Abstract
The initial adhesion of human umbilical vein endothelial cells (HUVECs), cord blood endothelial colony-forming cells (ECFCs), and human blood outgrowth endothelial cells (HBOECs) was studied under radial flow conditions. The surface of a variable shear-rate device was either coated with polymer films or covered by synthetic fibers. Spin-coating was applied to produce smooth polymer films, while fibrous scaffolds were generated by electrospinning. The polymer was composed of hexyl methacrylate, methyl methacrylate, poly(ethylene glycol) methacrylate (PEGMA), and CGRGDS peptide. The peptide was incorporated into the polymer system by coupling to an acrylate-PEG-N-hydroxysuccinimide comonomer. A shear-rate-dependent increase of the attached cells with time was observed with all cell types. The adhesion of ECs increased on RGD-linked polymer surfaces compared to polymers without adhesive peptides. The number of attached ECFCs and HBOECs are significantly higher than that of HUVECs within the entire shear-rate range and surfaces examined, especially on RGD-linked polymers at low shear rates. Their superior adhesion ability of endothelial progenitor cells under flow conditions suggests they are a promising source for in vivo seeding of vascular grafts and shows the potential to be used for self-endothelialized implants.
Introduction
The luminal surface of human blood vessel is covered with a monolayer of endothelial cells (ECs). It is known that the EC layer can significantly prevent blood protein deposition and the formation of thrombus, which makes the adhesion of ECs to artificial surfaces desirable in synthetic vascular grafts.1–3 There has been considerable research attempting to endothelialize material surfaces, such as EC seeding, employing EC-specific ligands, and pretreating surfaces with adhesive proteins.4–7 However, those methods require isolation of the host's ECs and the preculturing of such cells on the material surface before implantation. Furthermore, the endothelium often undergoes damage during implantation.
Recently, a particular group of cells known as Endothelial Progenitor Cells have shown to have great clinical potential. These are bone marrow-derived cells that have the capacity to differentiate into ECs.8 At least two distinct populations have been discovered: early outgrowth colony-forming cells and late outgrowth colony-forming cells.9 Late outgrowth endothelial colony-forming cells (ECFCs) are more EC like and they can be harvested from both cord and peripheral blood. ECFCs from peripheral blood are also known as human blood outgrowth endothelial cells (HBOECs).10 ECFCs possess higher proliferation potential and produce fewer growth factors than early outgrowth cells, which is less likely to promote neointimal hyperplasia.11 These findings suggest the use of ECFCs as a suitable source of cells for constructing functional tissue-engineered blood vessel replacements. However, those advantages can be offset by the current complex and time-consuming isolation procedures especially for HBOECs. Many animal species can endothelialize vascular grafts from adjacent tissue more readily. An alternative strategy for a human is to develop materials that self-endothelialize by harvesting ECFC from the circulation.
Previously, we used hexylmethacrylate (HMA), methylmethacrylate (MMA), and poly(ethylene glycol) methacrylate (PEGMA) to produce noncytotoxic and biostable surfaces for the growth and expansion of mature ECs (human umbilical vein endothelial cells [HUVECs]).12–14 Here, we wish to create a material tuned to capture circulating ECFCs and that has the potential to generate a confluent functioning endothelium in vivo. However, ECs are typically exposed to blood flow-induced shear forces under physiological conditions, and the effect of flow on the initial adhesion of ECs to surfaces has received little study. Since the response of ECs may differ substantially from those under static conditions,15–17 a radial flow chamber was used to study EC adhesion to surfaces under flow. Peptide incorporation was achieved by coupling with an acrylate-PEG-N-hydroxysuccinimide (NHS) spacer arm. The primary amine in the peptide can react with NHS ester producing stable amide bonds at the tip of the PEG arm.18 When exposed to hydrophilic media, the RGD group is expected to provide good ligand accessibility to cell receptors.
In this contribution, we examine the initial adhesion of HUVECs, cord blood ECFCs and HBOECs to surfaces under well-defined flow conditions. The influences of adhesive peptides were explored over a range of shear rates. Both cord blood ECFCs and HBOECs exhibited greater adhesion under flow especially on RGD containing polymers. In general, the EC adhesion was highly shear-rate-dependent and decreased markedly at higher shear rates.
Materials and Methods
Synthesis of polymers and the incorporation of peptides
Base polymer (H20) was copolymerized from 20 mol% HMA (Alfa Aesar) and 80 mol% MMA (ACROS Organics). Copolymerization took place in N,N-dimethylformamide (DMF) with 2,2-azobisisobutyronitrile (AIBN; Sigma-Aldrich) as the free radical initiator. The reaction proceeded for 2 days as previously described.19 At the end of the reaction, distilled water precipitated the polymer. The precipitate was washed, dried, and weighed.
Hydrophilic polymers (H20P15NHSRGD/RGE) were synthesized in two steps (Fig. 1). The acrylate-PEG-NHS (JenKem Technology USA, Inc.) was first combined with the peptide. The peptides used in this research were CGRGDS and CGRGES (Commonwealth Biotech, Inc.). RGD is adhesive to various types of cells, while RGE is not. The average molecular weight of acrylate-PEG-NHS is 2000. The reaction is conducted at a molar ratio of 1:1.2 PEG-NHS/Peptide. To prevent the production of acryloyl-PEG-OH, the product of the competing hydrolysis reaction, anhydrous DMF, was used as the solvent. Unacrylated peptides and NHS were separated from the desired product by dialysis (MWCO 2000) against deionized water for 24 h with periodic bath changes. The final acrylate-PEG-peptide monomer was lyophilized and stored at −20°C until use. In the second step, 20 mol% HMA, 65 mol% MMA, 14.8 mol% PEGMA, and 0.2 mol% acrylate-PEG-peptide are polymerized with AIBN. The average molecular weight of PEGMA is 500.The reaction proceeded for 2 days, and the polymer was collected by precipitation in distilled water. The chemical structure was confirmed by nuclear magnetic resonance, and the peptide content in the conjugate determined by amino acid analysis.
FIG. 1.
Polymerization process using N-hydroxysuccinimide (NHS) chemistry.
Composition analysis
The composition of the base polymer and the PEGylated polymer was determined through 1H Fourier transform nuclear magnetic resonance spectroscopy. Four hundred microliters of 0.05g/mL polymer in deuterated chloroform was analyzed on a 250-MHz NMR machine (Bruker DPX) at 300K. The peptides and the NHS molecules comprise only 0.2 mol% of the reactant. The detection of those compositions is beyond the sensitivity of the technique. The compositions of the polymers studied are summarized in Table 1.
Table 1.
Polymer Compositions and the Data of Water Contact Angle
| Polymer | Feed composition (mol% HMA/MMA/PEG) | Polymer composition (mol% HMA/MMA/PEG) | Average contact angle (°) | Amount of peptide incorporated (nmol peptide/mg polymer) |
|---|---|---|---|---|
| H20 | 20/80/0 | 23/77 | 80.1±2.1 | |
| H20P15NHSRGDa | 20/65/15 | 23/58/19 | 55.3±2.5 | 5.7±0.5 |
| H20P15NHSRGEa | 20/65/15 | 25/57/18 | 54.2±1.7 | 3.4±0.2 |
The feed compositions of these polymers was actually 20 mol% HMA, 65 mol% MMA, 14.8 mol% PEG, and 0.2 mol% acrylate-PEG-NHS, or 0.2 mol% acrylate-PEG-NHSRGD/RGE. The compositional analysis by NMR did not have the resolution to distinguish between PEG and PEG-NHSRGD/RGE.
HMA, hexylmethacrylate; MMA, methylmethacrylate; PEG, poly(ethylene glycol); NHS, N-hydroxysuccinimide.
Static contact angle
The polymer was coated onto a glass disk of 2 mm diameter by dip coating and dried under vacuum over night. Polymer films were pre-equilibrated with water for 3 h. A 2-μL droplet of deionized water was pipetted onto the polymer film, and the shape of the drop was captured using a camera. The contact angle was measured as the angle through the denser phase using ImageJ analysis software (Bethesda, MD).
Amino acid analysis
The amount of peptide incorporation was determined from amino acid analysis performed by Commonwealth Biotech, Inc.
Preparation of film surfaces for cellular adhesion and growth studies
Polymer samples were dissolved in 1:1 acetone:DMF at 5 wt%. Spin coating was performed in air by flooding the substrate surface with the solution (passed through a 0.2-μm filter) and spinning at 3500 rpm for 45 s. The films were then immediately dried in air for 5 min. Before contacting with cells, films were equilibrated with phosphate-buffered saline (PBS) for 1 h.
Preparation of fibrous surfaces for EC adhesion studies
Polymer samples were dissolved in a 1:1 volume ratio acetone:DMF mixture at 5 wt%. The polymer solution was fed by a syringe pump (Harvard Apparatus) into a steel capillary (inner diameter=0.047′) suspended vertically over the center of a glass disc. A combination of the three high-voltage generators (Gamma High-Voltage Research) were employed with a high-positive voltage (15 kV) to charge the steel capillary containing the polymer solution, and a negative voltage (−10 kV) was applied to the collector plate. The polymer solution was fed at a rate of 3 mL/h by the syringe pump. The component spacing and applied voltage were optimized to provide controlled deposition of fibrous matts. The fibrous matts were allowed to dry overnight at room temperature. Before contacting with cells, fibers were equilibrated with PBS for 1 h.
HUVEC and ECFC culture
HUVECs were obtained from American Type Culture Collection. The cells were cultured according to standard procedures. Briefly, the Dulbecco's modified Eagle's medium was supplemented with 0.1 mg/mL heparin (Sigma-Aldrich), 0.05 mg/mL EC growth supplement (Sigma-Aldrich), and 10 vol% fetal bovine serum. Cultures were incubated in a humidified environment at 37°C and 5% CO2. The culture medium was changed every 2 days, and cultures were passaged at 80% confluence to prevent contact inhibition. Passages 4 through 6 were used.
Cord blood ECFCs were bought from Lonza. Cells were cultured according to the standard procedure. The endothelial growth medium-2 (EGM-2) was supplemented with EGM-2 SingleQuots (Lonza) and 10 vol% fetal bovine. The cells were seeded onto collagen-coated T flasks. Cultures were incubated at 37°C with 5% CO2 and passaged at 80% confluence with 0.05% trypsin/ethylenediaminetetraacetic acid (EDTA). Passages 4 through 6 were used.
Adult peripheral blood was donated at the Ohio State Medical Center with the approval of an appropriate institutional review board. Buffy coat mononuclear cells were obtained from 30 to 50 mL fresh adult peripheral blood by a density gradient using the Lymphocyte Separation Medium (Mediatech, Inc.) according to the manufacturer's recommendations. The mononuclear fraction was resuspended in the supplemented EGM-2 medium and seeded into collagen-coated well plates at a density of 1×106 mL−1. Nonadherent cells were removed after 48 h and the medium was replaced every 2 days thereafter until the appearance of colonies with cobblestone-shaped cells. Colonies were split at 80% confluency. HBOECs were used between the third and fifth passages. For cell experiments, all cells were detached using 0.1 mM EDTA without trypsin.
Flow cytometry
Aliquots of 500,000 cells in 80 μL PBS containing 5% bovine serum albumin (BSA) were stained with 10 μL each of CD32 FcR blocker (Stem Cell Technologies), FITC CD34 (Miltenyi), PE CD133 (Miltenyi), and APC VGEFR-2 (Miltenyi). Isotype controls included APC IgG1, FITC IgG2a, and PE IgG (Miltenyi). HBOECs were incubated for 45 min at 4°C, and then washed twice with 0.5 mL PBS (1% BSA). Flow cytometry (BD LSR II) was conducted on live cells shortly after staining.
Radial flow chamber
Dickinson and Cooper described a radial flow chamber to study the adhesion of bacteria and neutrophils to polymer surfaces as a function of wall shear stress.20,21 In a single parallel plate flow chamber apparatus, a range of shear stresses can only be produced by varying the flow rate.22 It requires several individual runs to examine the effect of shear rates. In a radial flow chamber apparatus, the shear rate varies linearly with radial position. The influence of shear rates can be determined within one experiment significantly reducing the experimental error.
As shown in Figure 2, the radial flow chamber contains a Plexiglas housing with two optically flat glass disks, one 75 mm and the other 50 mm in diameter. The 50-mm glass disc is permanently cemented to the top of the chamber and bored with an inlet port in the center. The second disk is made of glass or tissue culture polystyrene (TCPS). It is removable and can be coated with a polymer test surface before its insertion into the chamber. After insertion, the chamber is sealed with O-rings and bolted together. The 75-mm disk sits at the bottom producing a narrow gap with the top chamber. The gap distance is roughly 300 μm. Fluid enters at the center of the cemented disc, flows over the removable one, and collects in an annular region surrounding the fixed disk. The fluid then exits the chamber via three equidistant ports in the Plexiglas housing of the chamber. The flow field within the radial flow cell can be analytically solved by assuming Newtonian flow between two parallel plates in cylindrical coordinates. The shear rate varies inversely with the radial distance in the chamber. The shear rate at the surface is defined as
 |
FIG. 2.
Structure of the radial flow chamber. (A) Schematic diagram of the radial flow chamber. (B) Schematic diagram of the scanned fields during experiments.
where S is the shear rate at the surface of the glass disk, Q is the volumetric flow rate, r is the radial position, and h the gap width.
The radial flow chamber was mounted on the motorized stage of a Nikon inverted microscope. A script in the ImagePro software was composed for the automated control of the stage and the rapid image archiving. Direct observation and counting of the attached cells was possible in real time. The observation field was located at several different positions within each radius as shown in Figure 1.
EC adhesion assay
HUVECs were bought from ATCC, while ECFCs were purchased from Lonza. HBOECs were derived from human blood samples following the Ohio State University protocols. The cells were cultured and proliferated in flasks and generations 4–6 were used in the experiments. The adhesions of HUVECs, cord blood ECFCs, and HBOECs under shear stress were determined using a radial flow chamber. Programmed software controlled the stage of a video-microscope for the rapid capturing of images. A background scan at every scanned flow filed was performed with only PBS flowing through the chamber. An EC suspension (6×105 cells/mL) was introduced into the chamber for the adhesion assay with a continuous field scan over time. The volumetric flow rate was maintained at 3 mL/min using the syringe pump. The resulting shear rate ranged from 0 to 40 s−1. Captured images were stored and analyzed. The number of the adherent cells as well as the cell morphology were determined. The duration of each independent experiment was about 15 min and at least two duplicates were carried out on each surface.
Tested surfaces include TCPS, H20, H20P15NHSRGD, and H20P15NHSRGE. The thickness of the polymer layer is less than 4 μm. Since the height of the flow channel is 300 μm, the reduction of the gap distance caused by coating with polymers is negligible. Polymer fibers were later generated by electrospinning and mounted in the flow cell. To visualize cells on polymer fibers, the cell tracker dye was applied to stain the cells.
Cell staining procedure for adhesion on fibrous scaffold
For the flow experiment on fibers, cells were detached and transferred in a 15 mL serum-free Dulbecco's modified Eagle's medium containing a 10 μL 5 μM cell tracker dye (Molecular Probes). Cells were incubated for 1 h at 37°C and stained blue. Centrifugation at 250 g for 5 min was subsequently performed. Cells were resuspended into a final concentration of 6×105 cells/mL and injected into the test chamber as described above.
Statistical analysis
Statistical software JMP (JMP) was used to compare data. One-way analysis of variance plus Tukey–Kramer analysis were conducted to determine which of the treatments were statistically different. In all experiments, a significance value of α<0.05 was used.
Results
Polymer characterization
The NMR spectra of the two functionalized polymers look similar to each other since the amount of peptide is beyond the detection range of NMR technology used. Figure 3 shows NMR spectra of the polymer without peptide incorporation. Allocating the peaks and analysis of the actual composition were followed as previously reported.19 Briefly, the chemical shift of the proton A in the HMA is 3.9 ppm. Proton B in the terminal methyl group of the MMA is the peak at 3.6 ppm. The peak at 3.4 ppm represents proton C in PEGMA. With respect to the analysis of H20P15 material, some of the signal from MMA overlaps with proton D from PEGMA. By studying the spectrum of pure PEG material, we can get the amount of area of PEG that is associated with the area of proton C. The area of protons in MMA is calculated by a simple deduction.
FIG. 3.
1H NMR spectra of H20P15.* *H20P15 is composed of 20 mol% hexylmethacrylate, 65 mol% methylmethacrylate, and 15 mol% PGMA.
Details of peptide incorporation along with the summary of the methacrylate copolymer compositions studied are shown in Table 1. A summary of the methacrylate copolymer compositions studied is shown in Table 1. In this report, the polymer is referred by the mole percent of the monomer and the composition MMA is omitted in the terminology. H20 refers to a base material synthesized from 20/80 mol% HMA/MMA. H20P15NHSRGD/RGE refers to a PEGylated polymer with the peptide incorporated by NHS chemistry, which was copolymerized from 20/65/14.8/0.2 mol% HMA/MMA/PEGMA/acrylate-PEG-RGD. The actual content of MMA is lower than the feed composition, which is consistent with a previous report.19,23 Even though there is a small deviation in MMA, the overall polymer composition is similar to that of the feed.
The base H20 material is hydrophobic and processes a significantly larger contact angle than other materials (Table 1). After incorporation of 15 mol% PEG units, the water contact angle decreased by about 30°. The two PEG-containing polymers are similar to each other with respect to the average contact angles.
Biological interactions
Human blood endothelial outgrowth cells from adult peripheral blood
After several days in the EGM-2 medium, a few adherent mononuclear cells from adult peripheral blood began to expand on collagen substrates. The cells formed mature colonies by 3 weeks. As these cells approached confluence, they exhibited typical cobblestone morphology. Flow cytometry of 4th passage adult HBOECs gave 0.3% CD133(+), 1.3% CD34(+), and 23% VEGFR-2(+). Lonza, Inc. reports that ECFC® expresses an identical amount of CD34.
Adhesion pattern and the effect of detaching conditions
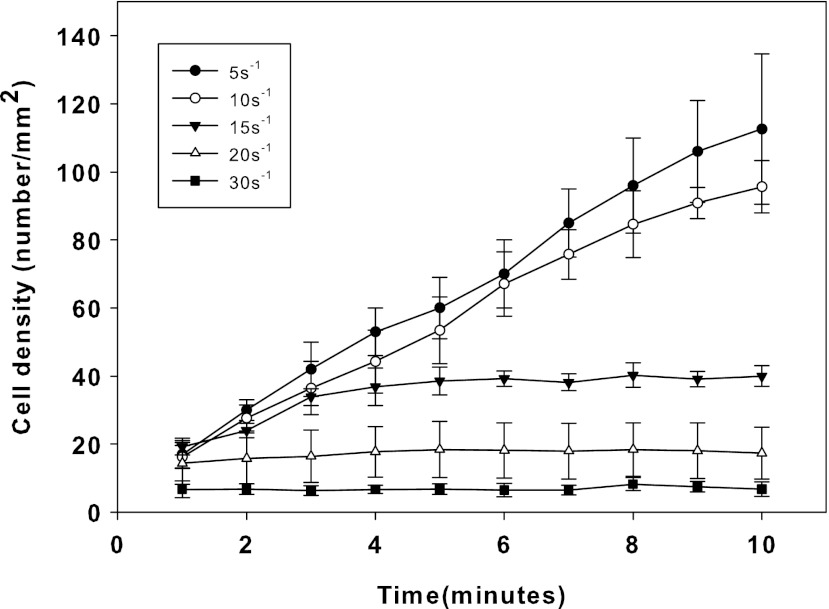
At low flow rates on TCPS, cord blood ECFCs adhered linearly with time, but at higher shear rates, a steady state was reached at lower cell densities illustrating shear-dependent cellular adhesion as observed in Figure 4. At 30 s−1 shear rate, cellular adhesion on TCPS was minimal. As the shear rate decreases, more and more cells attached in the observation area. At 5 s−1 shear rate, nearly 70% of the surface was covered by cells after 10 min. By counting the number of attached cells at 10 min, one can compare the degree of cell adhesion under different conditions.
FIG. 4.
Real-time shear-dependent cord blood endothelial colony-forming cells (ECFCs) adhesion to tissue culture polystyrene (TCPS).
Figure 5 summarizes the degree of cord blood ECFCs adhesion on TCPS using three cell detaching agents. It was observed even in the absence of serum proteins that the cell adhesion was highest on EDTA detached cells, somewhat lower for 0.025% trypsin/EDTA detached cells, and lowest on 0.25% trypsin/EDTA detached cells. At 5 s−1 shear rate, the difference between detaching conditions is not significant. However, at higher shear rates, trypsin detached cells had a much lower adhesion. In the subsequent experiments, all the cells were detached by trypsin-free EDTA.
FIG. 5.
Cord blood ECFC attachment on TCPS with different lifting conditions after 10 min.
Dynamic adhesion of HUVECs and cord blood ECFCs to unfunctionalized polymer films
A comparison of HUVEC and cord blood ECFC attachment at 10 min on TCPS is shown in Figure 6. The number of adherent HUVECs is less compared with cord blood ECFCs for the range of shear rates examined (5–30 s−1). At higher shear rates, the adhesion ability of cord blood ECFCs is higher by a factor of 3 to 4 compared with that of HUVECs. Figure 6 also shows that cord blood ECFCs are less sensitive to shear rate when adhering to H20. There are relatively more cord blood ECFCs on hydrophobic H20 surface especially at higher shear rates.
FIG. 6.
Dynamic adhesion of human umbilical vein endothelial cells (HUVECs) and cord blood ECFCs to TCPS and H20 after 10 min.
Dynamic adhesion of HUVECs and ECFCs to functionalized polymer films
Figure 7 shows the dynamic adhesion of HUVECs to the functionalized polymer surfaces at 10 min. The 30 s−1 shear rate is too high for any cells to adhere on all the surfaces examined. At lower shear rates, there are almost no cells on the H20P15NHSRGE substrate, indicating that the presence of PEG significantly reduced the degree of cell adhesion. At all shear rates, the cell density on TCPS, the positive control, is the highest. H20P15NHSRGD showed progressively more cell adhesion as the shear rate was lowered.
FIG. 7.
HUVEC adhesion on functionalized polymer surfaces after 10 min.
The comparison of HUVEC and ECFC attachment at 10 min on functionalized polymer films is shown in Figure 8. For all three cell types, the number of adhered cells increased as the flow rate decreased. Minimal cell adhesion was found on the H20P15NHSRGE surface. An interesting result is that the degree of cord blood ECFCs adhesion was much higher than that of HUVECs over the entire shear-rate range examined. The H20P15NHSRGD surface was almost saturated with cells at 5 s−1 shear rate after 10 min. The cell density of HBOECs was similar with that of cord blood ECFCs at low shear rates. A clear difference was observed between those two types of ECFCs at 15 s−1. HBOECs are less adhesive compared with cord blood cells, but still more adhesive than HUVECs.
FIG. 8.
Dynamic adhesion of HUVECs, cord blood ECFCs, and human blood outgrowth endothelial cells (HBOECs) to functionalized polymer films after 10 min. (A) Adhesion of HUVECs and both types of ECFCs on H20P15NHSRGD films. (B) Adhesion of HUVECs and both types of ECFCs on H20P15NHSRGE films.
Dynamic adhesion of HUVECs and ECFCs to functionalized polymer fibers
Figure 9A shows the scanning electron microscope (SEM) image of the electrospun H20P15NHSRGE polymer. The materials used in this research are produced by the copolymerization of several acrylate monomers, including the hydrophilic PEG chain. The generated fibers seem to coalesce resulting in a small pore size. All the fibers containing PEG with and without a peptide look similar to each other. They all have the sponge-like structure as illustrated in Figure 9A.
FIG. 9.

Scanning electron micrographs of polymer fibers and fluorescence microscope images of cells adherent on fibers. (A) SEM of electrospun H20P15NHSRGE polymer fibers at ×400 magnification. (B) Cord blood ECFCs on H20P15NHSRGD polymer fibers after 10 min. Color images available online at www.liebertpub.com/tea
A comparison of HUVEC, cord blood ECFC, and peripheral blood ECFC attachment after 10 min at the lowest shear rate on fibers is shown in Figure 10. Since the fibers are opaque using the bright field microscopy with the flow cell, the cells were stained blue to count them using fluorescent illumination. Similar trends were observed to that on films. The RGD peptide promoted the adhesion of all cell types and both ECFCs exhibited greater adhesion than HUVECs. There are no significant differences between cord blood ECFCs and HBOECs on fibers and films at a low shear rate. The number of attached cells was lower on fibers than what was found on films. Figure 9B also shows the adherent cells glowing on the polymer fibers at 5 s−1 shear rate after 10 min.
FIG. 10.
Dynamic adhesion of HUVECs, cord blood ECFCs, and HBOECs to functionalized polymer fibers after 10 min at 5 s−1*. *Cells tested on fibers were stained with cell tracker dye, while cells tested on films were not.
Discussion
ECFCs are a promising cell type in the field of tissue engineering as a means of improving biocompatibility of vascular grafts. The effectiveness of using ECFCs is limited by the time and complex procedures required to expand them in vitro. An alternative is to capture ECFCs directly from the blood. A radial flow chamber with peptide-linked polymers was fabricated in this contribution to understand the real-time adhesion of cells under flow. The results in this study demonstrated that the degree of EC adhesion on surfaces dynamically depends on material hydrophilicity, peptide density, cell detaching conditions, shear rate, and the type of cell.
The hydrophilicity of materials is determined by functional groups, mainly the PEG motif in this research. Even though some of the materials are functionalized by a peptide, the peptide density is too low to affect hydrophilcity. The base polymer H20 without any PEG is hydrophobic, which is demonstrated by the water contact angle in Table 1. A hydrophobic surface is usually adhesive for proteins and cells due to the entropy gain24 in the deposition process, which might be the reason that cord blood ECFCs had higher adhesion on H20 compared to TCPS as illustrated in Figure 6. One can tell that the dramatic increase of surface hydrophilicity from H20 to other materials is caused by the introduction of PEG. Since PEG is a well-known anti-fouling molecule, both HUVEC and ECFC adhesion was minimal even at the lowest shear rate examined.
The PEG containing polymers are intrinsically passive for the cellular attachment. On those surfaces, the peptide plays a major role in the cell capturing. In previous experiments on HUVECs, 2 nmol/mg RGD promoted robust HUVEC adhesion.14 The peptide density in present research is comparable and the results are consistent with these earlier observations. As RGE is nonadhesive, the PEG-RGE surface was nonadhesive to both types of cells at all shear rates. Compared to the PEG-RGE peptide containing surfaces, much higher HUVEC and ECFC adhesion was observed on the PEG-RGD surface, especially at low shear rates.
An important and often neglected factor in studies of cell adhesion is the cell detaching condition. The degree of cell adhesion was significantly reduced if the cells were detached by trypsin. In this study, the effect became more obvious with cells exposed to higher shear rates. While these changes in cell adhesion may be attributed to the damage of transmembrane proteins due to trypsin digestion, it was interesting to see an effect on adhesion even though there was minimal adsorbed protein on the polymers.25
Another important factor that affects cell adhesion is the shear rate. Dickinson and Cooper20 observed that the number of adherent bacteria on the surface increased as the shear rate decreased in the radial flow apparatus. In this research, consistent results are found. Relatively, more cells stayed on the surface in the area with a low flow rate as shown in Figures 7 and 8. Surfaces exposed to the highest shear rates barely support cell adhesion. The ligand receptor reaction requires time to take place,26 so a slower flow provides more time for cells to interact to peptides. In addition, higher shear rates were observed to remove cells due to the higher shear stresses on the cells. Both reasons can rationalize low cell adhesion and the plateau observed at higher shear rates. At lower flow rates, the number of attached cells increased linearly with time. The reverse relationship between the shear rate and the number of cells adherent indicates that convection dominates the process of cell transport. If cell adhesion was controlled by diffusion, a larger cell density should have been observed at the surrounding area of the inlet that is exposed to higher shear rates.27
This study also suggests an advantage of using ECFCs as an alternative cell source to endothelialize biomaterials for tissue engineering. The adhesion of HUVECs, cord blood ECFCs, and HBOECs was studied and compared to that of control polymer, TCPS, RGD films, and RGD fibers. In Figure 8, the number of adherent ECFCs on RGD films is three- to five-fold higher that of HUVECs. Both cord blood ECFCs and HBOECs are smaller in size and it may be that the forces required to stop them are significantly reduced. At low flow rates, most of the RGD surface can be endothelialized with ECFCs within 10 min. The difference between cord blood ECFC and HBOEC was minimal at low flow rates. Cord blood ECFC showed superior adhesion ability at higher shear rates. Compared to HUVECs, the procedure required to harvest cord blood ECFCs or HBOECs is complex and tedious and can take weeks of culture. HBOECs may express a more mature EC phenotype28 and become less robust in adhering compared with cord blood ECFCs. However, considering their long lifetime and fast expansion rate, both cord blood ECFCs and HBOECs are a promising cell source to create an endothelial layer under flow conditions. The shear rate examined in this research is less than that found in most arterial flow situations. Our aim is to overcome thrombosis in small diameter vascular grafts by creating an affinity surface where HBOECs could adhere to the surface and generate a fine endothelium after weeks or months.
For the cell adhesion on fibers, fewer cells attached on the fibrous mats. In a previous report, more cells were retained on the fibrous mats under static conditions since some of the cells were physically trapped.29 However, in the current work, the small pore size, flow, and the turbulence formed near the irregular surface may have decreased cell adhesion. In addition, for experiments using fibers, all of the cells were stained by the cell tracker dye, which might affect the adhesion characteristics of the cells.
Future work will employ phage display selected peptides directed to HBOECs7 with the aim of capturing circulating progenitor cells on a vascular graft construct.
Conclusions
This work is a preliminary study on developing a system that can capture ECFCs and self-endothelialize in vivo. Surface properties and the shear rate influence the degree of cell adhesion and highlight the superior adhesion ability of both types of ECFCs compared to HUVECs. Surface hydrophobicity, the presence of PEG moieties and RGD peptide, can significantly affect the efficiency of cell adhesion. The adhesion of cells to surfaces is the result of competition between cell-surface interactions and hydrodynamic forces. RGD promotes cell adhesion, while high shear rates reduce the number of attached cells. The cell density of both types of ECFCs was higher than that of HUVEC on any of the surfaces within the entire shear rate range examined. Similar trends were observed on electrospun fibers of the same materials.
Acknowledgments
The authors thank Dr. Vassiliki A. Tegoulia, who shared her expertise on the radial flow apparatus; Dr. Jianjun Guan, who provided access to his electrospinning device; and Dr. Nicanor Moldovan's group, which provided the HBOECs. This work was supported in part by NIH Grant RC2 AG036559.
Disclosure Statement
No competing financial interests exist.
References
- 1.Nerem R.M. Seliktar D. Vascular tissue engineering. Annu Rev Biomed Eng. 2001;3:225. doi: 10.1146/annurev.bioeng.3.1.225. [DOI] [PubMed] [Google Scholar]
- 2.Ratcliff A. Tissue engineering of vascular grafts. Matrix Biol. 2000;9:353. doi: 10.1016/s0945-053x(00)00080-9. [DOI] [PubMed] [Google Scholar]
- 3.Ratner B.D. Blood compatibility—a perspective. J Biomater Sci Polym Ed. 2000;11:1107. doi: 10.1163/156856200744219. [DOI] [PubMed] [Google Scholar]
- 4.Dichek D.A. Neville R.F. Zwiebel J.A. Freeman S.M. Leon M.B. Anderson W.F. Seeding of intravascular stents with genetically engineered endothelial cells. Circulation. 1989;80:1347. doi: 10.1161/01.cir.80.5.1347. [DOI] [PubMed] [Google Scholar]
- 5.Rosenman J.E. Kempczinski R.F. Pearce W.H. Silberstein E.B. Kinetics of endothelial cell seeding. J Vasc Surg. 1985;2:778. [PubMed] [Google Scholar]
- 6.Clark R. Folkvord J.M. Nielsen L.D. Either exogenous or endogenous fibronectin can promote adherence of human endothelial cells. J Cell Sci. 1986;82:263. doi: 10.1242/jcs.82.1.263. [DOI] [PubMed] [Google Scholar]
- 7.Veleva A.N. Heath D.E. Cooper S.L. Patterson C. Selective endothelial cell attachment to peptide-modified terpolymers. Biomaterials. 2008;29:3656. doi: 10.1016/j.biomaterials.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 8.Reed M.J. Karres N. Eyman D. Edelberg J. Endothelial precursor cells. Stem Cell Rev. 2007;3:218. doi: 10.1007/s12015-007-0007-5. [DOI] [PubMed] [Google Scholar]
- 9.Yoder M.C. Mead L.E. Prater D. Krier T.R. Mroueh K.N. Li F., et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin Y. Weisdorf D.J. Solovey A. Hebbel R.P. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murohara T. Cord blood-derived early outgrowth endothelial progenitor cells. Microvasc Res. 2010;79:174. doi: 10.1016/j.mvr.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Heath D.E. Cooper S.L. Interaction of endothelial cells with methacrylic terpolymer biomaterials. J Biomed Mater Res B Appl Biomater. 2010;92:289. doi: 10.1002/jbm.b.31514. [DOI] [PubMed] [Google Scholar]
- 13.Fussell G.W. Cooper S.L. Synthesis and characterization of acrylic terpolymers with RGD peptides for biomedical applications. Biomaterials. 2004;25:2971. doi: 10.1016/j.biomaterials.2003.09.062. [DOI] [PubMed] [Google Scholar]
- 14.Fussell G.W. Cooper S.L. Endothelial cell adhesion on RGD-containing methacrylate terpolymers. J Biomed Mater Res A. 2004;70:265. doi: 10.1002/jbm.a.30074. [DOI] [PubMed] [Google Scholar]
- 15.Levesque M.J. Nerem R.M. Sprague E.A. Vascular endothelial cell proliferation in culture and the influence of flow. Biomaterials. 1990;11:702. doi: 10.1016/0142-9612(90)90031-k. [DOI] [PubMed] [Google Scholar]
- 16.Brown M.A. Wallace C.S. Angelos M. Truskey G.A. Characterization of umbilical cord blood-derived late outgrowth endothelial progenitor cells exposed to laminar shear stress. Tissue Eng Part A. 2009;15:3575. doi: 10.1089/ten.tea.2008.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plouffe B.D. Njoka D.N. Harris J. Liao J. Horick N.K. Radisic M., et al. Peptide-mediated selective adhesion of smooth muscle and endothelial cells in microfluidic shear flow. Langmuir. 2007;23:5050. doi: 10.1021/la0700220. [DOI] [PubMed] [Google Scholar]
- 18.Laufer D.A. Chapman T.M. Marlborough D.I. Vaidya V.M. Blout E.R. A reagent for peptide synthesis. Copoly(ethylene-N-hydroxymaleimide) J Am Chem Soc. 1968;90:2696. doi: 10.1021/ja01012a045. [DOI] [PubMed] [Google Scholar]
- 19.Heath D.E. Cooper S.L. Design and characterization of PEGylated terpolymer biomaterials. J Biomed Mater Res A. 2010;94:1294. doi: 10.1002/jbm.a.32811. [DOI] [PubMed] [Google Scholar]
- 20.Dickinson R. Cooper S. Analysis of shear-dependent bacterial adhesion kinetics to biomaterial surfaces. AIChE J. 1995;41:2160. [Google Scholar]
- 21.Yung L.Y. Lim F. Khan M.M. Kunapuli S.P. Rick L. Colman R.W., et al. High-molecular-weight kininogen preadsorbed to glass surface markedly reduces neutrophil adhesion. Biomaterials. 2000;21:405. doi: 10.1016/s0142-9612(99)00203-3. [DOI] [PubMed] [Google Scholar]
- 22.Ling X. Ye J.F. Zheng X.X. Dynamic investigation of leukocyte-endothelial cell adhesion interaction under fluid shear stress in vitro. Acta Biochim Biophys Sin. 2003;35:567. [PubMed] [Google Scholar]
- 23.Wang X. Heath D.E. Cooper S.L. Endothelial cell adhesion and proliferation to PEGylated polymers with a covalently linked RGD peptide. J Biomed Mater Res Part A. 2012;100A:794. doi: 10.1002/jbm.a.34026. [DOI] [PubMed] [Google Scholar]
- 24.Roach P. Farrar D. Perry C.C. Interpretation of protein adsorption: surface-induced conformational changes. J Am Chem Soc. 2005;127:8168. doi: 10.1021/ja042898o. [DOI] [PubMed] [Google Scholar]
- 25.Brown M.A. Wallace C.S. Anamelechi C.C. Clermont E. Reichert W.M. Truskey G.A. The use of mild trypsinization conditions in the detachment of endothelial cells to promote subsequent endothelialization on synthetic surfaces. Biomaterials. 2007;28:3928. doi: 10.1016/j.biomaterials.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schuck P. Kinetics of ligand binding to receptor immobilized in a polymer matrix, as detected with an evanescent wave biosensor. I. A computer simulation of the influence of mass transport. Biophys J. 1996;70:1230. doi: 10.1016/S0006-3495(96)79681-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bird R.B. Stewart W.E. Lightfoot E.N. Transport Phenomena. New York: John Wiley & Sons; 1960. [Google Scholar]
- 28.Ingram D.A. Mead L.E. Tanaka H. Meade V. Fenoglio A. Mortell K., et al. Identification of a novel hierarchy of endothelialprogenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 29.Veleva A.N. Heath D.E. Johnson J.K. Nam J. Patterson C. Lannutti J.J., et al. Interactions between endothelial cells and electrospun methacrylic terpolymer fibers for engineered vascular replacements. J Biomed Mater Res A. 2009;91:1131. doi: 10.1002/jbm.a.32276. [DOI] [PMC free article] [PubMed] [Google Scholar]