Abstract
The aim of this study was to investigate the effects of serum and compressive dynamic loading on the cartilaginous matrix spatiotemporal distribution around chondrocytes in vitro. Murine chondrocytes suspended in agarose were cultured in serum-free media or in varying concentrations of serum with or without compressive dynamic loading. Gene expression was assayed by quantitative polymerase chain reaction. Immunohistochemistry was performed for type II collagen and type VI collagen, aggrecan, or cartilage oligomeric matrix protein (COMP) to study the effect of serum and dynamic loading on the spatiotemporal distribution of cartilage matrix components. Chondrocytes in serum-free culture exhibited negligible differences in type II collagen, aggrecan, and COMP mRNA expression levels over 15 days of cultivation. However, higher serum concentrations decreased matrix gene expression. Expression of the matrix metalloproteinases (MMP)-3 and MMP-13 mRNA increased over time in serum-free or reduced serum levels, but was significantly suppressed in 10% fetal bovine serum (FBS). Compressive loading significantly stimulated MMP-3 expression on days 7 and 15. Immunohistochemical analysis demonstrated that maximum pericellular matrix deposition was achieved in 10% FBS culture in the absence of compressive loading. The pericellular distribution of type II and VI collagens, aggrecan, and COMP proteins tended to be more co-localized in the pericellular region from day 9 to day 21; compressive loading helped promote this co-localization of matrix proteins. The results of this study suggest that the quantity, quality, and spatial distribution of cartilaginous matrix can be altered by serum concentrations and compressive loading.
Introduction
Articular cartilage is a specialized tissue that serves weight bearing and transition of force functions during normal joint movements. This functional property of articular cartilage is dictated by the highly specialized organization of the macromolecules in the extracellular matrix in response to mechanical loads applied to the tissue. A small population of chondrocytes maintains and remodels the matrix in response to the in vivo mechanochemical conditions through their anabolic and catabolic activities. Therefore, a continuous matrix slow turnover and reformation is observed during cartilage growth, maturation, and the aging process.1,2 Various matrix components like type II collagen, aggrecan, type VI collagen, and cartilage oligomeric matrix protein (COMP) are present in specific spatial arrangement and composition during assembly and turnover in the cartilagematrix.3–6 The chondrocyte response to mechanochemical stimuli and the assembly of the cell-associated cartilaginous matrix are therefore of significant interest in cartilage physiology and tissue engineering.
Numerous studies have made great efforts to optimize tissue engineering culture conditions with the aim of producing an articular cartilage substitute that will mimic the functional properties of the native cartilage for the treatment of articular cartilage defects. Both mechanical and biochemical stimuli have demonstrable effects on matrix metabolism.7–9 Specifically, mechanical stimuli like compressive dynamic loading have the capacity to enhance both anabolic and catabolic responses,10 improve organization of collagen network, and enhance the functional properties of the engineered tissue,11 whereas biochemical factors such as transforming growth factor (TGF)-β family and insulin-like growth factor 1 stimulate chondrocyte anabolic activities and decrease the catabolic activities thereby increasing matrix accumulation in tissues.12 While previous studies have focused on the utility of the cartilage constructs engineered by modulation of mechanical and biochemical stimuli,8,13–15 an understanding of the role of various mechanochemical culture conditions in the spatiotemporal deposition of cartilaginous matrix components remain incomplete.
The aim of this study was to examine the effects of serum supplement and compressive dynamic loading on chondrocyte metabolic responses and the spatiotemporal deposition of type II collagen, aggrecan, type VI collagen, and COMP around chondrocytes. We hypothesized that these four cell-associated matrix components will initially accumulate around the cells, but will be present in different distribution patterns over time in various mechanochemical culture conditions. To test this hypothesis, we seeded chondrocytes in agarose hydrogels in low density and maintained them in serum or serum-free culture media with or without compressive loading for 3 weeks. Gene expression profiling was used to monitor Col2a1, ACAN, Col6a1, COMP, and matrix metalloproteinases (MMP)-3 and MMP-13 at various time points. We utilized fluorescence deconvolution microscopy in conjunction with double-labeled immunohistochemistry (IHC) to measure the thickness of type II collagen pericellular distribution at days 9 and 21.
Materials and Methods
Tissue and cell culture
For each experiment rib cages were pooled from at least 50 five-day-old C57BL/6 mice (Jackson Laboratory) scarified,16 and ∼3×106 chondrocytes could be harvested from each animal, with a total at 1.5×108 cells obtained. All procedures were approved by the Washington University Animal Studies Committee. The agarose/culture medium mixture was prepared by mixing four volumes of 2.5% low melting agarose (Seaplaque; Cambrex Bioscience) with one volume of 5× basic culture medium to give a final concentration of 2% agarose in Dulbecco's modified Eagle's medium (DMEM)/Ham's F-12 containing 15 mM Hepes,100 U/mL penicillin, 100 μg/mL streptomycin, 0.1% fungizone, 50 μg/mL ascorbic acid, 1% nonessential amino acids, and 0.4 mM proline supplemented with 10% fetal bovine serum (FBS) (all products from Gibco). Cells were suspended in this agarose-DMEM culture medium at a density of 2×106 cells/mL.17 A 24-well plate was mounted into a custom-designed loading device (Fig. 1A), and the position of loading platform set to make a 2 mm space between the loading pin base and the well bottom. About 450 μL of the mixture was poured into each well, and chondrocyte/agarose constructs were allowed to solidify at room temperature for 10 min. Constructs were then punched out to obtain cylindrical gels (9 mm in diameter and 2 mm in height) that were placed in a 24-well plate, held in place by foam rings (Fig. 1B), placed in 5% CO2 at 37°C, and cultured for 3 days. At the end day of this preculture period, defined as day 0, chondrocyte/agarose constructs were grouped by culture conditions. We applied six different culture conditions: serum-free media (0% FBS) supplemented with 1% insulin-transferrin-selenium (ITS) (10 μg/mL insulin, 5.5 μg/mL transferrin, and 6.7 ng/mL selenium) (Gibco), 2% FBS+1% ITS, or 10% FBS supplemented in the basic culture medium, with or without compressive dynamic loading. Samples in loading groups were subjected to compressive loading at 10% peak-to-peak strain in unconfined compression, 1-Hz frequency, for 5 h/day and 7 days/week, at a duty cycle of 1 h of loading followed by 1 h of rest. Compressive loading was applied by a custom-designed device (Electronics & Machine Shop at Washington University School of Medicine) (Fig. 1) and was carried out at 37°C and 5% CO2 in a humidified incubator. Samples maintained under uncompressed conditions are referred to as free-swelling groups.
FIG. 1.
(A) Schematic of the custom-designed computer-controlled loading apparatus capable of simultaneously deforming multiple chondrocyte/agarose constructs. This bioreactor utilizes a programmable linear actuator connected to a loading platform that is equipped with 16 polysulfone loading pins. (B) Loading pin was used to impose the compressive dynamic loading on unconfined samples in a standard 24-well tissue culture plate.
In each study, from each group three samples were harvested for each test. On days 1, 7, and 15, samples were harvested for mRNA analysis by quantitative polymerase chain reaction (qPCR); on days 1, 9, and 21, samples were harvested for histological and immunohistochemical analysis. The entire study was repeated three times. Values reported were averaged across repeat studies.
RNA extraction and real-time qPCR
The protocol of RNA extraction was modified from the RNeasy-mini kit (Qiagen) procedure as per Bougault.17 Briefly, each chondrocyte/agarose construct was diced in a mixture of 0.75 mL QG buffer (Qiagen) and 1 mL RTL buffer (Qiagen), and the samples were kept at room temperature until complete dissolution and then supplemented in 1 mL 70% ethanol. The subsequent RNA purification steps were performed as described by the manufacturer. RNA quantity and quality were assessed by using a nanodrop 2000 (Thermo Scientific) and a capillary electrophoresis system (RNA Nano kit and 2100 BioAnalyzer; Agilent). Total RNA (200 ng) was reverse-transcribed with SuperScript™ II Reverse Transcriptase to synthesize cDNA. The cDNA was then used for real-time qPCR. qPCR was performed in a total volume of 20 μL reaction mixture containing 10 μL of SYBR Green PCR Master Mix (Applied Biosystems), 1.5 μL of cDNA, and 200 nM of primers using a 7500 Real-Time PCR System (Applied Biosystems). All cDNA samples were analyzed in duplicate.
The primer sequences for the following murine genes are as follows: glyceraldehyde-3-phosphate dehydrogenase (Gapdh), 5′-AGGTCGGTGTGAACGGATTTG-3′ and 5′-TGT AGACCATGTAGTTGAGGTCA-3′; type II collagen (Col2a1), 5′-CAGGATGCCCGAAAATTAGGG-3′ and 5′-ACCACGAT CACCTCTGGGT-3′; aggrecan (Acan), 5′-CCTGCTACTTCAT CGACCCC-3′ and 5′-AGATGCTGTTGACTCGAACCT-3′; type VI collagen (Col6a1), 5′-CTGCTGCTACAAGCCTGCT-3′ and 5′-CCCCATAAGGTTTCAGCCTCA-3′; cartilage oligomeric matrix protein (COMP), 5′-ACTGCCTGCGTTCTAG TGC-3′ and 5′-CGCCGCATTAGTCTCCTGAA-3′; MMP-3, 5′-AGTCTACAAGTCCTCCACAG-3′ and 5′-TTGGTGATGT CTCAGGTTCC-3′; MMP-13, 5′-GATTCTTCTGGCGCCTGC AC-3′ and 5′-CGCAGCGTCCAGTCTCTTCA-3′ (Invitrogen).
Levels of gene expression were determined by using the comparative 2−ΔΔCt method with normalization to Gapdh as the endogenous control, and the significant differences were compared to control groups (calibrator).
Histological analysis
For histology, constructs were fixed in 4% formaldehyde for at least 24 h, dehydrated in a graded series of ethanol, cleared, embedded in paraffin, and sectioned into 10-μm-thick slices. The slices were fixed on glass slides and then either stained with picrosirius red to visualize collagen distribution or toluidine blue to analyze glycosaminoglycans distribution.
Immunohistochemistry
For IHC, sections were deparaffinized and rehydrated and digested with hyaluronidase (1% for 30 min at 37°C) to enhance antibody penetration. The sections were washed in phosphate buffered saline (PBS) and then blocked with 10% serum in PBS for 1 h at room temperature. Next, the slides were incubated with the II fibrillar collagen (IIF) antibody against the type II collagen triple helical domain18 (1:100) at 4°C overnight in the blocking solution, washed, and incubated with a second primary antibody against either aggrecan (gift from Dr. Kurt Doege, Louisiana State University) (1:100), type VI collagen (ab6588, Abcam) (1:200), or COMP (gift from Dr. Dick Heinegard, University of Lund, Sweden) (1:100). Fluorescent detection of each protein was achieved using either secondary goat Alexa Fluor 488-conjugated anti-rabbit IgG (1:250) (Invitrogen) or goat Alexa Fluor 594-conjugated anti-rat IgG antibodies (1:250) (Invitrogen), and counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (1:1000) (Vector Laboratories). The images were captured using a 40×, 1.4 numerical aperture oil immersion objective mounted on an Eclipse E800 microscope (Nikon), QImaging Retiga 2000R Fast 1394 camera, and MetaMorph software v 7.7 (Molecular Devices). All images were deconvoluted for five iterations to remove extraneous fluorescence.
Analysis of fluorescently labeled proteins
The spatial distribution of matrix proteins around individual chondrocytes in double-labeled IHC images was analyzed using ImageJ (NIH) (Fig. 4). The maximum fluorescence intensity of each labeled matrix protein was standardized to the same level. This technique allowed for the measurement of the extent of pericellular distribution of type II collagen and also to evaluate its relative spatial relationship to aggrecan, type VI collagen, or COMP in the pericellular region of a single cell. The pericellular distributions around at least 90 different cells were analyzed for this study.
FIG. 4.
An illustration of the assessment of spatial distribution of type II collagen, COMP, type VI collagen, and aggrecan via labeling-associated fluorescence around exemplary chondrocytes from C10F (10% FBS with compressive loading) group on day 21 (A, C, E). The fluorescence intensity was profiled along a manually drawn line (yellow overlay, A, C, E) that perpendicularly intersected the fluorescence as shown. At each intersection, the relative spatial relationship between two matrix components was profiled (B, D, F). The location of type II collagen was used as the reference (r in B, D, F, G) and its fluorescence intensity profile was superimposed on those of the other three matrix proteins (G). The red dotted lines indicate the range of standard deviation of these three exemplary cells. Color images available online at www.liebertpub.com/tea
Statistical analysis
Statistics were performed with SPSS Software (SPSS, Inc.). Each data point represents the mean and standard deviation. Student t-tests or one-way ANOVA followed by Tukey's post hoc test were carried out with a statistical significance set at p=0.05.
Results
Mechanochemical cultures modulate expression profiles of anabolic and catabolic genes
Chondrocyte agarose constructs were transferred at day 0 from the preculture conditions of 10% FBS to serum-free or reduced-serum conditions (2% FBS) or to 10% FBS culture media in the presence or absence of compressive dynamic loading. In the absence of loading (the free-swelling group) in a serum-free culture (F0F group), chondrocytes showed minimal changes in expression for Col2a1 and Col6a1 over 15 days of cultivation, while expression levels of Acan, COMP, and MMP-13 were observed to increase on day 9, the expression levels of MMP-3 increased on day 15 (Fig. 2A). At day 1, after the deprivation of serum, the expression of the anabolic genes Col2a1, Acan, Col6a1, and COMP is significantly less in the serum-free (F0F group) and reduced-serum (F2F group) conditions than in the culture conditions with the full complement of 10% FBS (F10F group) (Fig. 2B). Extended culturing (day 7 and day 15), however, drastically reduced the expression of these anabolic genes (p<0.01) to a level below than that observed for the serum-free group (F0F) on that day. Interestingly, the presence of 10% serum appeared to significantly suppress MMP-3 and MMP-13 expression (p<0.01) in the F10F group over 15 days of cultivation compared to the absence of serum in F0F (Fig. 2B).The MMP-3 and MMP-13 expression profiles in the F2F group were close to that seen in the F0F group (Fig. 2B).
FIG. 2.
(A) Gene expression profiles of chondrocytes embedded in agarose for 15 days in the F0F group (0% fetal bovine serum [FBS] without loading). Levels of gene expression were normalized to Gapdh. (B) Effects of serum supplement on gene expression from day 1 to day 15. Levels of gene expression at each time point in F2F (2% FBS without loading) and F10F (10% FBS without loading) groups were determined by using the comparative 2−ΔΔCt method with normalization to Gapdh and the significant differences were compared to the F0F control group. (C, D, E) Effects of compressive dynamic loading on gene expression from day 1 to day 15. Levels of gene expression at each time point were determined by using the comparative 2−ΔΔCt method with normalization to Gapdh and the significant differences were compared to the irresponsive free-swelling counterpart groups. *p<0.05; **p<0.01.
To isolate and understand the effects of compressive dynamic loading from the serum treatment, agarose/chondrocyte constructs treated with compressive loading were compared to their free-swelling counterpart groups with the same serum concentration and culture time (Fig. 2C, D, E). With dynamic compressive loading (C) in the absence of serum in C0F groups, Col2a1 mRNA expression was upregulated at day 15 compared to the F0F groups (without compressive loading) (p=0.03) (Fig. 2C). Application of compressive loading to 2% (C2F group, Fig. 2D) or 10% (C10F group, Fig. 2E) serum-supplemented culture conditions did not significantly alter Col2a1 expression in the C2F (p=0.128) and C10F groups (p=0.387), although some expression for Col2a1 is seen on day 15 in the C2F conditions (Fig. 2D). Compressive loading appeared to significantly reduce Acan expression in all cultures at days 7 and 15 (p<0.01) (Fig. 2C, D, E). Col6a1 mRNA expression was not significantly affected by loading in C0F and C2F groups (Fig. 2C, D), but was downregulated by loading in the C10F group on day 15 (Fig. 2E). However, compressive loading significantly upregulated COMP expression on day 15 in the C0F and C10F groups (p=0.047 and p=0.048, respectively) (Fig. 2C, E).
With compressive loading, the most significant effect was seen in the expression of the catabolic gene MMP-3. Compressive loading upregulated the expression of MMP-3 irrespective of the serum conditions at days 1, 7, and 15 as seen in C0F (p=0.012, p=0.006, and p=0.008, respectively), C2F (p=0.068, p=0.039, and p=0.153, respectively), and C10F (p=0.315, p=0.027, and p=0.049, respectively) groups compared to the free-swelling counterpart groups (Fig. 2C, D, E). However, with compressive loading, MMP-13 expression profile was close to that seen in the free-swelling groups and marginally downregulated by compressive loading in C10F and C0F groups at day 15 (p<0.01) (Fig. 2C, E).
Serum-free culture and compressive loading result in smaller cell size and less matrix deposition
To investigate the distribution of proteoglycans and collagen, chondrocyte–agarose constructs were sectioned and stained with picrosirius red and toluidine blue, respectively, at days 1, 9, and 21. We set the histology study time points later than that of the mRNA expression study, since the changes of mRNA expression would be earlier than the corresponding changes in matrix protein synthesis, assembly, and deposition. In addition during early culture, the deposition of matrix proteins would be increased dramatically, while in the later culture period, the rate of matrix reformation would slow and take more time to observe differences in matrix deposition, so we increased the interval period, set day 21 as the last time point in histological studies. As shown in Figure 3 for the six different culture conditions at the three time points, cells were observed scattered and often grouped in small clusters of 3–6 cells, with no specific topographic location within the agarose gel. At day 1, after the preculture period, little matrix accumulation either on the cell surface or within the cell was detected for most cells. Cell size enlargement and matrix accumulation was observed to a large extent in the 10% serum-supplemented culture in the absence of compressive loading (F10F group) by day 21, while in the other groups no obvious matrix deposition increment was observed from day 9 to day 21. Interestingly, compressive dynamic loading appeared to result in smaller cell sizes and less matrix deposition compared to the free-swelling groups at days 9 and 21.
FIG. 3.
Tissue sections on days 1, 9, and 21 were stained with picrosirius red and toluidine blue to visualize collagen and glycosaminoglycans distribution. High-level serum supplement culture without compressive loading (F10F group) increased cell size and matrix deposition, while serum-free culture and compressive loading resulted in smaller cell size and less matrix deposition. Scale bar: 200 μm. Color images available online at www.liebertpub.com/tea
Spatiotemporal distribution of four matrix proteins around single chondrocytes
To analyze the changes in the matrix, especially the changes in the spatial distribution of matrix proteins, we evaluated the changes in the extent of pericellular distribution of type II collagen combined with aggrecan, type VI collagen, or COMP proteins around single cells, using IHC visualized with fluorescence deconvolution microscopy. The localization of type II collagen was regarded as a landmark to identify the relative spatial relationship with aggrecan, type VI collagen, or COMP. In this analysis illustrated in Figure 4, the fluorescence intensity of a protein is profiled along a manually drawn line (shown as a yellow line in Fig. 4A, C, E) that perpendicularly intersected the fluorescence from an arbitrary, fluorescence-free site just within the ring of fluorescence to an arbitrary, fluorescence-free site just outside. At each intersection, the relative spatial relationship between two matrix components was profiled (Fig. 4B, D, F) with that for type II collagen used as a reference (r in Fig. 4B, D, F, G) and its fluorescence distribution profile superimposed on those of type VI collagen, aggrecan, and COMP proteins.
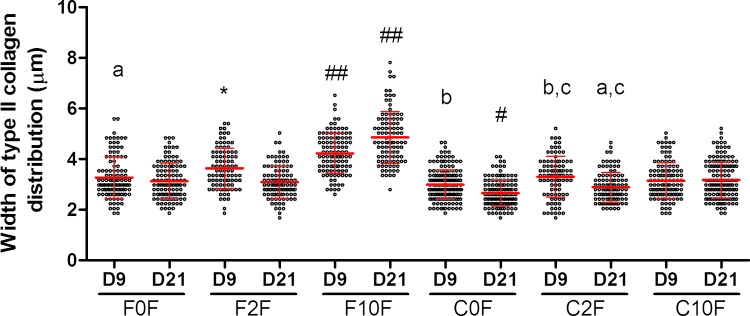
Our analysis showed that serum was beneficial to type II collagen deposition, but compressive loading can negate the benefits of serum. By day 9, type II collagen was uniformly distributed around the cells and no significant differences were detected in the extent of pericellular deposition in the serum-deprived cultures with (C0F, C2F) or without (F0F, F2F) compressive loading (Fig. 5). The quantification of type II collagen depositions are shown in Table 1 for all culture conditions at day 9 and 21. The thickest type II collagen deposition on day 9 was observed in the presence of the full complement of 10% serum and in the absence of compressive dynamic loading in the F10F group, averaging 4.2±0.8 μm. Between day 9 and day 21, only in the F10F group, the deposition of type II collagen extended further into the matrix, reaching 4.8 μm in thickness. However in the F2F, C0F, and C2F groups, the thickness of type II collagen distribution decreased by 0.5, 0.3, and 0.4 μm (p<0.01) over time, and it did not vary significantly over time in F0F and C10F groups. On day 21 (Table 1), the thinnest type II collagen accumulation was observed in the C0F (2.7 μm±0.5 μm) group, while the thickest one was still in the absence of compressive loading in the F10F (4.9 μm±1.0 μm) group. No significant differences were detected between F0F, F2F, C2F, and C10F groups.
FIG. 5.
The thickness of pericellular distribution of type II collagen on days 9 and 21. Measurements were based on at least 90 cells stained with type II collagen antibody in each group. ##Significantly different (p<0.01) from all groups; #significantly different (p<0.01) from all groups expect for C2F, on day 21 (D21); *significantly different (p<0.01) from all groups expect for C2F, on day 9 (D9); a, b, c: groups labeled with the same letter were significantly different (p<0.01) from each other. Color images available online at www.liebertpub.com/tea
Table 1.
Quantification of Type II Collagen Deposition Under Various Culture Conditions at Days 9 and 21
| Free-swelling culture conditions (no compressive loading) | Thickness of type II collagen deposition (μm) | Culture conditions with compressive loading | Thickness of type II collagen deposition (μm) |
|---|---|---|---|
| Day 9 | |||
| F0F | 3.3±0.8 | C0F | 3.0±0.6 |
| F2F | 3.6±0.8 | C2F | 3.3±0.8 |
| F10F | 4.2±0.8 | C10F | 3.1±0.7 |
| Day 21 | |||
| F0F | 3.1±0.7 | C0F | 2.7±0.5 |
| F2F | 3.1±0.6 | C2F | 2.9±0.6 |
| F10F | 4.9±1.0 | C10F | 3.2±0.7 |
Compressive loading also dramatically changed the distribution of the matrix proteins inducing more colocalization of the proteins studied. On day 9, pericellular distribution of aggrecan could be observed in all the groups, which generally co-localized with type VI collagen and partially overlapped with type II collagen (Fig. 6). However, with compressive loading in the C0F, C2F, and C10F groups, intracellular retention of aggrecan was observed (Fig. 6D, E, F). By day 21, in the C0F and C2F groups, aggrecan showed more pericellular accumulation than that on day 9, while aggrecan intracellular retention was still observed in all the loading groups. However, now the pericellular distribution of aggrecan overlapped with the collagen network (Fig. 6J, K, L).
FIG. 6.
Spatial distribution profiles of type II collagen (red lines), aggrecan (blue lines), type VI collagen (green lines), and COMP (yellow lines) on days 9 and 21 with (D–F, J–L) and without (A–C, G–I) dynamic compressive loading. Solid lines indicate average values; dotted lines indicate the range of standard deviation. Insets in each panel show representative dual-labeled deconvoluted immunohistochemical images. Scale bar: 20 μm. Color images available online at www.liebertpub.com/tea
Interestingly, type VI collagen was visible as a two-layer distribution around the cells in all groups (Fig. 6). The inner layer appeared as a densely stained region surrounding the cells, while the outer layer appeared as a less intense granular staining, which extended away from the inner layer to a distance of about 2 μm. The distribution of type II collagen partially overlapped with the inner layer of type VI collagen and was sandwiched between the two layers of type VI collagen. The spatial distribution of the inner layer of type VI collagen appeared to change little over time in all cultures (Fig. 6A–L).
COMP generally showed a two-layer distribution around cells in all groups on day 9 (Fig. 6A–F). The inner layer overlapped with type II collagen, while the outer layer appeared to be more extensive and distributed out of the range of type II collagen distribution. With compressive loading in the C0F, C2F, and C10F groups, COMP accumulated closer to the cell (Fig. 6D–F) than in the free-swelling cultures at day 9 (Fig. 6A–C). Notice the decrease in COMP deposition with compressive loading than that seen in the free-swelling cultures at day 9. However, by day 21, the range of COMP distribution was dramatically redistributed in all the groups, reconfigured to a single layer with more co-localization with type II collagen (Fig. 6G–L). However, with full serum complement of 10% FBS in the F10F group, the distribution of COMP was still beyond the range of type II collagen at day 21 but to a less extent than that at day 9 (Fig. 6C, I).
Discussion
This study was designed to examine the effects of serum and compressive dynamic loading on the spatiotemporal deposition of the main cartilaginous matrix proteins around single chondrocytes in vitro over 3-week cultivation period. Our study showed that high-level serum supplement could significantly suppress catabolic activities in matrix formation and result in a net increase in matrix deposition, while dynamic compressive loading could increase catabolic activities and potentially promote a rearrangement of matrix components. As to cell-associated matrix assembly, type II and VI collagen, aggrecan, and COMP formed a pericellular matrix network and showed different spatial distribution patterns. These matrix components exhibited more colocalization in the pericellular region over time in vitro. Dynamic compressive loading promoted this co-localization compared to free-swelling groups.
Our study showed that the thickness of type II collagen pericellular distribution around chondrocytes in low-serum supplemented groups was significantly thinner than that of the cells with full serum complement as in the F10F group, similar to that demonstrated in previous studies.13,14 However, this result appears to be at odds with the observed decrease in gene expression profiles in chondrocytes in high levels of serum supplementation even in 3D cultures coupled with the changes in cell morphologic features such as increase in cell size, which may not be favorable for engineering cartilage. Combined with the gene expression result, we found that the continuous increment in type II collagen deposition in F10F group was possibly due to the significant downregulation in MMP-3 and MMP-13 expression throughout the culture in the presence of serum. Many studies have implicated members of the TGFβ superfamily and insulin-like growth factor-1 in serum7,19,20; thus, 10% FBS in the culture medium could decrease the catabolic activity of numerous catabolic cytokines,12 while low levels of FBS (less than 5%) would not be able to inhibit the collagenase effectively.21 Our data also showed that dynamic compressive loading resulted in decreased type II collagen deposition in the C10F group compared to that in F10F, which indicated that loading could counteract the effect of 10% FBS on collagenase inhibition. Specifically, MMP-3 expression was increased by loading in all groups at days 7 and 15. However, in C0F and C2F groups, dynamic compressive loading, combined with low serum cultures, could result in higher MMP activities that eventually would lead to progressive matrix degradation. Kelly et al. reported that with compressive loading the functional properties of chondrocyte/agarose constructs in low serum culture were significantly lower than in free-swelling controls by day 42.13 Thus, in engineered cultures both mechanical and biochemical stimuli contribute to a fine balance between the continuous process of degradation and synthesis of extracellular matrix components to maintain the integrity of normal tissue.
One of the aims of our study was to investigate the spatiotemporal distribution of type II and VI collagen, aggrecan, and COMP around single cells in vitro, using IHC visualized by fluorescence deconvolution microscopy. In our histological analysis, we have chosen type II collagen as a reference. Even though the distribution of type II collagen is not uniform through the pericellular matrix, the spatial distribution of type II collagen is relatively more stable with a one-peak pericellular distribution pattern rather than the two-peak distribution pattern shown by COMP and type VI collagen or a possible intracellular distribution pattern shown by aggrecan. Therefore, type II collagen provided an ideal reference protein for our image analysis. Furthermore, the spatial distribution relationship of type II collagen with discussed matrix proteins is relatively consistent through the pericellular matrix, which made it an ideal spatial reference for this analysis. A potential drawback of this study is that we did not quantify the matrix using a 3D plot by computing the fluorescence around the entire cells, and may have allowed for a better plot with no sampling artifact. This, however, would have made data analysis of matrix proteins in relation to type II collagen more difficult to visualize and perform. Generating a radial line from the chondrocyte to the pericellular matrix allowed the spatial distribution profile to be averaged for each matrix protein, otherwise difficult to merge and average in 3D data.
Our studies demonstrated that the distribution of type II and VI collagen, aggrecan, and COMP was seen, forming a pericellular matrix network that tended to be more co-localized close to the cell on day 21 than on day 9. This indicated that it takes time for the generation of complex interactions among matrix components and the assembly of functional multimolecular network in vitro. This pericellular distribution is comparable to the pericellular matrix seen in vivo, which has been shown to be rich in proteoglycans and collagen.22 Furthermore, dynamic compressive loading promoted this co-localization compared to the free-swelling culture conditions. Specifically, dynamic loading promoted the redistribution of COMP around cells. COMP colocalized with type II collagen and accumulated closer to the cell than that seen in free-swelling cultures at day 9. However, dynamic compressive loading presumably also promoted the remodeling of proteoglycan. With loading, intracellular accumulation of aggrecan was observed to a large extent at days 9 and 21 by a mechanism yet unknown. It would seem that aggrecan fragments generated in vitro, by either aggrecanases or MMPs, are internalized, which may be the final pathway for complete catabolism of aggrecan.23 In contrast, type VI collagen showed a two-layer distribution around the cells in all groups where the spatial distribution of the inner layer changed little over time. Taken together, COMP and aggrecan showed a rapid turnover than collagen in chondrocyte/agarose constructs. Mechanical regulation of matrix metalloproteinases most probably played a key role in promoting the catabolic rates of pericelluar matrix network in vitro, as seen by the upregulation of MMP-3 on dynamic loading.
The cell density used in our current studies was 2×106 cells/mL for the purpose of investigation of matrix formation around single cells, reducing the influence from neighboring cells in the matrix rebuilding process. This cell density is much lower than commonly used densities of 6–10×106 cells/mL for tissue engineering purposes. Therefore, one drawback in our study is that we did not take into account the involvement of the cell density in matrix network establishment. In a recent study by Moreira et al., it is stated that chondrocytes cultured in microaggregates showed lower MMP-1, 9, and 13 expression levels and enhanced matrix deposition in constructs compared to single-cell-seeded constructs.24 Additionally, due to the low cell density in constructs, the quantity of newly synthesized matrix is too small to improve gross material properties, which would not make our samples suitable for material testing.
In tissue-engineered cartilage, matrix develops in response to the in vitro mechanochemical conditions. Our study proposed a new way to evaluate different culture conditions by comparing the subsequent redistribution patterns of the major cartilaginous matrix components around single cells. Matrix synthesis and deposition depends on many factors, protein synthesis, processing, assembly, and remodeling. The interplay between matrix proteins and enzymes activity is complex with enzymes involved in both the establishment and degradation of the matrix. Our study showed that serum-free culture conditions do not support an efficient matrix increment over 21 days of culture. High-level serum supplement was able to significantly suppress catabolic activities and appears to result in a net increase in matrix deposition. Dynamic compressive loading increases catabolic activities, leading to a denser and potentially more rigid matrix. Our ongoing studies are focused on investigating the maturation of matrix in various culture conditions along with structured characteristics of native tissue to determine which synthetic matrix reflects the functional matrix in vivo.
Author Contribution
P.W., E.D., and L.S.: experimental concept and design, analysis, interpretation of data, and article preparation. D.P.: data analysis, intellectual inputs, and article preparation. W.L.: experimental design. P.W., D.P., and L.S. were involved in the drafting, critical editing of the article, and final approval of the version to be submitted. L.S. and W.L. were responsible for obtaining funding for this work.
Acknowledgments
This work was supported, in whole or in part, by National Institutes of Health Grants 5R01AR045550-14 (to L.S.), Core Center Grant P30AR057235(to L.S.), and by China Scholarship Council 2011638072 (to P.W.).
Disclosure Statement
No competing financial interests exist. The study sponsors had no involvement in the study design, collection, analysis, and interpretation of data; in the writing of the article; or in the decision to submit the article for publication.
References
- 1.Gepstein A. Shapiro S. Arbel G. Lahat N. Livne E. Expression of matrix metalloproteinases in articular cartilage of temporomandibular and knee joints of mice during growth, maturation, and aging. Arthritis Rheum. 2002;46:3240. doi: 10.1002/art.10690. [DOI] [PubMed] [Google Scholar]
- 2.Hyttinen M.M. Holopainen J. van Weeren P.R. Firth E.C. Helminen H.J. Brama P.A. Changes in collagen fibril network organization and proteoglycan distribution in equine articular cartilage during maturation and growth. J Anat. 2009;215:584. doi: 10.1111/j.1469-7580.2009.01140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayes A.J. Hall A. Brown L. Tubo R. Caterson B. Macromolecular organization and in vitro growth characteristics of scaffold-free neocartilage grafts. J Histochem Cytochem. 2007;55:853. doi: 10.1369/jhc.7A7210.2007. [DOI] [PubMed] [Google Scholar]
- 4.Wilson R. Norris E.L. Brachvogel B. Angelucci C. Zivkovic S. Gordon L., et al. Changes in the chondrocyte, extracellular matrix proteome during post-natal mouse cartilage development. Mol Cell Proteomics. 2012;11:M111 014159. doi: 10.1074/mcp.M111.014159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halasz K. Kassner A. Morgelin M. Heinegard D. COMP acts as a catalyst in collagen fibrillogenesis. J Biol Chem. 2007;282:31166. doi: 10.1074/jbc.M705735200. [DOI] [PubMed] [Google Scholar]
- 6.Wilusz R.E. Defrate L.E. Guilak F. Immunofluorescence-guided atomic force microscopy to measure the micromechanical properties of the pericellular matrix of porcine articular cartilage. J R Soc Interface. 2012;9:2997. doi: 10.1098/rsif.2012.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mauck R.L. Nicoll S.B. Seyhan S.L. Ateshian G.A. Hung C.T. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9:597. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 8.Elder B.D. Athanasiou K.A. Systematic assessment of growth factor treatment on biochemical and biomechanical properties of engineered articular cartilage constructs. Osteoarthritis Cartilage. 2009;17:114. doi: 10.1016/j.joca.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blain E.J. Mechanical regulation of matrix metalloproteinases. Front Biosci. 2007;12:507. doi: 10.2741/2078. [DOI] [PubMed] [Google Scholar]
- 10.Nebelung S. Gavenis K. Luring C. Zhou B. Mueller-Rath R. Stoffel M., et al. Simultaneous anabolic and catabolic responses of human chondrocytes seeded in collagen hydrogels to long-term continuous dynamic compression. Ann Anat. 2012;194:351. doi: 10.1016/j.aanat.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Kelly T.A. Ng K.W. Wang C.C. Ateshian G.A. Hung C.T. Spatial and temporal development of chondrocyte-seeded agarose constructs in free-swelling and dynamically loaded cultures. J Biomech. 2006;39:1489. doi: 10.1016/j.jbiomech.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 12.Fortier L.A. Barker J.U. Strauss E.J. McCarrel T.M. Cole B.J. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469:2706. doi: 10.1007/s11999-011-1857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly T.A. Fisher M.B. Oswald E.S. Tai T. Mauck R.L. Ateshian G.A., et al. Low-serum media and dynamic deformational loading in tissue engineering of articular cartilage. Ann Biomed Eng. 2008;36:769. doi: 10.1007/s10439-008-9476-1. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y.H. Barabino G.A. Requirement for serum in medium supplemented with insulin-transferrin-selenium for hydrodynamic cultivation of engineered cartilage. Tissue Eng Part A. 2011;17:2025. doi: 10.1089/ten.TEA.2010.0415. [DOI] [PubMed] [Google Scholar]
- 15.Wan L.Q. Jiang J. Miller D.E. Guo X.E. Mow V.C. Lu H.H. Matrix deposition modulates the viscoelastic shear properties of hydrogel-based cartilage grafts. Tissue Eng Part A. 2011;17:1111. doi: 10.1089/ten.tea.2010.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gosset M. Berenbaum F. Thirion S. Jacques C. Primary culture and phenotyping of murine chondrocytes. Nat Protoc. 2008;3:1253. doi: 10.1038/nprot.2008.95. [DOI] [PubMed] [Google Scholar]
- 17.Bougault C. Paumier A. Aubert-Foucher E. Mallein-Gerin F. Investigating conversion of mechanical force into biochemical signaling in three-dimensional chondrocyte cultures. Nat Protoc. 2009;4:928. doi: 10.1038/nprot.2009.63. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Y. Oganesian A. Keene D.R. Sandell L.J. Type IIA procollagen containing the cysteine-rich amino propeptide is deposited in the extracellular matrix of prechondrogenic tissue and binds to TGF-beta1 and BMP-2. J Cell Biol. 1999;144:1069. doi: 10.1083/jcb.144.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madry H. Zurakowski D. Trippel S.B. Overexpression of human insulin-like growth factor-I promotes new tissue formation in an ex vivo model of articular chondrocyte transplantation. Gene Ther. 2001;8:1443. doi: 10.1038/sj.gt.3301535. [DOI] [PubMed] [Google Scholar]
- 20.Madry H. Padera R. Seidel J. Langer R. Freed L.E. Trippel S.B., et al. Gene transfer of a human insulin-like growth factor I cDNA enhances tissue engineering of cartilage. Hum Gene Ther. 2002;13:1621. doi: 10.1089/10430340260201716. [DOI] [PubMed] [Google Scholar]
- 21.Kandel R.A. Pritzker K.P. Mills G.B. Cruz T.F. Fetal bovine serum inhibits chondrocyte collagenase production: interleukin 1 reverses this effect. Biochim Biophys Acta. 1990;1053:130. doi: 10.1016/0167-4889(90)90004-w. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z. Jin W. Beckett J. Otto T. Moed B. A proteomic approach for identification and localization of the pericellular components of chondrocytes. Histochem Cell Biol. 2011;136:153. doi: 10.1007/s00418-011-0834-y. [DOI] [PubMed] [Google Scholar]
- 23.Embry Flory J.J. Fosang A.J. Knudson W. The accumulation of intracellular ITEGE and DIPEN neoepitopes in bovine articular chondrocytes is mediated by CD44 internalization of hyaluronan. Arthritis Rheum. 2006;54:443. doi: 10.1002/art.21623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moreira Teixeira L.S. Leijten J. Sobral J. Jin R. van Apeldoorn A.A. Feijen J., et al. High throughput generated micro-aggregates of chondrocytes stimulate cartilage formation in vitro and in vivo. Eur Cell Mater. 2012;23:387. doi: 10.22203/ecm.v023a30. [DOI] [PubMed] [Google Scholar]