supported a significantly increased collagen synthesis compared to the negative control, while the other three groups promoted minimal changes from the baseline. Closer examination of these results revealed some intriguing trends relating tenocyte proliferation and functional phenotype (using collagen synthesis as a proxy). For example, the highest PDGF-BB doses significantly stimulated proliferation, resulting in ∼15 times the number of cells as the nonsupplemented control, but only ∼2.5 times the amount of soluble collagen synthesized. A similar trend was previously observed for PDGF-BB supplementation of tenocytes in 2D culture, but these results were not investigated further.22 We hypothesized that the observed trade-off between tenocyte proliferation and collagen synthesis on a per cell basis may be indicative of greater changes in the tenocyte phenotype and pursued in depth analysis of resultant gene expression profiles.
We first looked at the transcript levels of tenocyte-associated structural protein genes for type I collagen (COL1A2), type III collagen (COL3A1), COMP, and DCN after 7 days (Fig. 3). While type I collagen constitutes the majority of the protein content of many tissues in the body, including tendon, type III collagen, COMP, and DCN play important roles in maintaining mechanical integrity and directing collagen fibrillogenesis.61–63 We observed dose-dependent downregulation of COL1A2, COL3A1, COMP, and DCN for the PDGF-BB groups, supporting the hypothesis that increased tenocyte proliferation leads to a decrease in collagen synthesis on a per cell basis. In contrast, IGF-1 promoted upregulation of COL1A2 and COL3A1. GDF-5 also upregulated COMP for all three doses. We next investigated the expression profiles of two phenotypic markers of tenocytes: SCX, a basic-helix-loop-helix transcription factor found both in immature and adult tendon,56,64 and TNC, a protein associated with normal collagen fibril organization and expressed in mechanically dynamic regions of tendon65,66 (Fig. 4). We again found dose-dependent downregulation with PDGF-BB supplementation. However, we also observed significant upregulation of both SCXB and TNC for the bFGF and GDF-5 groups.
Together, the outcomes of the proliferation, collagen synthesis, and gene expression analyses demonstrate the capacity of soluble factor supplementation to elicit complex phenotypic changes for tenocytes within an anisotropic biomaterial. While PDGF-BB and IGF-1 supported a pro-proliferation phenotype with significantly increased tenocyte chemotaxis, metabolic activity, and number, they also synthesized less collagen on a per cell basis. Critically, PDGF-BB supplementation induced significant and dose-dependent downregulation of several key tenogenic genes. In contrast, bFGF and GDF-5 had little impact on tenocyte chemotaxis, proliferation, or collagen synthesis, but upregulated tenocyte markers SCXB and TNC, indicating a pro-tenocyte phenotype. These results suggested a trade-off between increased proliferation and maintenance of a healthy tenocyte phenotype, so we performed a series of rescue experiments. We hypothesized that pairing either bFGF or GDF-5 with PDGF-BB or IGF-1 would have synergistic effects on measures of tenocyte viability, soluble collagen synthesis, and transcriptomic stability. We chose bioactive levels of all groups (PDGF-BB: 50 ng/mL, IGF-1: 50 ng/mL, bFGF: 5 ng/mL, GDF-5: 500 ng/mL) and examined tenocyte bioactivity after 7 days in culture. For all four pairings tested, tenocyte proliferation, metabolic activity, and soluble collagen synthesis were significantly increased over the nonsupplemented control (Fig. 5). Gene expression results revealed that pairing either bFGF or GDF-5 with PDGF-BB was unable to rescue the tendon phenotype (Fig. 6). In contrast, the IGF-1/GDF-5 pairing promoted increased expression of COL1A2, COMP, and SCXB, while also inducing significantly more collagen synthesis than the other groups. These results suggest that it is possible to design supplementation schemes to both drive tenocyte proliferation, while maintaining transcriptomic stability.
While soluble supplementation allows us to screen biomolecule dosages, this strategy will likely be less impactful for clinical translation due to short biomolecule half-life in vivo, rapid loss of factor via diffusion or internalization, and inability to easily provide additional doses. Inspired by the sequestration of biomolecules within the native ECM,41 we explored immobilizing these factors within the anisotropic CG scaffold to evaluate relative biomolecule bioactivity as a function of presentation modality. Using a carbodiimide crosslinking approach previously described for ubiquitous attachment of multiple biomolecules within biomaterials,42,52 we showed that efficient factor immobilization could be achieved within the CG scaffold. We determined immobilization efficiency with both a model protein (BSA594) as well as one of our factors of interest (PDGF-BB), and demonstrated both were immobilized within CG scaffolds with efficiencies of ∼50% (Fig. 7A, B). By increasing the amount of the NHS stabilization agent used during carbodiimide crosslinking, we improved the efficiency of immobilization (data not shown) and observed little effect on overall scaffold mechanics.
We explored the ability for scaffold-immobilized factors to drive tenocyte bioactivity in a manner similar to that observed for soluble media supplementation. While there are likely some minor variations in immobilization efficiency based on the protein size, conformation, and amine/carboxyl content, based on our results with PDGF-BB and BSA594 (Fig. 7A, B), we assumed an immobilization efficiency of 50% for subsequent immobilization experiments using PDGF-BB, IGF-1, bFGF, and GDF-5. Gene expression trends for these immobilized factors largely mirrored the results from the soluble experiments (Fig. 7C, D), including increased expression of COMP, SCXB, and TNC for GDF-5, decreased expression of COMP and SCXB for PDGF-BB, and significant upregulation of TNC for bFGF. Interestingly, we also observed a significant upregulation of COL1A2 for the PDGF-BB group. While soluble PDGF-BB did not have as significant an inhibitory effect on COL1A2 expression as on other markers, downregulation was observed for higher doses. The differences found here may be due to reduced levels of tenocyte proliferation induced by a single, immobilized dose of PDGF-BB compared to repeated soluble doses over the course of a long-term experiment. While we were able to confirm that repeated soluble supplementation of PDGF-BB resulted in increased tenocyte proliferation and metabolic activity compared to a single immobilized dose (data not shown), we used a slightly modified approach to compare the metabolic activity of tenocytes grown on scaffolds supplemented with a single, equivalent dose of PDGF-BB (either soluble or immobilized) over the course of 7 days (Fig. 8). We immobilized PDGF-BB such that the total amount immobilized on the scaffold was equivalent to the total soluble biomolecule content in the soluble experimental group. This experiment revealed that as expected, the freely soluble group initially induced higher levels of metabolic activity. However, by day 7, the sustained, localized effect of the immobilized PDGF-BB allowed that group to surpass the soluble group, leading to a significant increase in tenocyte metabolic activity.
Conclusions
We have demonstrated the dose-dependent effects of soluble factors PDGF-BB, IGF-1, bFGF, SDF-1α, and GDF-5 on tenocyte migration, viability, collagen synthesis, and gene expression within anisotropic CG scaffolds as a model system for tendon repair. Taken together, our work suggests a critical trade-off between induction of rapid cell migration/proliferation and the expression of normal tenocyte phenotypic markers. Pairing proliferative (IGF-1) and phenotypic (GDF-5) factors can support significantly increased tenocyte proliferation and soluble collagen synthesis in addition to upregulation of key genetic markers (COL1A2, COMP, and SCXB), indicating that combining factor pairs with an engineered CG scaffold may be an approach toward optimizing a construct for tendon tissue engineering. Facile immobilization of these factors within scaffolds was accomplished using carbodiimide chemistry and induced similar effects as soluble supplementation, but in a manner more amenable to in vivo translation. Improved understanding of the individual and combined effects of the soluble factors investigated here is motivating ongoing work in our laboratory integrating immobilized and patterned factor presentation within scaffolds for functional musculoskeletal tissue engineering applications.
Acknowledgments
The authors would like to acknowledge Dr. Allison Stewart (Veterinary Sciences, UIUC) for providing equine tendon cells, Dr. Hyunjoon Kong (ChBE, UIUC) for use of the mechanical testing equipment, Dr. Sandra McMasters (SCS, UIUC) for preparation of culture media, Mr. Manuel Ramirez for assistance with mechanical testing, and the IGB Core Facilities for assistance with real-time PCR. We are grateful for the funding for this study provided by the Chemistry–Biology Interface Training Program NIH NIGMS T32GM070421 (SRC), the Chemical and Biomolecular Engineering Dept. (BAH), and the Institute for Genomic Biology (BAH) at the University of Illinois at Urbana-Champaign.
Disclosure Statement
No competing financial interests exist.
References
- 1.Butler D.L. Juncosa-Melvin N. Boivin G.P. Galloway M.T. Shearn J.T. Gooch C., et al. Functional tissue engineering for tendon repair: a multidisciplinary strategy using mesenchymal stem cells, bioscaffolds, and mechanical stimulation. J Orthop Res. 2008;26:1. doi: 10.1002/jor.20456. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y. Ramanath H.S. Wang D.A. Tendon tissue engineering using scaffold enhancing strategies. Trends Biotechnol. 2008;26:201. doi: 10.1016/j.tibtech.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Xu Y. Murrell G.A. The basic science of tendinopathy. Clin Orthop Relat Res. 2008;466:1528. doi: 10.1007/s11999-008-0286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.James R. Kesturu G. Balian G. Chhabra A.B. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg-Am. 2008;33A:102. doi: 10.1016/j.jhsa.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Schoen D.C. Injuries of the wrist. Orthop Nurs. 2005;24:304. doi: 10.1097/00006416-200507000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Vitale M.A. Vitale M.G. Zivin J.G. Braman J.P. Bigliani L.U. Flatow E.L. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16:181. doi: 10.1016/j.jse.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Cole D.W. Ginn T.A. Chen G.J. Smith B.P. Curl W.W. Martin D.F., et al. Cost comparison of anterior cruciate ligament reconstruction: autograft versus allograft. Arthroscopy. 2005;21:786. doi: 10.1016/j.arthro.2005.04.102. [DOI] [PubMed] [Google Scholar]
- 8.Gulotta L.V. Rodeo S.A. Emerging ideas: evaluation of stem cells genetically modified with scleraxis to improve rotator cuff healing. Clin Orthop Relat Res. 2011;469:2977. doi: 10.1007/s11999-010-1727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yannas I.V. Lee E. Orgill D.P. Skrabut E.M. Murphy G.F. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc Natl Acad Sci U S A. 1989;86:933. doi: 10.1073/pnas.86.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harley B.A. Spilker M.H. Wu J.W. Asano K. Hsu H.P. Spector M., et al. Optimal degradation rate for collagen chambers used for regeneration of peripheral nerves over long gaps. Cells Tissues Organs. 2004;176:153. doi: 10.1159/000075035. [DOI] [PubMed] [Google Scholar]
- 11.Yannas I.V. Tissue and Organ Regeneration in Adults. New York: Springer; 2001. [Google Scholar]
- 12.Huang K.F. Hsu W.C. Chiu W.T. Wang J.Y. Functional improvement and neurogenesis after collagen-GAG matrix implantation into surgical brain trauma. Biomaterials. 2012;33:2067. doi: 10.1016/j.biomaterials.2011.11.040. [DOI] [PubMed] [Google Scholar]
- 13.Harley B.A.C. Gibson L.J. In vivo and in vitro applications of collagen-GAG scaffolds. Chem Eng J. 2008;137:102. [Google Scholar]
- 14.O'Brien F.J. Harley B.A. Yannas I.V. Gibson L.J. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials. 2005;26:433. doi: 10.1016/j.biomaterials.2004.02.052. [DOI] [PubMed] [Google Scholar]
- 15.Duffy G.P. McFadden T.M. Byrne E.M. Gill S.L. Farrell E. O'Brien F.J. Towards in vitro vascularisation of collagen-GAG scaffolds. Eur Cell Mater. 2011;21:15. doi: 10.22203/ecm.v021a02. [DOI] [PubMed] [Google Scholar]
- 16.Farrell E. O'Brien F.J. Doyle P. Fischer J. Yannas I. Harley B.A., et al. A collagen-glycosaminoglycan scaffold supports adult rat mesenchymal stem cell differentiation along osteogenic and chondrogenic routes. Tissue Eng. 2006;12:459. doi: 10.1089/ten.2006.12.459. [DOI] [PubMed] [Google Scholar]
- 17.Harley B.A.C. Kim H.D. Zaman M.H. Yannas I.V. Lauffenburger D.A. Gibson L.J. Microarchitecture of three-dimensional scaffolds influences cell migration behavior via junction interactions. Biophys J. 2008;95:4013. doi: 10.1529/biophysj.107.122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harley B.A. Lynn A.K. Wissner-Gross Z. Bonfield W. Yannas I.V. Gibson L.J. Design of a multiphase osteochondral scaffold III: fabrication of layered scaffolds with continuous interfaces. J Biomed Mater Res A. 2010;92:1078. doi: 10.1002/jbm.a.32387. [DOI] [PubMed] [Google Scholar]
- 19.Harley B.A. Lynn A.K. Wissner-Gross Z. Bonfield W. Yannas I.V. Gibson L.J. Design of a multiphase osteochondral scaffold. II. Fabrication of a mineralized collagen-glycosaminoglycan scaffold. J Biomed Mater Res A. 2010;92:1066. doi: 10.1002/jbm.a.32361. [DOI] [PubMed] [Google Scholar]
- 20.Caliari S.R. Ramirez M.A. Harley B.A.C. The development of collagen-GAG scaffold-membrane composites for tendon tissue engineering. Biomaterials. 2011;32:8990. doi: 10.1016/j.biomaterials.2011.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caliari S.R. Harley B.A.C. The effect of anisotropic collagen-GAG scaffolds and growth factor supplementation on tendon cell recruitment, alignment, and metabolic activity. Biomaterials. 2011;32:5330. doi: 10.1016/j.biomaterials.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomopoulos S. Harwood F.L. Silva M.J. Amiel D. Gelberman R.H. Effect of several growth factors on canine flexor tendon fibroblast proliferation and collagen synthesis in vitro. J Hand Surg Am. 2005;30A:441. doi: 10.1016/j.jhsa.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Gulotta L.V. Rodeo S.A. Growth factors for rotator cuff repair. Clin Sports Med. 2009;28:13. doi: 10.1016/j.csm.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Molloy T. Wang Y. Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med. 2003;33:381. doi: 10.2165/00007256-200333050-00004. [DOI] [PubMed] [Google Scholar]
- 25.Yoshikawa Y. Abrahamsson S.O. Dose-related cellular effects of platelet-derived growth factor-BB differ in various types of rabbit tendons in vitro. Acta Orthop Scand. 2001;72:287. doi: 10.1080/00016470152846646. [DOI] [PubMed] [Google Scholar]
- 26.Abrahamsson S.O. Lundborg G. Lohmander L.S. Recombinant human insulin-like growth factor-I stimulates in vitro matrix synthesis and cell proliferation in rabbit flexor tendon. J Orthop Res. 1991;9:495. doi: 10.1002/jor.1100090405. [DOI] [PubMed] [Google Scholar]
- 27.Costa M.A. Wu C. Pham B.V. Chong A.K.S. Pham H.M. Chang J. Tissue engineering of flexor tendons: optimization of tenocyte proliferation using growth factor supplementation. Tissue Eng. 2006;12:1937. doi: 10.1089/ten.2006.12.1937. [DOI] [PubMed] [Google Scholar]
- 28.Li Y. Yu X. Lin S. Li X. Zhang S. Song Y.H. Insulin-like growth factor 1 enhances the migratory capacity of mesenchymal stem cells. Biochem Biophys Res Commun. 2007;356:780. doi: 10.1016/j.bbrc.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 29.Ponte A.L. Marais E. Gallay N. Langonne A. Delorme B. Herault O., et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 30.Ozaki Y. Nishimura M. Sekiya K. Suehiro F. Kanawa M. Nikawa H., et al. Comprehensive analysis of chemotactic factors for bone marrow mesenchymal stem cells. Stem Cells Dev. 2007;16:119. doi: 10.1089/scd.2006.0032. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt A. Ladage D. Schinkothe T. Klausmann U. Ulrichs C. Klinz F.J., et al. Basic fibroblast growth factor controls migration in human mesenchymal stem cells. Stem Cells. 2006;24:1750. doi: 10.1634/stemcells.2005-0191. [DOI] [PubMed] [Google Scholar]
- 32.Ker E.D.F. Chu B. Phillippi J.A. Gharaibeh B. Huard J. Weiss L.E., et al. Engineering spatial control of multiple differentiation fates within a stem cell population. Biomaterials. 2011;32:3413. doi: 10.1016/j.biomaterials.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sahoo S. Toh S.L. Goh J.C. A bFGF-releasing silk/PLGA-based biohybrid scaffold for ligament/tendon tissue engineering using mesenchymal progenitor cells. Biomaterials. 2010;31:2990. doi: 10.1016/j.biomaterials.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Son B.R. Marquez-Curtis L.A. Kucia M. Wysoczynski M. Turner A.R. Ratajczak J., et al. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24:1254. doi: 10.1634/stemcells.2005-0271. [DOI] [PubMed] [Google Scholar]
- 35.Du L. Yang P. Ge S. Stromal cell-derived factor-1 significantly induces proliferation, migration, and collagen type I expression in a human periodontal ligament stem cell subpopulation. J Periodontol. 2012;83:379. doi: 10.1902/jop.2011.110201. [DOI] [PubMed] [Google Scholar]
- 36.Shen W. Chen X. Chen J. Yin Z. Heng B.C. Chen W., et al. The effect of incorporation of exogenous stromal cell-derived factor-1 alpha within a knitted silk-collagen sponge scaffold on tendon regeneration. Biomaterials. 2010;31:7239. doi: 10.1016/j.biomaterials.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 37.Park A. Hogan M.V. Kesturu G.S. James R. Balian G. Chhabra A.B. Adipose-derived mesenchymal stem cells treated with growth differentiation factor-5 express tendon-specific markers. Tissue Eng Part A. 2010;16:2941. doi: 10.1089/ten.tea.2009.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keller T.C. Hogan M.V. Kesturu G. James R. Balian G. Chhabra A.B. Growth/differentiation factor-5 modulates the synthesis and expression of extracellular matrix and cell-adhesion-related molecules of rat Achilles tendon fibroblasts. Connect Tissue Res. 2011;52:353. doi: 10.3109/03008207.2010.534208. [DOI] [PubMed] [Google Scholar]
- 39.James R. Kumbar S.G. Laurencin C.T. Balian G. Chhabra A.B. Tendon tissue engineering: adipose-derived stem cell and GDF-5 mediated regeneration using electrospun matrix systems. Biomed Mater. 2011;6 doi: 10.1088/1748-6041/6/2/025011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolfman N.M. Hattersley G. Cox K. Celeste A.J. Nelson R. Yamaji N., et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest. 1997;100:321. doi: 10.1172/JCI119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu H.W. Chen C.H. Tsai C.L. Hsiue G.H. Targeted delivery system for juxtacrine signaling growth factor based on rhBMP-2-mediated carrier-protein conjugation. Bone. 2006;39:825. doi: 10.1016/j.bone.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 42.Shen Y.H. Shoichet M.S. Radisic M. Vascular endothelial growth factor immobilized in collagen scaffold promotes penetration and proliferation of endothelial cells. Acta Biomater. 2008;4:477. doi: 10.1016/j.actbio.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 43.Oh S.H. Kim T.H. Lee J.H. Creating growth factor gradients in three dimensional porous matrix by centrifugation and surface immobilization. Biomaterials. 2011;32:8254. doi: 10.1016/j.biomaterials.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 44.Anderson S.M. Chen T.T. Iruela-Arispe M.L. Segura T. The phosphorylation of vascular endothelial growth factor receptor-2 (VEGFR-2) by engineered surfaces with electrostatically or covalently immobilized VEGF. Biomaterials. 2009;30:4618. doi: 10.1016/j.biomaterials.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin T.A. Caliari S.R. Williford P.D. Harley B.A. Bailey R.C. The generation of biomolecular patterns in highly porous collagen-GAG scaffolds using direct photolithography. Biomaterials. 2011;32:3949. doi: 10.1016/j.biomaterials.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Brien F.J. Harley B.A. Yannas I.V. Gibson L. Influence of freezing rate on pore structure in freeze-dried collagen-GAG scaffolds. Biomaterials. 2004;25:1077. doi: 10.1016/s0142-9612(03)00630-6. [DOI] [PubMed] [Google Scholar]
- 47.Caliari S.R. Weisgerber D.W. Ramirez M.A. Kelkhoff D.O. Harley B.A.C. The influence of collagen–glycosaminoglycan scaffold relative density and microstructural anisotropy on tenocyte bioactivity and transcriptomic stability. J Mech Behav Biomed Mater. 2012;11:27. doi: 10.1016/j.jmbbm.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olde Damink L.H. Dijkstra P.J. van Luyn M.J. van Wachem P.B. Nieuwenhuis P. Feijen J. Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials. 1996;17:765. doi: 10.1016/0142-9612(96)81413-x. [DOI] [PubMed] [Google Scholar]
- 49.Harley B.A. Leung J.H. Silva E.C. Gibson L.J. Mechanical characterization of collagen-glycosaminoglycan scaffolds. Acta Biomater. 2007;3:463. doi: 10.1016/j.actbio.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 50.Kapoor A. Caporali E.H. Kenis P.J. Stewart M.C. Microtopographically patterned surfaces promote the alignment of tenocytes and extracellular collagen. Acta Biomater. 2010;6:2580. doi: 10.1016/j.actbio.2009.12.047. [DOI] [PubMed] [Google Scholar]
- 51.Freyman T.M. Yannas I.V. Gibson L.J. Cellular materials as porous scaffolds for tissue engineering. Prog Mater Sci. 2001;46:273. [Google Scholar]
- 52.Odedra D. Chiu L.L. Shoichet M. Radisic M. Endothelial cells guided by immobilized gradients of vascular endothelial growth factor on porous collagen scaffolds. Acta Biomater. 2011;7:3027. doi: 10.1016/j.actbio.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 53.Gibson L.J. Ashby M.F. Harley B.A. Cellular Materials in Nature and Medicine. Cambridge, United Kingdom: Cambridge University Press; 2010. [Google Scholar]
- 54.Kim Y.J. Sah R.L. Doong J.Y. Grodzinsky A.J. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988;174:168. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- 55.Tierney C.M. Jaasma M.J. O'Brien F.J. Osteoblast activity on collagen-GAG scaffolds is affected by collagen and GAG concentrations. J Biomed Mater Res A. 2009;91:92. doi: 10.1002/jbm.a.32207. [DOI] [PubMed] [Google Scholar]
- 56.Taylor S.E. Vaughan-Thomas A. Clements D.N. Pinchbeck G. Macrory L.C. Smith R.K., et al. Gene expression markers of tendon fibroblasts in normal, diseased tissue compared to monolayer, three dimensional culture systems. BMC Musculoskelet Disord. 2009;10:27. doi: 10.1186/1471-2474-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wagenhauser M.U. Pietschmann M.F. Sievers B. Docheva D. Schieker M. Jansson V., et al. Collagen type I and decorin expression in tenocytes depend on the cell isolation method. BMC Musculoskelet Disord. 2012;13:140. doi: 10.1186/1471-2474-13-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wynn R.F. Hart C.A. Corradi-Perini C. O'Neill L. Evans C.A. Wraith J.E., et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643. doi: 10.1182/blood-2004-02-0526. [DOI] [PubMed] [Google Scholar]
- 59.Pek Y.S. Spector M. Yannas I.V. Gibson L.J. Degradation of a collagen-chondroitin-6-sulfate matrix by collagenase and by chondroitinase. Biomaterials. 2004;25:473. doi: 10.1016/s0142-9612(03)00541-6. [DOI] [PubMed] [Google Scholar]
- 60.McCarrel T. Fortier L. Temporal growth factor release from platelet-rich plasma, trehalose lyophilized platelets, and bone marrow aspirate and their effect on tendon and ligament gene expression. J Orthop Res. 2009;27:1033. doi: 10.1002/jor.20853. [DOI] [PubMed] [Google Scholar]
- 61.Halasz K. Kassner A. Morgelin M. Heinegard D. COMP acts as a catalyst in collagen fibrillogenesis. J Biol Chem. 2007;282:31166. doi: 10.1074/jbc.M705735200. [DOI] [PubMed] [Google Scholar]
- 62.Yoon J.H. Halper J. Tendon proteoglycans: biochemistry and function. J Musculoskelet Neuronal Interact. 2005;5:22. [PubMed] [Google Scholar]
- 63.Liu C.F. Aschbacher-Smith L. Barthelery N.J. Dyment N. Butler D. Wylie C. Spatial and temporal expression of molecular markers and cell signals during normal development of the mouse patellar tendon. Tissue Eng Part A. 2012;18:598. doi: 10.1089/ten.tea.2011.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuo C.K. Tuan R.S. Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A. 2008;14:1615. doi: 10.1089/ten.tea.2006.0415. [DOI] [PubMed] [Google Scholar]
- 65.Riley G.P. Harrall R.L. Cawston T.E. Hazleman B.L. Mackie E.J. Tenascin-C and human tendon degeneration. Am J Pathol. 1996;149:933. [PMC free article] [PubMed] [Google Scholar]
- 66.Doroski D.M. Levenston M.E. Temenoff J.S. Cyclic tensile culture promotes fibroblastic differentiation of marrow stromal cells encapsulated in poly(ethylene glycol)-based hydrogels. Tissue Eng Part A. 2010;16:3457. doi: 10.1089/ten.tea.2010.0233. [DOI] [PMC free article] [PubMed] [Google Scholar]