Abstract
Background: Variable health literacy and genetic knowledge may pose significant challenges to engaging the general public in personal genomics, specifically with respect to promoting risk comprehension and healthy behaviors. Methods: We are conducting a multistage study of individual responses to genomic risk information for Type 2 diabetes mellitus. A total of 300 individuals were recruited from the general public in Durham, North Carolina: 60% self-identified as White; 70% female; and 65% have a college degree. As part of the baseline survey, we assessed genetic knowledge and attitudes toward genetic testing. Results: Scores of factual knowledge of genetics ranged from 50% to 100% (average=84%), with significant differences in relation to racial groups, the education level, and age. Scores were significantly higher on questions pertaining to the inheritance and causes of disease (mean score 90%) compared to scientific questions (mean score 77.4%). Scores on the knowledge survey were significantly higher than scores from European populations. Participants' perceived knowledge of the social consequences of genetic testing was significantly lower than their perceived knowledge of the medical uses of testing. More than half agreed with the statement that testing may affect a person's ability to obtain health insurance (51.3%) and 16% were worried about the consequences of testing for chances of finding a job. Conclusions: Despite the relatively high educational status and genetic knowledge of the study population, we find an imbalance of knowledge between scientific and medical concepts related to genetics as well as between the medical applications and societal consequences of testing, suggesting that more effort is needed to present the benefits, risks, and limitations of genetic testing, particularly, at the social and personal levels, to ensure informed decision making.
Introduction
Over the last decade, genetic testing has been transformed by an explosion of genomic data, powerful new technologies and analytical approaches (Zhao and Grant, 2011). Increasingly, risk information generated from a genome analysis for a range of conditions, such as heart disease, cancer, and Type 2 diabetes mellitus (T2DM), will inform disease prevention efforts (Bloss et al., 2011; Chan and Ginsburg, 2011; Kingsmore and Saunders, 2011). Future promise notwithstanding, the current clinical utility (usefulness of information to improve health outcomes) of this information remains a subject of debate and appears to be a major obstacle to further translation or adoption (Hunter et al., 2008; Rogowski et al., 2009; Yang et al., 2009; Khoury, 2010). However, even once demonstrated, true clinical utility cannot be achieved if patients/consumers are unable to correctly interpret and understand the significance of genomic risk information, either in the specific context of health care or for one's overall sense of personal well-being.
Studies suggest that health literacy may impact the understanding of personal genomic risk (Lea et al., 2011). For example, it has been reported that women with lower health literacy recalled less information about a genetic test to predict breast cancer recurrence (although participants in these studies did not actually undergo genetic testing) (Lillie et al., 2007; Brewer et al., 2009). Likewise, genetic literacy can also affect public attitudes, interest, and understanding. Genetic literacy refers to one's knowledge and appreciation of basic genetic (and, in the modern context, genomic) principles, as they inform personal decision making and underlie effective participation in public debates on genetic or genomic issues (McInerney, 2002; Bowling et al., 2008). Some studies have reported low levels of public understanding of genetic concepts (e.g., location of genes) and applications (e.g., newborn screening), although participants displayed familiarity with genetic terminology (Lanie et al., 2004; Miller, 2004; Catz et al., 2005; Lea et al., 2011). In contrast, other studies have shown some public understanding of genetic concepts and genetics research, such as the meaning of a reported genetic association (Bates et al., 2003; Miller, 2004; Levitt et al., 2005). Longitudinal survey data suggest that awareness and understanding may be increasing (Miller, 2004; Singer et al., 2008). The increased participation of the public in personal genomics activities, including research and direct-to-consumer genomic services (Eriksson et al., 2010; Do et al., 2011; Tung et al., 2011) may be due, in part, to the public's increased awareness and understanding of genetics.
While strong factual knowledge of genetics seems likely to result in higher levels of comprehension of genomic risk, it is unclear whether this is an essential component for understanding risk and/or adopting healthy behavior (McBride et al., 2010). We conducted a study to investigate the impact of genetic knowledge and other variables on comprehension and perception of genomic risk and on both intended and reported changes in one's health behaviors. In this report, we present data collected during the baseline survey characterizing participants' health literacy, genetic knowledge, and attitudes toward genetic testing.
Materials and Methods
Overall study design
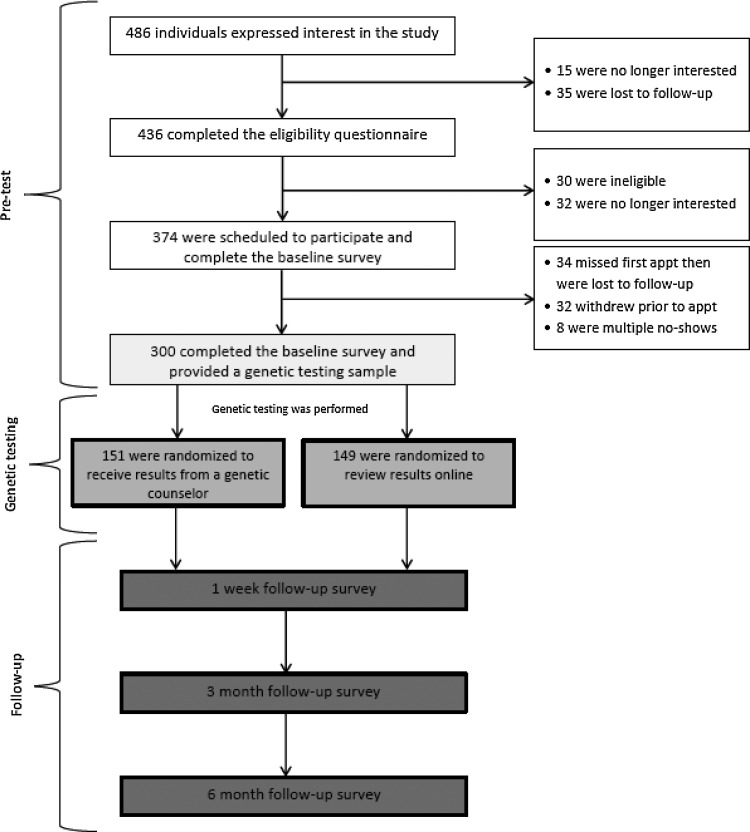
We measured participants' health literacy, actual and perceived genetic knowledge and attitudes about genetic testing as part of the baseline assessment for a randomized clinical study. The overall goals of the randomized study were to explore the impact of health literacy, genetic knowledge, and the method of risk communication on risk comprehension and perception, and health behaviors in a community-based population for genomic risk of T2DM. Enrolled participants were required to (1) complete a pretest baseline screening for knowledge of and attitudes about genetics and genetic testing; (2) undergo genomic testing for risk of T2DM and (3) complete post-test follow-up assessments at intervals up to 6 months post-testing, to assess the comprehension and impact of knowledge of genomic risk on perceptions of risk and health behaviors (Fig. 1). This study was approved by the Institutional Review Board of the Duke University Health System.
FIG. 1.
Schematic of the study design.
Participant recruitment
Participants for this study were recruited from Durham, NC through newspaper advertisements, flyers on the Duke University's campus and throughout the community, posters on public transit buses, and online advertisements. Eligible participants must have been at least 18 years of age, English-speaking, have Internet access, no personal history of T2DM, and not had a genetic test for T2DM.
Surveys
The baseline survey gathered information on participant demographics, health literacy, and genetic knowledge and attitudes about genetic testing. Although an instrument has been developed to assess literacy in a genetic context (Erby et al., 2008), we decided to conduct separate assessments of health literacy and genetic knowledge to avoid any confounding between basic health literacy skills (including numeracy) with participants' actual or perceived knowledge of genetics. Numerous measures of health literacy in general and specific to disease populations, including T2DM, have been developed (Al Sayah et al., 2012). We used the well-validated Short Test of Functional Health Literacy in Adults (S-TOFHLA) to measure health literacy (Parker et al., 1995; Baker et al., 1999). The test has been shown to have good reliability and validity compared to the other commonly used health literacy tool, the Rapid Estimate of Adult Literacy in Medicine (REALM) (Baker et al., 1999). We used two validated instruments to assess actual and perceived genetic knowledge. A 16-item survey was used to measure actual knowledge about the association between genes, chromosomes, and cells and the body and diseases (Jallinoja and Aro, 1999). A second 11-item survey was used to assess perceived knowledge of medical possibilities and social consequences of genetic testing (Morren et al., 2007).
To ascertain participants' attitudes and expectations about the future of genetic testing, we used a survey developed by Morren et al. (2007). In this survey, participants were asked to indicate their level of agreement with 13 statements regarding anticipation of the impact of genetic testing on society, use of genetic information, and the importance of genetic aspects of diseases. After completion of the baseline surveys, participants were provided educational resources about T2DM and genomics (NIH, 2006; NDIC, 2007).
Survey scoring
Overall health literacy scores (combined reading comprehension and numeracy scores) were categorized as inadequate (0–53), marginal (54–66), or adequate (67–100). Since only a single participant fell outside of the adequate functional health literacy range (score: 40), this variable was excluded from the statistical analysis. Perceived knowledge of genetics was scored as 1=none; 2=a little; 3=a lot. Genetic attitudes (favorable (positive) and reserved (negative)) were scored on a 5-point scale (1=totally disagree; 2=disagree; 3=don't know; 4=agree; 5=totally agree).
Survey analysis
In this article, we present data collected during the baseline survey characterizing participants' genetic knowledge and attitudes toward genetics. Descriptive statistics were used to summarize the demographic factors of the study sample and their association with baseline measures of knowledge. For each survey question, responses were compared to early reports (Jallinoja and Aro, 1999; Calsbeek et al., 2007; Morren et al., 2007) using Pearson chi-squared tests, with a Bonferonni correction for the number of questions in each measure of knowledge or attitudes. Kruskal–Wallis tests were used to evaluate the associations between the demographic factors and literacy and to compare subscales of literacy. Multivariate linear regression models for genetic knowledge were constructed from full models that included all participant characteristics with univariate p<0.2, and then reduced in a step-down manner with Likelihood Ratio tests. Mean Score Chi-squared tests were used to evaluate different attitudes toward genetics among patient groups. Two-sided p-values are reported for all tests using a Type I error level of 0.05.
Results
Participant characteristics
Overall, 300 individuals were enrolled in the study (Fig. 1). Seventy percent of participants were female, 60% self-identified as White, and 65% reported a college degree or higher (Table 1). The number of participants with a college degree or higher is substantially higher than reported for the Durham region (40%), state of North Carolina (23.6%), and the U.S. (25%) (American Community Survey, 2005–2009). Forty-four percent of participants were between 20 to 29 years of age. Seventy percent indicated that they had a family history of T2DM. Comparatively, about 30% of the general U.S. population has a family history of T2DM (Valdez et al., 2007).
Table 1.
Characteristics of Enrolled Participants (N=300)
| N (%) | |
|---|---|
| Sex | |
| Female | 210 (70) |
| Race | |
| Black/African-American | 86 (29) |
| White | 179 (60) |
| Other | 29 (9.7) |
| Prefer not to answer | 4 (1.3) |
| Unsure | 2 (0.7) |
| Age | |
| 18–29 years old | 131 (44) |
| 30–39 years old | 58 (19) |
| 40–49 years old | 48 (16) |
| 50–59 years old | 34 (11) |
| 60–69 years old | 28 (9) |
| 70 years or older | 1 (0.3) |
| Education | |
| Some high school or high-school graduate | 29 (10) |
| Some college, but no degree or Associate's degree | 74 (25) |
| Bachelors degree or higher | 196 (65) |
| Missing response | 1 (0.3) |
| Family history of T2DM | 210 (70) |
| Annual household income | |
| Less than $20,000 | 65 (22) |
| $20,000 to $39,000 | 80 (27) |
| $40,000 to $49,000 | 38 (13) |
| $60,000 or more | 99 (33) |
| Missing response | 18 (6) |
Actual genetic knowledge
Scores of factual knowledge of genetics ranged from 50% to 100% (mean=83.6%; median=87.5%) (Table 2). Participants scored significantly higher on questions pertaining to the inheritance and causes of disease (mean score 94.6%) compared to questions on genes, chromosomes, and cells (mean score 78.6%) (p<0.0001). No differences were noted between scores for scientific questions compared to inheritance-related questions with respect to respondent demographics. Overall differences in genetic knowledge scores were observed among the racial groups (4 df; p=0.0001) with average scores of 13.7 (±1.4) in White and 12.8 (±1.8) in non-White participants. In addition, differences in genetic knowledge were observed among education levels (6 df; p=0.0001) with increased genetic knowledge in participants with higher education levels (Spearman rho=0.22) and among age groups (4 df; p=0.004), with a slight downward trend with age deciles (Spearman rho=−0.17). In the multivariate model, the age, racial group, and education level remained statistically significant after adjusting for the other demographic factors, while no pairwise interactions were found to be significant (data not shown). No significant difference was observed in the genetic knowledge scores for participants who reported a family history of T2DM as compared to those who did not report a family history (1 df; p=0.2913).
Table 2.
General Knowledge of Genes and Disease (% of Participants Answering Questions Correctly)
| |
(General population) |
(Patient population) |
|||
|---|---|---|---|---|---|
| Current study population (n=300) | Jallinoja and Aro, 1999 [40] (n=1216)a | p-Valueb | Calsbeek et al., 2007 [39] (n=306)c | p-Valueb | |
| [Q1–Q11: Scientific facts] | |||||
| 1. One can see a gene with a naked eye. | 99 | 87 | <0.001 | 75 | <0.001 |
| 2. A gene is a disease. | 98 | 87 | <0.001 | 71 | <0.001 |
| 3. A gene is a molecule that controls hereditary characteristics. | 84 | 63 | <0.001 | 52 | <0.001 |
| 4. Genes are inside cells. | 91 | 55 | <0.001 | 46 | <0.001 |
| 5. A gene is a piece of DNA. | 93 | 57 | <0.001 | 42 | <0.001 |
| 6. A gene is a cell. | 74 | 51 | <0.001 | 29 | <0.001 |
| 7. A gene is a part of a chromosome. | 91 | 45 | <0.001 | 34 | <0.001 |
| 8. Different body parts include different genes. | 67 | 36 | <0.001 | 23 | <0.001 |
| 9. Genes are bigger than chromosomes. | 83 | 41 | <0.001 | 21 | <0.001 |
| 10. The genotype is not susceptible to human intervention.d | 25 | 77 | 1.0 | 16 | 0.008 |
| 11. It has been estimated that a person has 22,000 genes. | 60 | 18 | <0.001 | 8 | <0.001 |
| Average subsection score | 78.6 | 56.1 | - | 37.9 | - |
| [Q12–Q16: Disease-related concepts] | |||||
| 12. Healthy parents can have a child with a hereditary disease. | 97 | 85 | <0.001 | 75 | <0.001 |
| 13. The onset of certain diseases is due to genes, environment, and lifestyle. | 98 | 88 | <0.001 | 75 | <0.001 |
| 14. The carrier of a disease gene may be completely healthy. | 95 | 83 | <0.001 | 66 | <0.001 |
| 15. All serious diseases are hereditary. | 98 | 83 | <0.001 | 59 | <0.001 |
| 16. The child of a disease gene carrier is always also a carrier of the same disease gene. | 85 | 60 | <0.001 | 41 | <0.001 |
| Average subsection score | 94.6 | 79.8 | <0.001 | 63.2 | <0.001 |
| Overall average score | 83.6 | 63.5 | - | 45.8 | - |
Study population for Jallinoja and Aro (1999) consisted of 1,216 participants randomly selected from the general population in Finland.
p-values for increased knowledge are computed under the Pearson's Chi-squared test with Yates' continuity correction.
Study population for Calsbeek et al. (2007) consisted of 306 participants enrolled in the Panel of Patients with Chronic Diseases in the Netherlands and diagnosed with a chronic disease.
Perceived genetic knowledge
The majority of participants (79%) indicated that they had some knowledge (they answered either “a lot” or “a little”) of the medical applications of genetics (Table 3). A significantly lower proportion (64%) reported having some knowledge of the social implications (p<0.0001). However, despite their high education status, more participants indicated they knew nothing about the medical possibilities or social consequences of genetic testing than those who indicated they knew a lot. For example, 36% indicated they knew nothing about the social consequences of genetic testing. A higher proportion (45%) reported having no knowledge about the potential consequences of testing on their job and 50% reported having no knowledge about the rights of third parties to inquire about the results of a DNA test. No significant differences were noted between the overall perceived genetic knowledge with respect to respondent demographics. In comparison to a previous study (Morren et al., 2007), a significantly larger proportion of our study population reported having some or a lot of knowledge of each possibility or issue.
Table 3.
Perceived Genetic Knowledge of Participants (Percentage Response)
| |
A lot |
A little |
None |
|||
|---|---|---|---|---|---|---|
| Current study | Morren et al., 2007a | Current study | Morren et al., 2007a | Current study | Morren et al., 2007a | |
| Medical possibilities/uses of genetic testing | ||||||
| 1. The possibility of early detection of certain disorders using DNA-testing | 18 | 17 | 68 | 47 | 14 | 36 |
| 2. The significance of DNA-testing for my relatives | 15 | 12 | 64 | 32 | 21 | 56 |
| 3. The significance of DNA-testing for my offspring | 19 | 12 | 63 | 30 | 18 | 58 |
| 4. The possibility to use genetic knowledge to prevent or treat a disorder | 19 | 11 | 67 | 38 | 15 | 52 |
| 5. The possibilities and risks of gene therapy | 11 | 6 | 51 | 24 | 38 | 70 |
| Mean response for medical possibilities scale: | 16 | 12 | 63 | 34 | 21 | 54 |
| Social consequences | ||||||
| 6. Your rights to refuse DNA testing | 32 | 8 | 49 | 18 | 19 | 74 |
| 7. The consequences of DNA testing for my daily life | 17 | 7 | 54 | 17 | 29 | 76 |
| 8. The consequences of DNA testing for my work | 15 | 6 | 40 | 14 | 45 | 79 |
| 9. The consequences of DNA testing for affecting health insurance | 17 | 6 | 48 | 17 | 34 | 76 |
| 10. Your own possibilities to apply for a DNA test | 15 | 5 | 46 | 18 | 39 | 77 |
| 11. The rights of third parties to inquire about the results of a DNA test | 12 | 5 | 38 | 15 | 50 | 80 |
| Mean response for social consequences scale: | 18 | 6 | 46 | 16 | 36 | 77 |
| Total mean response | 17 | 9 | 54 | 25 | 28 | 66 |
Study population consisted of 1,496 participants enrolled in the Panel of Patients with Chronic Diseases in the Netherlands and diagnosed with a chronic disease.
Interest and attitudes toward genetics
When asked about their general interest in genetic testing, 52% of participants indicated they were somewhat interested in the topic of genetic testing and 45% indicated they were extremely interested. Most participants expressed positive attitudes toward the goals of genetics research and uses of genetic testing (Table 4). For example, 92% indicated that they agreed or strongly agreed with the use of DNA testing for early detection of diseases. Participants in our study had significantly more positive attitudes than those in the two European studies of patient populations that used the same survey instrument (Jallinoja and Aro, 1999; Calsbeek et al., 2007) (p<0.003).
Table 4.
Attitudes Toward Genetics Research and Testing
| % Strongly agree/agree | Mean scorea | |
|---|---|---|
| Favorable attitudes | ||
| 1. I think the development of DNA research is hopeful for the treatment of diseases. | 93.3 | 4.4 |
| 2. I think that the development of DNA research is a positive medical progress. | 93.7 | 4.5 |
| 3. I approve of using DNA-testing for early detection of diseases. | 91.7 | 4.4 |
| 4. I would inform my children about the results of a DNA-test for a specific disease. | 76.3 | 4.1 |
| 5. I want to know whether my disease is hereditary. | 94.3 | 4.5 |
| 6. I would inform my siblings about the results of a DNA-test for a specific disease. | 89.7 | 4.4 |
| Reserved attitudes | ||
| 7. I worry about the consequences of DNA-testing for being able to affect health insurance. | 51.3 | 3.4 |
| 8. The possibility of a DNA-test will change one's future. | 56.3 | 3.6 |
| 9. As long as a disease cannot be treated, I don't want a DNA-test. | 7.3 | 2.1 |
| 10. If I had a DNA-test done, my family does not need to know about the result. | 22.7 | 2.6 |
| 11. I don't want a DNA-test to tell me that I am at risk for a certain disease. | 7.7 | 2.0 |
| 12. I worry about the consequences of DNA-testing for the chances of finding a job. | 15.7 | 2.4 |
| 13. The idea of a DNA-test frightens me. | 5.3 | 1.7 |
Mean Score (items were answered on a 5-point scale: 1=totally disagree to 5=totally agree).
Attitudes were mixed regarding the consequences of testing. More than half of the participants agreed with the possibility that a DNA test will change a person's future (56.3%) or affect a person's ability to obtain health insurance (51.3%), and 16% were worried about the consequences of testing for chances of finding a job. Less than 10% of the participants agreed with the statement that they would not want to know their risk for a certain disease (not specified) or an untreatable disease, with the greatest disparities in attitudes between our population and the two other European populations for these two questions (p<0.0001). Five percent indicated that the idea of a DNA test frightens them, substantially fewer than reported in the studies of European populations. Overall, our population was less skeptical in their attitudes about testing than the European populations (17.87±3.7, p<0.0001) (data not shown).
Among study participants, males were more likely than females to believe that DNA research is hopeful for treatment of diseases (p=0.0408) and that the development of DNA research represents a positive medical progress (0.0511). Participants who self-reported as White (p=0.0004), as well as those who have a higher education status (p=0.0431), were more likely to believe that the possibility of a DNA test will change a person's future. Younger individuals were more likely to indicate that they worried about the consequences of DNA testing for finding a job (p=0.0082). We did not observe any association between genetic knowledge and positive attitudes. However, participants with higher genetic knowledge (≥87.5%) were more likely to express uncertainty about the impact of genetic testing on a person's future than those with a lower level of genetic knowledge (p=0.02) and also more likely to agree with the statement that DNA testing is frightening (p=0.04).
Discussion
The adoption of personalized medicine will be driven, in part, by the public's understanding and interest in new clinical genetic applications (Syurina et al., 2011). However, this area has been understudied with respect to the association of genetic knowledge, attitudes about genetic testing, actual comprehension of personal genomic risks and its impact on health behaviors. In this article, we describe genetic knowledge and baseline attitudes toward genetic testing of participants enrolled in a study investigating the impact of genomic risk testing for T2DM on comprehension of personal genomic risk and behavior change. In summary, our U.S.-based study population demonstrated high genetic knowledge and positive attitudes about genetic research and testing, although knowledge of potential consequences of genetic testing varied.
Overall, participants demonstrated higher scores than published reports on European populations using the same survey instruments in a patient (Calsbeek et al., 2007) and general public population (Jallinoja and Aro, 1999). The demographics of each of the survey study populations varied with respect to gender, age, and education status (race was not a variable in the European studies). However, our study as well as the two European studies (Jallinoja and Aro, 1999; Calsbeek et al., 2007) reported similar associations between age, education, and genetic knowledge. Cultural differences may account for disparities in knowledge as well as differing perceptions of the role of genes in disease, and national differences between the U.S. and Europe in science education curricula, and health systems.
The higher knowledge levels in our study may also be due to increased reporting of genetic and genomic research in recent years and the permeation of genetics into our culture, resulting in greater public familiarity (Bates, 2005). However, some studies suggest that public familiarity does not necessarily correlate with understanding (Morris and Adley, 2001; Lanie et al., 2004). The illusion of knowing or perceived comprehension of a specific term or concept due to widespread media reporting may create a false sense of reassurance that will inhibit individuals from further seeking information (Glenberg et al., 1982; Park, 2001). Even if individuals could accurately define terms or describe scientific concepts, translating basic knowledge to decisions regarding a genetic test may not be possible (Lanie et al., 2004).
Study participants demonstrated a greater knowledge of genetic disease-related concepts than scientific facts, consistent with published findings (Jallinoja and Aro, 1999; Calsbeek et al., 2007; Smerecnik et al., 2008; Condit, 2010). Despite their knowledge of the medical applications of genetics, one third of participants indicated they had no knowledge of some of the adverse societal consequences of testing. This inconsistency may be due to biased reporting of the benefits of genetics research for medical applications in the news compared to potential harms. Similarly, other articles have reported that less than half of the survey populations of the public (Allain et al., 2012) and health professionals (Laedtke et al., 2012) were aware of the existing federal legislation (GINA) protecting against the use of genetic information by health insurers and employers. Before the passage of the federal legislation prohibiting genetic discrimination, individuals often cited this issue as a primary concern and/or reason not to have genetic testing (Lapham et al., 1996; Hadley et al., 2003; Apse et al., 2004; Hall et al., 2005). Therefore, the lower perceived knowledge of the social consequences, and potentially of existing protections, remains a concern as some individuals may not be making informed decisions regarding clinical uses of genetic or genomic testing. Efforts are needed to educate clinicians about some of these issues, so that they may appropriately inform their patients in addition to the risks and benefits of testing.
Participants in our study also had significantly more positive attitudes than the two European patient study populations (Jallinoja and Aro, 1999; Calsbeek et al., 2007). The more negative attitudes of the European study populations may be attributed to national attitudes toward biotechnology, genetic testing, and genetically modified foods (Davison et al., 1997; Gaskell et al., 1999; Bonfadelli, 2005). Although higher knowledge has been associated with positive attitudes toward genetic testing (Davison et al., 1997; Rose et al., 2005), we found little or no relationship between knowledge and attitudes, comporting with other published findings (Macnicol et al., 1991; Singer, 1991; Decruyenaere et al., 1992). We observed an inverse correlation to a small degree with some of the more negative attitudes toward testing also reported elsewhere (Jallinoja and Aro, 2000). These results may not be surprising for participants enrolled in a genetic testing study, but remain consistent with previous reports of the public's knowledge and attitudes toward genetics.
Some limitations of our findings should be noted. Although our population included a substantial proportion of African-Americans and other minorities, it was relatively young and highly educated. However, the make-up of our study population is similar to other reported studies of early adopters of genomic technologies (McGuire et al., 2009; Bloss et al., 2011) and participants of clinical studies on personalized genomic risk (Gollust et al., 2012). As a result of the high education status, we were unable to assess the association with health literacy and may have had a more limited range of genetic knowledge and attitudes. The high levels of genetic knowledge and positive attitudes may also be attributed to the participants' interest/willingness to participate in a study about genetic testing and therefore, may not be representative of the general population. The high rate of family history of T2DM may also have served as a motivator for participation and biasing responses. However, this may also be considered a strength of the study, since this is a population who may represent patient populations likely to have T2DM testing. The administration of the surveys (i.e., the order of the surveys, the knowledge surveys came before the attitudes surveys) may also have affected participant responses. Given these limitations, further research is needed to fully inform the development of educational and health interventions designed to enhance genetic health decision making.
This study provides insight regarding the imbalance of knowledge between scientific and medical concepts related to genetics as well as between the medical applications and social consequences of testing. Our finding of different levels of knowledge regarding scientific concepts and medical uses or societal implications of genomic testing may affect the study participants' ability to comprehend genomic risk information and potentially bias behavior outcomes, particularly if they value their personal genomic risk information differently. The association of genetic knowledge with race and education may also impact behavior outcomes as these characteristics may be linked to knowledge about healthy lifestyles and economic feasibility.
Although it is still unclear what level of knowledge of genetics is needed or desired to ensure informed decision making and optimize the understanding of genomic risk, the biased knowledge suggests that more effort is needed to present the benefits, risks, and limitations of genetic testing to ensure informed decision making, both in the context of health care and in terms of one's overall sense of personal well-being and social identity. Our follow-up reports will explore the relationship between genetic knowledge, associated factors like race and education, and delivery models of risk information on comprehension and behavior, providing further insight about what level of knowledge patients/consumers should be equipped with to optimize their understanding and test utility.
Acknowledgment
This work was funded by the U.S. National Institutes of Health (1R21HL096573-01A1). This study is registered in clinicaltrials.gov as # NCT01186354.
Author Disclosure Statement
No competing financial interests exist.
References
- Al Sayah F. Williams B. Johnson JA. Measuring health literacy in individuals with diabetes: a systematic review and evaluation of available measures. Health Educ Behav. 2012. [Epub ahead of print] [DOI] [PubMed]
- Allain DC. Friedman S. Senter L. Consumer awareness and attitudes about insurance discrimination post enactment of the genetic information nondiscrimination Act. Familial cancer. 2012;11:637–644. doi: 10.1007/s10689-012-9564-0. [DOI] [PubMed] [Google Scholar]
- Apse KA. Biesecker BB. Giardiello FM, et al. Perceptions of genetic discrimination among at-risk relatives of colorectal cancer patients. Genet Med. 2004;6:510–516. doi: 10.1097/01.gim.0000144013.96456.6c. [DOI] [PubMed] [Google Scholar]
- Baker DW. Williams MV. Parker RM, et al. Development of a brief test to measure functional health literacy. Patient Educ Couns. 1999;38:33–42. doi: 10.1016/s0738-3991(98)00116-5. [DOI] [PubMed] [Google Scholar]
- Bates BR. Public culture and public understanding of genetics: a focus group study. Public Underst Sci. 2005;14:47–65. doi: 10.1177/0963662505048409. [DOI] [PubMed] [Google Scholar]
- Bates BR. Templeton A. Achter PJ, et al. What does “a gene for heart disease” mean? A focus group study of public understandings of genetic risk factors. Am J Med Genet A. 2003;119A:156–161. doi: 10.1002/ajmg.a.20113. [DOI] [PubMed] [Google Scholar]
- Bloss CS. Jeste DV. Schork NJ. Genomics for disease treatment and prevention. Psychiatr Clin North Am. 2011;34:147–166. doi: 10.1016/j.psc.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloss CS. Schork NJ. Topol EJ. Effect of direct-to-consumer genomewide profiling to assess disease risk. N Engl J Med. 2011;364:524–534. doi: 10.1056/NEJMoa1011893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfadelli H. Mass media and biotechnology: Knowledge gaps within and between European countries. Int J Public Opin Res. 2005;17:42–62. [Google Scholar]
- Bowling BV. Acra EE. Wang L, et al. Development and evaluation of a genetics literacy assessment instrument for undergraduates. Genetics. 2008;178:15–22. doi: 10.1534/genetics.107.079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer NT. Tzeng JP. Lillie SE, et al. Health literacy and cancer risk perception: implications for genomic risk communication. Med Decis Making. 2009;29:157–166. doi: 10.1177/0272989X08327111. [DOI] [PubMed] [Google Scholar]
- Calsbeek H. Morren M. Bensing J, et al. Knowledge and attitudes towards genetic testing: a two year follow-up study in patients with asthma, diabetes mellitus and cardiovascular disease. J Genet Couns. 2007;16:493–504. doi: 10.1007/s10897-006-9085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catz DS. Green NS. Tobin JN, et al. Attitudes about genetics in underserved, culturally diverse populations. Community Genet. 2005;8:161–172. doi: 10.1159/000086759. [DOI] [PubMed] [Google Scholar]
- Chan IS. Ginsburg GS. Personalized medicine: progress and promise. Annu Rev Genomics Hum Genet. 2011;12:217–244. doi: 10.1146/annurev-genom-082410-101446. [DOI] [PubMed] [Google Scholar]
- Condit CM. Public understandings of genetics and health. Clin Genet. 2010;77:1–9. doi: 10.1111/j.1399-0004.2009.01316.x. [DOI] [PubMed] [Google Scholar]
- Davison A. Barns I. Schibeci R. Problematic publics: a critical review of surveys of public attitudes to biotechnology. Sci Technol Hum Values. 1997;22:317–348. [Google Scholar]
- Decruyenaere M. Evers-Kiebooms G. Denayer L, et al. Cystic fibrosis: community knowledge and attitudes towards carrier screening and prenatal diagnosis. Clin Genet. 1992;41:189–196. doi: 10.1111/j.1399-0004.1992.tb03661.x. [DOI] [PubMed] [Google Scholar]
- Do CB. Tung JY. Dorfman E, et al. Web-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson's disease. PLoS Genet. 2011;7:e1002141. doi: 10.1371/journal.pgen.1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erby LH. Roter D. Larson S, et al. The rapid estimate of adult literacy in genetics (REAL-G): a means to assess literacy deficits in the context of genetics. Am J Med Genet A. 2008;146A:174–181. doi: 10.1002/ajmg.a.32068. [DOI] [PubMed] [Google Scholar]
- Eriksson N. Macpherson JM. Tung JY, et al. Web-based, participant-driven studies yield novel genetic associations for common traits. PLoS Genet. 2010;6:e1000993. doi: 10.1371/journal.pgen.1000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskell G. Bauer MW. Durant J, et al. Worlds apart? the reception of genetically modified foods in Europe and the U.S. Science. 1999;285:384–387. doi: 10.1126/science.285.5426.384. [DOI] [PubMed] [Google Scholar]
- Glenberg AM. Wilkinson AC. Epstein W. The illusion of knowing - failure in the self-assessment of comprehension. Mem Cognit. 1982;10:597–602. [Google Scholar]
- Gollust SE. Gordon ES. Zayac C, et al. Motivations and perceptions of early adopters of personalized genomics: perspectives from research participants. Public Health Genomics. 2012;15:22–30. doi: 10.1159/000327296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley DW. Jenkins J. Dimond E, et al. Genetic counseling and testing in families with hereditary nonpolyposis colorectal cancer. Arch Intern Med. 2003;163:573–582. doi: 10.1001/archinte.163.5.573. [DOI] [PubMed] [Google Scholar]
- Hall MA. McEwen JE. Barton JC, et al. Concerns in a primary care population about genetic discrimination by insurers. Genet Med. 2005;7:311–316. doi: 10.1097/01.gim.0000162874.58370.c0. [DOI] [PubMed] [Google Scholar]
- Hunter DJ. Khoury MJ. Drazen JM. Letting the genome out of the bottle—will we get our wish? N Engl J Med. 2008;358:105–107. doi: 10.1056/NEJMp0708162. [DOI] [PubMed] [Google Scholar]
- Jallinoja P. Aro AR. Knowledge about genes and heredity among Finns. New Genet Soc. 1999;18:101–110. [Google Scholar]
- Jallinoja P. Aro AR. Does knowledge make a difference? The association between knowledge about genes and attitudes toward gene tests. J Health Commun. 2000;5:29–39. doi: 10.1080/10810730050019546. [DOI] [PubMed] [Google Scholar]
- Khoury MJ. Dealing with the evidence dilemma in genomics and personalized medicine. Clin Pharmacol Ther. 2010;87:635–638. doi: 10.1038/clpt.2010.4. [DOI] [PubMed] [Google Scholar]
- Kingsmore SF. Saunders CJ. Deep sequencing of patient genomes for disease diagnosis: when will it become routine? Sci Transl Med. 2011;3:87ps23. doi: 10.1126/scitranslmed.3002695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laedtke AL. O'Neill SM. Rubinstein WS, et al. Family physicians' awareness and knowledge of the Genetic Information Non-Discrimination Act (GINA) J Genet Couns. 2012;21:345–352. doi: 10.1007/s10897-011-9405-6. [DOI] [PubMed] [Google Scholar]
- Lanie AD. Jayaratne TE. Sheldon JP, et al. Exploring the public understanding of basic genetic concepts. J Genet Couns. 2004;13:305–320. doi: 10.1023/b:jogc.0000035524.66944.6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapham EV. Kozma C. Weiss JO. Genetic discrimination: perspectives of consumers. Science. 1996;274:621–624. doi: 10.1126/science.274.5287.621. [DOI] [PubMed] [Google Scholar]
- Lea DH. Kaphingst KA. Bowen D, et al. Communicating genetic and genomic information: health literacy and numeracy considerations. Public Health Genomics. 2011;14:279–289. doi: 10.1159/000294191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt M. Weiner K. Goodacre J. Gene Week: a novel way of consulting the public. Public Underst Sci. 2005;14:67–79. [Google Scholar]
- Lillie SE. Brewer NT. O'Neill SC, et al. Retention and use of breast cancer recurrence risk information from genomic tests: the role of health literacy. Cancer Epidemiol Biomarkers Prev. 2007;16:249–255. doi: 10.1158/1055-9965.EPI-06-0525. [DOI] [PubMed] [Google Scholar]
- Macnicol AM. Wright AF. Watson ML. Education and attitudes in families with adult polycystic kidney disease. Nephrol Dial Transplant. 1991;6:27–30. doi: 10.1093/ndt/6.1.27. [DOI] [PubMed] [Google Scholar]
- McBride CM. Koehly LM. Sanderson SC, et al. The behavioral response to personalized genetic information: will genetic risk profiles motivate individuals and families to choose more healthful behaviors? Annu Rev Public Health. 2010;31:89–103. doi: 10.1146/annurev.publhealth.012809.103532. [DOI] [PubMed] [Google Scholar]
- McGuire AL. Diaz CM. Wang T, et al. Social networkers' attitudes toward direct-to-consumer personal genome testing. Am J Bioeth. 2009;9:3–10. doi: 10.1080/15265160902928209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInerney JD. Education in a genomic world. J Med Philosophy. 2002;27:369–390. doi: 10.1076/jmep.27.3.369.2977. [DOI] [PubMed] [Google Scholar]
- Miller JD. Public understanding of, and attitudes toward, scientific research: what we know and what we need to know. Public Underst Sci. 2004;13:273–294. [Google Scholar]
- Morren M. Rijken M. Baanders AN, et al. Perceived genetic knowledge, attitudes towards genetic testing, and the relationship between these among patients with a chronic disease. Patient Educ Couns. 2007;65:197–204. doi: 10.1016/j.pec.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Morris SH. Adley CC. Irish public perceptions and attitudes to modern biotechnology: an overview with a focus on GM foods. Trends Biotechnol. 2001;19:43–48. doi: 10.1016/s0167-7799(00)01527-4. [DOI] [PubMed] [Google Scholar]
- National Diabetes Information Clearinghouse (NDIC) http://diabetes.niddk.nih.gov/dm/pubs/type2_ES/WYNTK_type2_508.pdf Type 2 Diabetes: What You Need to Know. 2007 NIH Publication No. 12-6129. [Google Scholar]
- National Institutes of Health (NIH) http://ndep.nih.gov/media/GP_Toolkit.pdf Small Steps, Big Rewards: Your Game Plan to Prevent Type 2 Diabetes. National Diabetes Education Program. 2006 NIH Publication No.03-5334. [Google Scholar]
- Park CY. News media exposure and self-perceived knowledge: the illusion of knowing. Int J Public Opin Res. 2001;13:419–425. [Google Scholar]
- Parker RM. Baker DW. Williams MV, et al. The test of functional health literacy in adults: a new instrument for measuring patients' literacy skills. J Gen Intern Med. 1995;10:537–541. doi: 10.1007/BF02640361. [DOI] [PubMed] [Google Scholar]
- Rogowski WH. Grosse SD. Khoury MJ. Challenges of translating genetic tests into clinical and public health practice. Nat Rev Genet. 2009;10:489–495. doi: 10.1038/nrg2606. [DOI] [PubMed] [Google Scholar]
- Rose A. Peters N. Shea JA, et al. The association between knowledge and attitudes about genetic testing for cancer risk in the United States. J Health Commun. 2005;10:309–321. doi: 10.1080/10810730590950039. [DOI] [PubMed] [Google Scholar]
- Singer E. Public attitudes toward genetic testing. Popul Res Policy Rev. 1991;10:235–255. doi: 10.1007/BF00141952. [DOI] [PubMed] [Google Scholar]
- Singer E. Couper MP. Raghunathan TE, et al. Trends in U.S. Attitudes Toward Genetic Testing, 1990–2004. Public Opin Q. 2008;72:446–458. doi: 10.1093/poq/nfn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smerecnik CM. Mesters I. de Vries NK, et al. Educating the general public about multifactorial genetic disease: applying a theory-based framework to understand current public knowledge. Genet Med. 2008;10:251–258. doi: 10.1097/GIM.0b013e31816b4ffd. [DOI] [PubMed] [Google Scholar]
- Syurina EV. Brankovic I. Probst-Hensch N, et al. Genome-based health literacy: a new challenge for public health genomics. Public Health Genomics. 2011;14:201–210. doi: 10.1159/000324238. [DOI] [PubMed] [Google Scholar]
- Tung JY. Do CB. Hinds DA, et al. Efficient replication of over 180 genetic associations with self-reported medical data. PLoS One. 2011;6:e23473. doi: 10.1371/journal.pone.0023473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez R. Yoon PW. Liu T, et al. Family history and prevalence of diabetes in the U.S. population: the 6-year results from the National Health and Nutrition Examination Survey (1999–2004) Diabetes Care. 2007;30:2517–2522. doi: 10.2337/dc07-0720. [DOI] [PubMed] [Google Scholar]
- Yang Q. Flanders WD. Moonesinghe R, et al. Using lifetime risk estimates in personal genomic profiles: estimation of uncertainty. Am J Hum Genet. 2009;85:786–800. doi: 10.1016/j.ajhg.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J. Grant SF. Advances in whole genome sequencing technology. Curr Pharm Biotechnol. 2011;12:293–305. doi: 10.2174/138920111794295729. [DOI] [PubMed] [Google Scholar]