Abstract
Poly(l-lactide) (PLLA) microfibrous scaffolds produced by electrospinning were treated with mild Ar or Ar-NH3/H2 plasmas to enhance cell attachment, growth, and infiltration. Goniometry, atomic force microscopy (AFM), and X-ray photoelectron spectroscopy (XPS) measurements were used to evaluate the modification of the scaffold surface chemistry by plasma treatment. AFM and XPS measurements showed that both plasma treatments increased the hydrophilicity without affecting the integrity of the fibrous structure and the fiber roughness, whereas Ar-NH3/H2 plasma treatment also resulted in surface functionalization with amine groups. Culture studies of bovine aorta endothelial cells and bovine smooth muscle cells on the plasma-treated PLLA scaffolds revealed that both Ar and Ar-NH3/H2 plasma treatments promoted cell spreading during the initial stage of cell attachment and, more importantly, increased the cell growth rate, especially for Ar plasma treatment. In vitro cell infiltration studies showed that both plasma treatments effectively enhanced cell migration into the microfibrous scaffolds. In vivo experiments involving the subcutaneous implantation of plasma-treated PLLA scaffolds under the skin of Sprague-Dawley rats also showed increased cell infiltration. The results of this study indicate that surface treatment of PLLA microfibrous scaffolds with mild Ar or Ar-NH3/H2 plasmas may have important implications in tissue engineering. Further modifications with bioactive factors should improve the functions of the scaffolds for specific applications.
Introduction
Microfibrous structures synthesized by electrospinning are of particular interest in bioengineering due to their high porosity and biodegradability that make them ideal candidates for polymer scaffolds. However, because polymer surfaces (solid or fibrous), such as poly(l-lactide) (PLLA), are hydrophobic, cell attachment and growth on polymer scaffolds is limited. Therefore, various surface treatments have been used to modify the chemical behavior of PLLA surfaces in order to improve biocompatibility.1 Plasma-assisted surface modification is a common method of tuning biochemical surface properties to specific application needs. This method provides a wide range of surface functionalities, which can improve biocompatibility either directly or indirectly through biomolecule surface immobilization. For instance, surface functionalization with hydrophilic chemical groups (e.g.,−COOH and −NH2) by reactive gas plasma treatment or surface chemical modification by film deposition2–11 and coating of polymer surfaces by various extracellular matrix proteins (e.g., collagen, gelatin, and laminin)12–18 and other bioactive molecules by plasma treatment19,20 have been shown to improve the biocompatibility of polymer materials.
In addition to studies devoted to the increase of the surface hydrophilicity of biopolymers for promoting bioactive molecular and protein attachment, the direct effect of plasma surface treatment on biocompatibility has also received significant attention. Surface treatment with simple plasmas (e.g., air, Ar, O2, and NH3) has been reported to enhance cell growth.3–8 NH3 plasma treatment resulting in −NH2 surface functionalization has been proven to be more effective in improving cell growth on polymer surfaces than O2 and SO2 plasma treaments.2,3,8 Furthermore, plasma-synthesized polymer coatings rich in −NH2 and−COOH surface groups have been reported to promote cell growth on scaffold surfaces.9–11 However, relatively less is known about the effect of inert gas plasma treatment of polymers on cell growth,5,7 and information about the effect of inert or reactive gas plasmas on cell infiltration in three-dimensional structures is sparse. The intense conditions of the inert gas plasmas used in previous studies to produce detectable chemistry modification induced structural damage and/or roughening of the polymer fibers due to thermal heating and excessive plasma etching, respectively. Since intense plasma treatment of polymers leads to both morphological and surface chemical changes, it is difficult to determine the effect of each type of modification on the resulting biocompatibility characteristics and the effect of possible changes in the fiber structure and morphology on the mechanical strength of the scaffolds. Mild plasma conditions conducive to only chemical surface modification are preferred because they do not damage the scaffold material, enabling the effects of plasma-induced surface chemical changes on biocompatibility to be examined. However, indepth studies of in vivo cell infiltration, such as characterization of infiltrated cells and cell proliferation in vivo, have not been reported.
The main objective of this investigation was to examine the surface chemical modification of PLLA microfibrous scaffolds induced by different plasma treatments under relatively mild conditions. Inert (Ar) plasma treatment was selected to remove any surface contaminants and to produce ex situ oxygen surface functionalities (e.g., −OH and −COOH) upon the exposure of the activated PLLA surfaces to the ambient.21 Reactive (NH3) plasma treatment was used to produce in situ nitrogen-containing surface functionalities, such as amine (−NH2) groups. The NH3 plasma treatment was optimized by mixing NH3 with Ar gas, followed by a post-treatment with H2 plasma to maximize the fraction of −NH2 surface functionalities. Plasma-treated scaffold surfaces were characterized by goniometry, scanning electron microscopy (SEM), atomic force microscopy (AFM), and X-ray photoelectron spectroscopy (XPS) measurements. Surface chemical modification by NH3 plasma treatment was studied by tracking the nitrogen surface concentration, while the incorporation of −NH2 functional groups was examined with a chemical derivative method described in a previous study.22 Bovine aorta endothelial cells (BAECs) and bovine smooth muscle cells (BSMCs) were used as representative cell lines to examine the effect of plasma treatment on cell attachment and growth. Both in vitro and in vivo experiments were performed to gain insight into the direct effect of plasma treatment on cell proliferation and cell infiltration into the electrospun PLLA microfibrous scaffolds.
Experimental Procedures
Sample preparation
PLLA of an inherent viscosity equal to 1.09 dL/g (Lactel Absorbable Polymers, Pelham, AL) was used to fabricate microfibrous scaffolds by electrospinning, as described previously.23 PLLA pellets were dissolved in 19% (w/v) hexafluoroisopropanol. The solution was then delivered by a programmable pump to an electrically charged needle under a high voltage (12 kV), causing the ejection of polymer fibers of diameter between hundreds of nanometers to 1 μm. The electrostatically charged fibers were collected onto the surface of a grounded drum kept at a fixed distance (8 cm) from the needle tip, resulting in the formation of a nonwoven microfibrous scaffold on the drum surface. Fiber alignment during electrospinning was controlled by adjusting the rotational speed of the drum, while the scaffold thickness was varied by adjusting the electrospinning time. The low rotational speed (150 rpm) used in this study resulted in randomly oriented fibers. The thickness of the scaffolds used for surface topography and chemistry characterization and for cell culture was ∼100 μm, whereas that of the scaffolds used in the in vitro and in vivo cell infiltration studies was ∼250 μm. Film thickness measurements were obtained with a thickness gauge (Mitutoyo America, Aurora, IL).
Plasma treatment of PLLA microfibrous scaffolds
Surface modification of the PLLA microfibrous scaffolds was accomplished with a radio-frequency capacitively coupled plasma reactor (Plasmalab 80plus, Oxford Instruments, Oxfordshire, United Kingdom) with a plate of diameter equal to 20 cm and a plate-to-plate distance fixed at 2 cm. Before processing, the chamber was cleaned for 5 min with Ar plasma (300 W power, 100 sccm Ar gas flow rate, and 0.9 Torr pressure). Ar plasma treatment was performed for 2 min under conditions of 30 W power, 100 sccm Ar gas flow rate, and 0.5 Torr pressure. NH3 plasma treatment comprised a 5-min treatment with a mixture of Ar and NH3 (50 W power, 30/70 sccm Ar/NH3 gas flow rate, and 0.5 Torr pressure) followed by a 0.5-min treatment with H2 (10 W power, 50 sccm H2 gas flow rate, and 0.5 Torr pressure) to maximize the incorporation of −NH2 groups at the scaffold surface. Hereafter, the latter treatment will be referred to as the Ar-NH3/H2 plasma treatment. Before removing the plasma-treated samples from the chamber, an ambient pressure was established by backfilling the chamber with N2 gas.
Characterization of plasma-treated surfaces
Surface morphology
The surface morphology of the plasma-treated microfibrous scaffolds was examined with a field emission SEM (TM-1000, Hitachi, Pleasanton, CA) and an AFM (Dimension 3100, Veeco Instruments, Plainview, NY), operated in the tapping mode to avoid surface damage of the soft sample surfaces. AFM imaging was performed with 10-nm-radius silicon tips attached to silicon cantilevers of spring constant equal to 46 N/m (NSC15/AlBS, MicroMasch, Wilsonville, OR).
Contact angle measurements
Static contact angle measurements were used to study the effect of plasma treatment on the hydrophilic behavior of electrospun PLLA microfibrous scaffolds, as well as PLLA solid membranes fabricated by thermal molding, for comparison. The surface wetting characteristics were examined with a drop-shape analysis system (DSA10, Krüss GmbH, Hamburg, Germany). Deionized water droplets (∼6 μL) were delivered to the sample surface by a syringe at room temperature and the droplet configuration was captured with a camera. From the measured angle between the droplet baseline and the tangent at the water/air boundary, a contact angle was calculated as the average of the measured left and right contact angles. For statistical analysis, six contact angle measurements were obtained from three different surface regions of two identical samples.
Chemical analysis
The surface chemical composition was studied with an XPS system (Perkin-Elmer PHI 5400 ESCA), without charge neutralization or monochromator, equipped with an Al-Kα X-ray source of photon energy equal to 1486.6 eV. All of the XPS spectra were obtained with a take-off angle of 54.7°, measured from the analyzer axis. During spectral acquisition, the pressure in the main chamber was maintained at ∼10–7 Torr. Survey spectra were acquired in the binding energy range of 0–1100 eV with pass energy of 178.95 eV. To determine the surface chemical composition of the PLLA microfibrous scaffolds before and after plasma treatment, high-resolution XPS spectra of the C1s, O1s, and N1s core level peaks were collected with pass energy of 35.75 eV. XPS results were deduced from at least three measurements obtained from different surface regions of each sample. To detect the presence of −NH2 groups using the XPS, untreated and plasma-treated scaffolds were first exposed for 45 min to trifluoromethyl benzaldehyde (TFBA) vapor (Fisher Scientific, Pittsburgh, PA), as described previously,22 and then degased for 1 h in a vacuum of ∼2 Torr.
Cell spreading and proliferation
Cell adhesion and proliferation on ∼100-μm-thick plasma-treated scaffolds were studied using BAECs and BSMCs. Both cell lines were isolated from bovine aorta, as described in previous studies.24,25 Before cell seeding, the untreated (control) scaffolds were sterilized for 30 min in 70% ethanol under ultraviolet (UV) light and then washed five times with sterile phosphate buffered saline (PBS). Cells were seeded on different substrates with a serum medium consisting of Dulbecco's modified Eagle's medium, 10% fetal bovine serum, and 1% penicillin/streptomycin. Seeded cells were kept at 37°C for 5 or 24 h in a humidified incubator with 5% CO2. To study cell adhesion and spreading, cells incubated for 5 or 24 h were fixed with 4% paraformaldehyde (PFA), permeabilized with 0.5% Triton X-100, and cell actin was stained with Alexa-Phalloidin 488. Fluorescence images of the stained cells were obtained with an upright fluorescence microscope (Zeiss HAL 100, Carl Zeiss MicroImaging, Thornwood, NY).
For cell proliferation studies, BAECs and BSMCs seeded on different surfaces and cultured for 24 h were incubated for 1 h with 10 μM EdU (Invitrogen, Carlsbad, CA). Then, the samples were fixed with 4% PFA, permeabilized with 0.5% Triton X-100, blocked with 3 mg/mL bovine serum albumin, and stained with click-it EdU kit (Invitrogen). The percentage of BAECs and BSMCs that incorporated EdU (i.e., cells exhibiting DNA synthesis) was correlated to the proliferation rate of each cell line. To ensure repeatability, each cell proliferation experiment was repeated three times.
In vitro cell infiltration
Plasma-treated scaffolds of ∼250 μm thickness were cut into 0.7×0.7 cm2 samples. Untreated (control) scaffolds were sterilized in 70% ethanol for 30 min while they were exposed to UV light and then washed five times in PBS. However, the plasma-treated scaffolds were not treated with ethanol in order to preserve the surface chemical behavior imparted by plasma treatment. Subsequently, three scaffolds from each group were attached to non-tissue-culture-treated polystyrene dishes by sterile double-sided tape. BAECs were seeded at 100% confluency on the scaffold surfaces and incubated for 5 days in the serum medium. Sufficient media were used for each dish to avoid changing the medium during the 5-day incubation.
At the fifth day of incubation, the whole scaffold was fixed and stained with 4′,6-diamidino-2-phenylindole (DAPI) and then placed in optimal cutting temperature (OCT) compound (TissueTek, Elkhart, IN) on dry ice. Transverse cross sections of 20 μm thickness were obtained with a cryosectioner at −20°C. DAPI fluorescent signals of the cells in these cryosections were viewed with the previously mentioned Zeiss microscope. A minimum of 30 cryosections of each scaffold were examined to ensure consistency.
In vivo cell infiltration
All of the experimental procedures pertaining to animal samples were approved by the Animal Care and Use Committee at the University of California, Berkeley. To investigate the effect of plasma treatment on cell infiltration in vivo, six scaffolds of each group were implanted in the subcutaneous cavity of Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) for 5 or 14 days. First, the rats were anesthetized with isofluorane and the incision site was marked and disinfected with 70% ethanol. Then, three incisions were made on both sides and the middle of the abdominal wall, and scaffolds of different plasma treatment were implanted to one side of each incision and tucked subcutaneously away from the incision. Finally, the incisions were sewed with interrupted 5–0 Monocryl (Ethicon, Somerville, NJ) mattress sutures. All animals were monitored on a daily basis. No adverse events were noted with any of the animals. After 5 or 14 days, the rats were returned to the operating room where they were given general anesthesia and an overdose of euthanasia solution. Subsequently, the implants and surrounding tissue were removed and embedded in OCT on dry ice. Transverse cross sections of 10 μm thickness were obtained with a cryosectioner at −20°C. These cross sections were fixed with 4% PFA and immunostained for pan macrophages using CD68 (Serotec, Raleigh, NC) and for proliferation marker Ki67 (Abcam, Cambridge, MA). The 14-day samples were also immunostained for myofibroblasts using α-actin (Epitomics, Burlingame, CA) and for endothelial cells using CD31 (BD Pharmingen, San Diego, CA). All samples were then counterstained for cell nuclei using DAPI. Finally, the samples were observed under the Zeiss microscope. A minimum of 30 cryosections of each scaffold were examined to ensure consistency between sections.
Statistical analysis
Results are presented in the form of mean and standard deviation (error bar) data, calculated under the assumption that they followed normal distributions. All data were compared by using one-way ANOVA tests. Two-tailed Student's t-test was used to assess significant differences between groups of the same plasma treatment. Multiple comparisons against control-sample data were then performed using Holm's t-test to determine significant differences between control and plasma-treated samples. A p-value of <0.05 was considered to indicate statistically significant differences.
Results and Discussion
Contact angle and surface morphology
Contact angle measurements of untreated and plasma-treated electrospun PLLA microfibrous scaffolds and PLLA membranes fabricated by thermal molding are given in Table 1. The significant decrease of the contact angle after plasma treatment indicates a more hydrophilic surface. The nearly zero apparent contact angle of the Ar-NH3/H2 plasma-treated scaffolds may be due to capillary effects and the highly porous fibrous structure. Contact angle measurements obtained with nonfibrous PLLA membranes confirmed that surface treatment with Ar and Ar-NH3/H2 plasmas enhanced the surface hydrophilicity, although the decrease of the contact angle was not as pronounced as for the fibrous scaffolds. AFM measurements revealed insignificant differences in surface roughness between untreated and Ar or Ar-NH3/H2 plasma-treated membranes (the root-mean-square roughness was found equal to about 9.5, 26.8, and 6.7 nm, respectively). Therefore, these contact angle differences may be correlated to differences in the modification of the surface chemistry by the Ar and Ar-NH3/H2 plasmas, with the dramatic decrease of the contact angle of the scaffold surfaces attributed to the effects of surface roughness and porosity on the contact angle measurements. The results given in Table 1 demonstrate a general trend for Ar-NH3/H2 plasma treatment to produce more hydrophilic PLLA surfaces than Ar plasma treatment.
Table 1.
Contact Angle of Untreated and Plasma-Treated PLLA Microfibrous Scaffolds and PLLA Membranes Fabricated by Thermal Molding
| |
|
Plasma treated |
|
|---|---|---|---|
| PLLA material | Untreated | Ar | Ar-NH3/H2 |
| Thermally molded membrane | 67.7±2.6 | 53.6±0.6 | 48.8±0.8 |
| Electrospun microfibrous scaffold | 116.2±3.6 | 85.0±4.3 | ∼0 |
PLLA, poly(l-lactide).
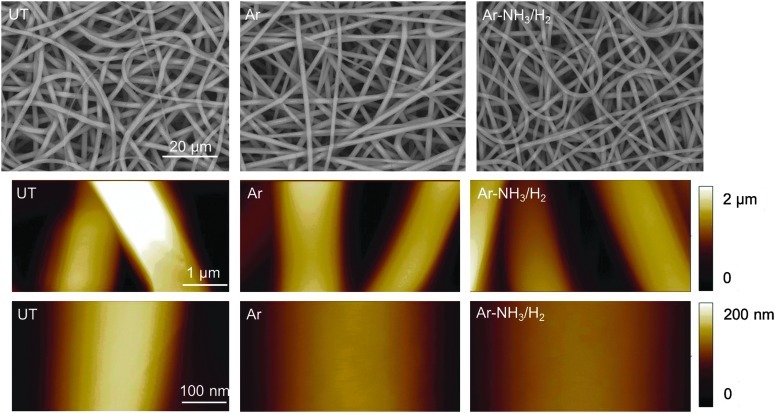
Figure 1 shows SEM and AFM images of the surface morphologies of untreated and plasma-treated scaffolds. A comparison of the SEM images shown in Figure 1 (top row) does not show any discernible structural changes as a result of plasma treatment, indicating that the plasma conditions used to alter the surface chemical characteristics did not physically change the fibers. The AFM images shown in Figure 1 (second and third row) reveal very smooth fiber surfaces for both untreated and plasma-treated scaffolds. Ion etching during plasma treatment usually roughens the polymer surface.21,26 However, such roughening effect was not observed in the present study, presumably because of the structure of the electrospun PLLA fibers consisting of stretched polymer chains oriented parallel to the longitudinal fiber direction.27 In fact, the fibrous structure produced a uniform and smooth surface exhibiting a higher etch resistance than polymer surfaces consisting of randomly oriented, recoiled molecular chains. Thus, the relatively mild plasma conditions of this study could only change the chemical behavior of the scaffold surfaces.
FIG. 1.
Scanning electron microscopy (first row) and atomic force microscopy (second and third row) images of untreated and plasma-treated poly(l-lactide) (PLLA) microfibrous scaffolds. UT, Ar, and Ar-NH3/H2 indicate untreated, Ar plasma-treated, and Ar-NH3/H2 plasma-treated scaffolds, respectively.
Surface chemical modification by plasma treatment
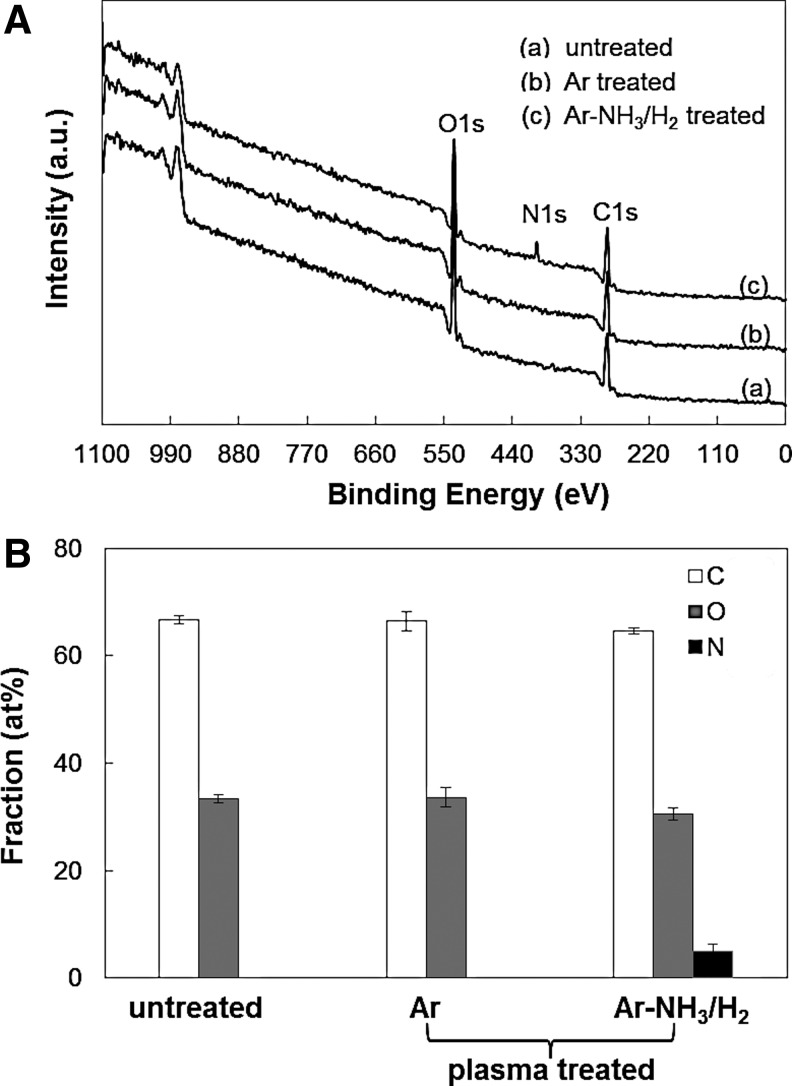
Figure 2 shows XPS results of untreated and Ar or Ar-NH3/H2 plasma-treated scaffold surfaces. The XPS survey spectra shown in Figure 2A indicate that treatment with Ar plasma did not produce any new peaks, presumably because of the mild plasma conditions. However, the N1s peak in the XPS survey spectrum of the scaffolds subjected to the Ar-NH3/H2 plasma treatment indicates the incorporation of nitrogen in the scaffold surface. Figure 2B shows the C, O, and N contents (calculated from the C1s, O1s, and N1s core level peaks of the XPS survey spectra, respectively) of untreated and plasma-treated scaffolds. The similar C and O contents of the untreated and Ar plasma-treated scaffolds provide further evidence that the mild Ar plasma conditions did not produce any detectable chemical changes on the scaffold surfaces.
FIG. 2.
(A) X-ray photoelectron spectroscopy (XPS) survey spectra and (B) surface concentration of C, O, and N for untreated and plasma-treated PLLA microfibrous scaffolds. Error bars in (B) indicate one standard deviation above and below the corresponding mean value, calculated from at least three measurements.
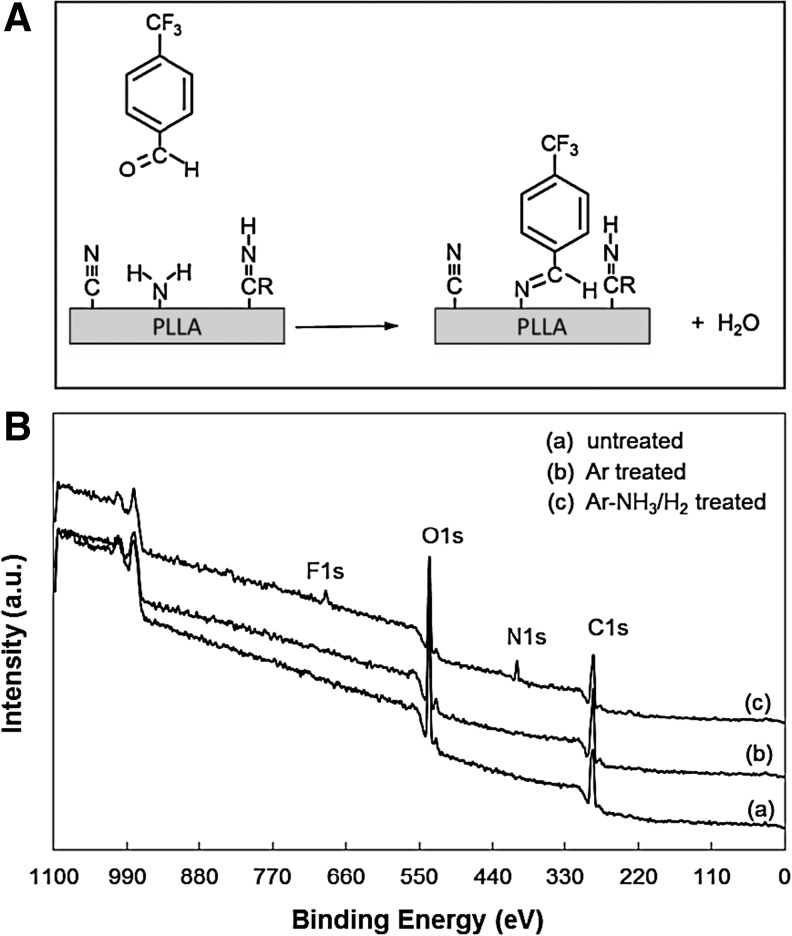
XPS survey spectra of scaffolds that were chemically treated with TFBA were used to determine if the N1s peak in the XPS survey spectra of the Ar-NH3/H2 plasma-treated scaffolds was due to the presence of −NH2 surface groups. The TFBA chemical derivatization process, shown schematically in Figure 3A, uses F to selectively label the −NH2 groups from all other nitrogen-containing groups. The presence of the F1s peak only in the XPS spectrum of the Ar-NH3/H2 plasma-treated scaffolds, shown in Figure 3B, confirms the incorporation of primary −NH2 groups on these scaffold surfaces. Using a previous method,22 the NH2/C ratio was found equal to ∼1.5%.
FIG. 3.
(A) Schematic of trifluoromethyl benzaldehyde (TFBA) labeling of −NH2 surface groups on Ar-NH3/H2 plasma-treated PLLA microfibrous scaffolds, and (B) XPS survey spectra of untreated and plasma-treated PLLA microfibrous scaffolds obtained after treatment with TFBA.
The above results indicate that the applied plasma treatments were conducive to chemical surface modification. While both plasma treatments enhanced the surface hydrophilicity (Table 1), Ar plasma did not produce any detectable changes in the surface composition, specifically O and N content of the treated scaffolds, whereas Ar-NH3/H2 plasma treatment resulted in surface functionalization with −NH2 groups.
Cell culture and effect of plasma treatment on cell morphology
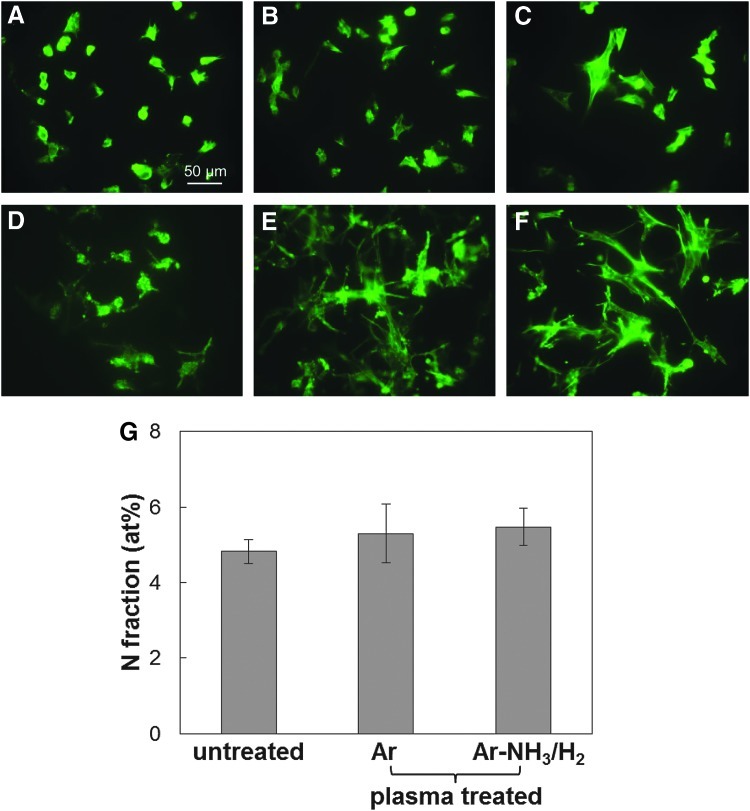
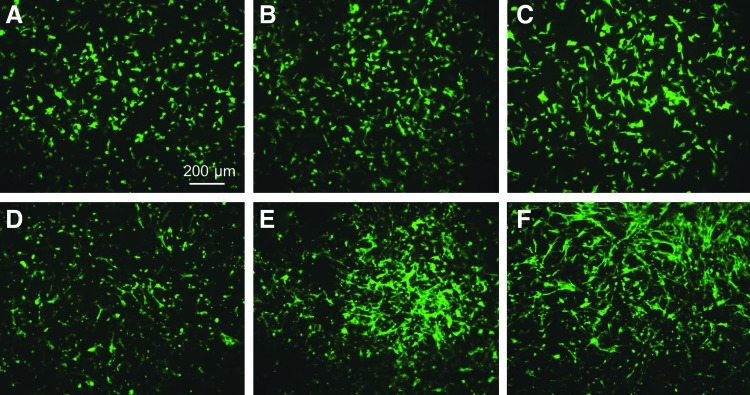
Staining for cell actin was used to examine the cell morphology after incubation for 5 h. Figure 4A–F shows that during the initial stage of cell attachment, both BAECs and BSMCs attached and spread more on the plasma-treated scaffold surfaces than the untreated surfaces. To examine if this higher surface affinity of the cells was due to the increased adsorption of serum protein on the plasma-treated surfaces, the nitrogen fraction determined from the XPS measurements was used to calculate the amount of protein adsorbed on the untreated and plasma-treated surfaces after incubation in the serum medium. Figure 4G shows statistically insignificant differences in protein adsorption between untreated and plasma-treated scaffold surfaces. Because the surface morphology measurements did not reveal a significant effect of plasma treatment on the surface roughness, it may be inferred that the only factors responsible for the enhancement of cell spreading on the plasma-treated surfaces were the increased hydrophilicity and the incorporation of O and N functionalities (e.g., −OH, −COOH, and −NH2 groups). One explanation is that surface modification might promote serum protein adsorption in configurations that are conducive to cell attachment and spreading. Therefore, although the amount of protein absorbed onto the fibrous scaffold surfaces was similar, differences in absorbed protein configurations could have influenced cell spreading, consistent with a previous study showing that plasma treatment affects protein adsorption on a polymer surface.28
FIG. 4.
Morphologies of bovine aorta endothelial cells (BAECs) (A–C) and bovine smooth muscle cells (BSMCs) (D–F) seeded on untreated and plasma-treated PLLA microfibrous scaffolds obtained after incubation in the serum medium for 5 h. (A, D) Untreated, (B, E) Ar plasma-treated, and (C, F) Ar-NH3/H2 plasma-treated scaffold surfaces. (G) Atomic percentage of N indicating the amount of serum protein adsorbed on untreated and plasma-treated PLLA microfibrous scaffolds after incubation in the 10% fetal bovine serum medium for 5 h. Error bars in (G) indicate one standard deviation above and below the corresponding mean value, calculated from at least three measurements.
A similar trend in cell spreading was observed with both BAECs and BSMCs after relatively long-term incubation (24 h). Figure 5 shows that more cell spreading occurred on the Ar-NH3/H2 plasma-treated surfaces than the Ar plasma-treated surfaces. This may be indicative of the long-term beneficial effect of the −NH2 surface groups and is in agreement with a previous study showing that NH3 plasma treatment enhances the adhesion of cells subjected to shear stresses.29
FIG. 5.
Morphologies of BAECs (A–C) and BSMCs (D–F) seeded on untreated and plasma-treated PLLA microfibrous scaffolds obtained after incubation in the serum medium for 24 h. (A, D) Untreated, (B, E) Ar plasma-treated, and (C, F) Ar-NH3/H2 plasma-treated scaffold surfaces.
Effect of plasma treatment on cell proliferation
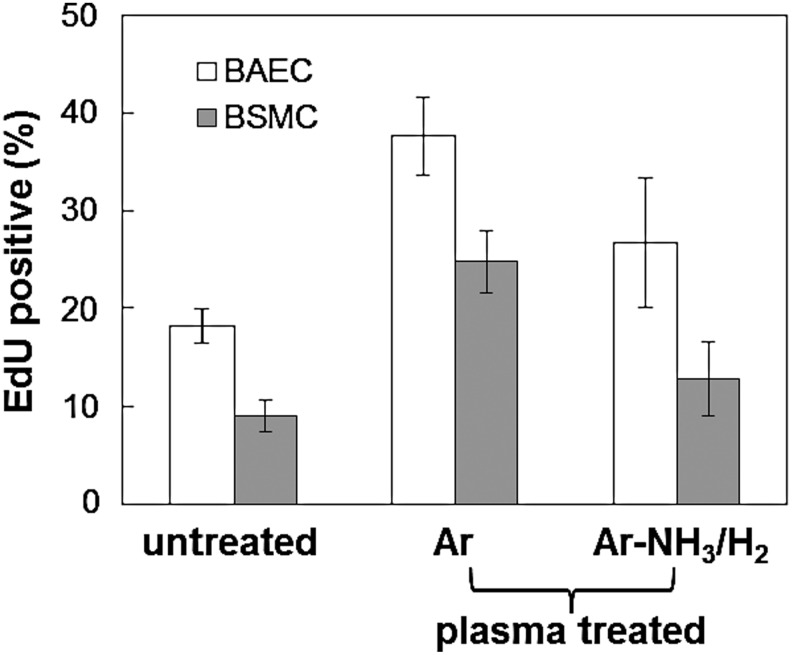
Figure 6 shows that both plasma treatments significantly increased the cell proliferation rate compared to the control (untreated) scaffolds. This is attributed to plasma-induced surface chemical modification (functionalization) and is consistent with the results shown in Figures 4 and 5. However, it appears that Ar plasma treatment was more effective in increasing the proliferation rate of both BAECS and BSMCs than Ar-NH3/H2 plasma treatment, despite the fact that the latter plasma treatment was more effective in promoting cell spreading (Fig. 5). This finding is in agreement with a previous study3 showing that Ar plasma promotes cell growth more than other plasma treatments, although the Ar plasma conditions of that study were significantly more intense than those of the present study. In the absence of any surface morphology (roughness) changes due to plasma treatment, a plausible explanation for the different proliferation rates after these plasma treatments is that the surface chemical modification produced by the Ar plasma not only enhanced hydrophilicity but also resulted in serum protein adsorption in a configuration that enhanced more cell growth, despite the fact that the Ar-NH3/H2 plasma treatment was more effective in promoting cell spreading initially. It appears that surface activation by Ar plasma was more conducive to cell growth than surface functionalization with −NH2 groups, which is widely known to enhance surface biocompatibility. Clearly, further studies must be performed to fully understand the effect of these plasma conditions on the biochemical characteristics of microfibrous scaffolds.
FIG. 6.
Proliferation rates of BAECs and BSMCs seeded on untreated and plasma-treated scaffold surfaces obtained after incubation in the serum medium for 24 h. The proliferation rate of each treatment differs statistically from the other two treatments for the same cell type (p<0.05, repeated three times). Error bars indicate one standard deviation above and below the corresponding mean value, calculated from at least eight different surface sites of each sample.
Effect of plasma treatment on cell infiltration
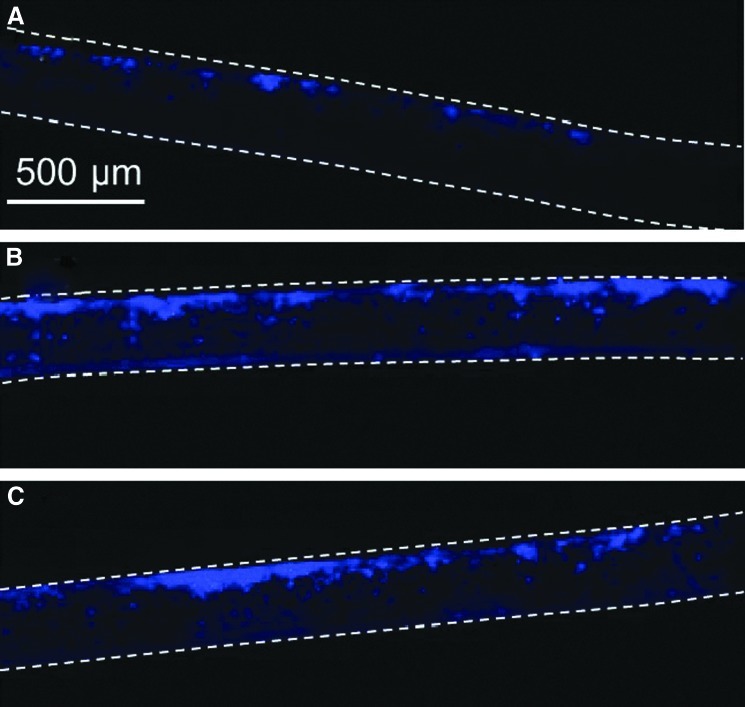
Both in vitro and in vivo experiments were conducted to elucidate the effect of plasma treatment on cell infiltration in the three-dimensional microfibrous structure of the PLLA scaffolds. Figure 7 shows representative cross-sectional images of scaffolds seeded with BAECs, which were stained with DAPI after incubation for 5 days. The cells were seeded at the top surface of each scaffold. The cell density on the untreated scaffold surface was low and cell infiltration in the thickness direction was very limited (Fig. 7A). After Ar or Ar-NH3/H2 plasma treatment, however, significantly more cells were observed at the top scaffold surfaces and cell infiltration in the thickness direction was more apparent (Fig. 7B, C). This finding is consistent with the cell spreading and proliferation results presented above. Thus, in addition to improving cell spreading and proliferation, both plasma treatments improved in vitro cell infiltration into the scaffold structure. Because of the highly porous structure of the thin scaffolds and the fact that plasma treatment was performed in the gas phase, it is likely that plasma-induced surface activation occurred throughout the scaffold thickness, which explains the increased cell infiltration in the plasma-treated scaffolds. However, because it is difficult to quantify cell ingrowth through the scaffold thickness, it is not possible to distinguish differences in cell infiltration between the two plasma treatments.
FIG. 7.
Cross-sectional images of (A) untreated, (B) Ar plasma-treated, and (C) Ar-NH3/H2 plasma-treated scaffolds obtained after in vitro culture with BAECs in the serum medium for 5 days. Cells were stained with 4′,6-diamidino-2-phenylindole (DAPI). Top and bottom scaffold surfaces are distinguished by dashed lines.
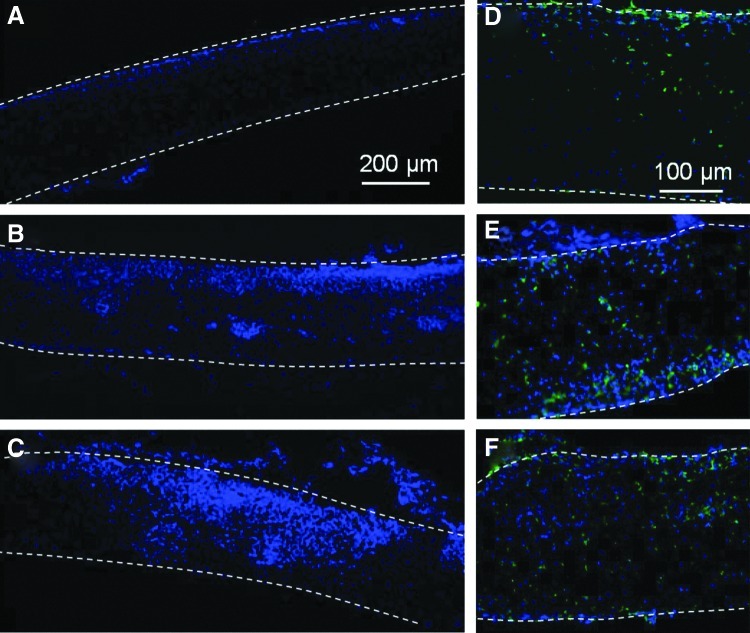
Figure 8 shows representative cross-sectional images of scaffolds implanted under the skin of Sprague-Dawley rats for 5 days. Untreated scaffolds demonstrated very low cell density and minimal cell infiltration (Fig. 8A). Plasma treatment promoted cell infiltration, significantly increasing the cell density and the ingrowth depth (Fig. 8B, C). Positive CD68 staining revealed monocytes/macrophages (Fig. 8D, E); however, differences in their percentages were insignificant. It appears that plasma treatment enhanced infiltration of several cell types, including immune cells. In addition, although positive Ki67 (a proliferation marker) staining cells were not observed with untreated samples, a few positive staining cells were detected in the plasma-treated samples, indicating that plasma-induced enhancement of cell proliferation in vivo was not as significant as in vitro (data not shown).
FIG. 8.
Cross-sectional images of (A, D) untreated, (B, E) Ar plasma-treated, and (C, F) Ar-NH3/H2 plasma-treated scaffolds obtained after in vivo implantation under the skin of Sprague-Dawley rats for 5 days. Cells in (A–C) were stained with DAPI, while cells in (D–F) were stained with DAPI (blue) and CD68 (green). Top and bottom scaffold surfaces are distinguished by dashed lines.
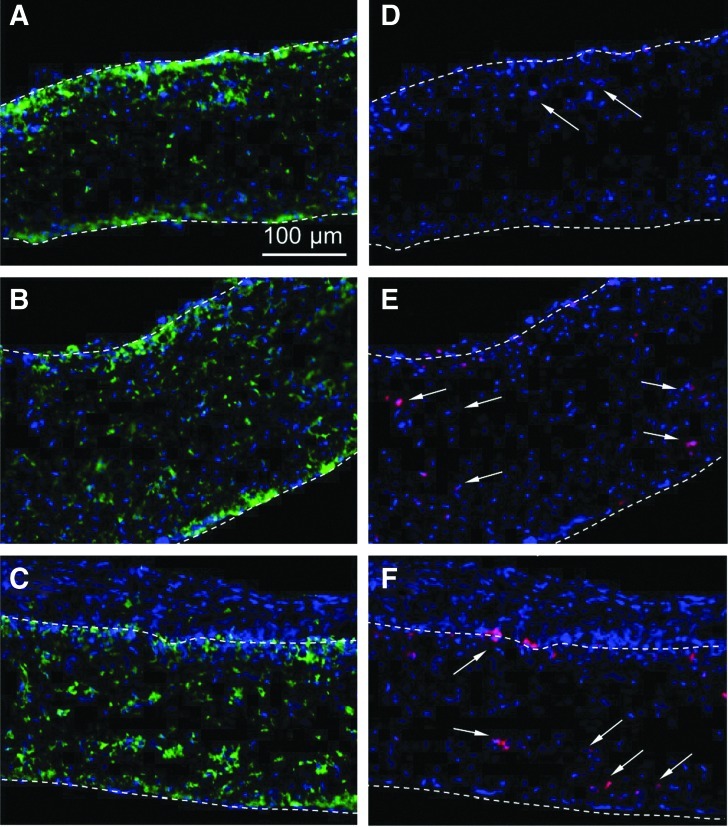
The 14-day samples were stained with DAPI, CD68, and Ki67. After long-term implantation, differences in cell density and the extent of cell infiltration between untreated and plasma-treated samples became less significant, as seen in Figure 9. In addition, similar to the results shown in Figure 8, differences in monocyte/macrophage percentages between untreated and plasma-treated samples were not significant. However, more Ki67-positive cells were observed with both types of plasma-treated samples, with an approximate quantification of cell proliferation equal to 0.8% for untreated, 5% for Ar plasma-treated, and 4.8% for Ar-NH3/H2 plasma-treated scaffolds. Even though such effect on cell proliferation is consistent with the results of the in vitro cell studies, cell proliferation on Ar plasma-treated samples was not significantly higher than that on Ar-NH3/H2 plasma-treated samples in vivo.
FIG. 9.
Cross-sectional images of (A, D) untreated, (B, E) Ar plasma-treated, and (C, F) Ar-NH3/H2 plasma-treated scaffolds obtained after in vivo implantation under the skin of Sprague-Dawley rats for 14 days. Cells in (A–C) were stained with DAPI (blue) and CD68 (green), while cells in (D–F) were stained with DAPI (blue) and Ki67 (red). Positive Ki67 staining cells in (D–F) are indicated by arrows. Top and bottom scaffold surfaces are distinguished by dashed lines.
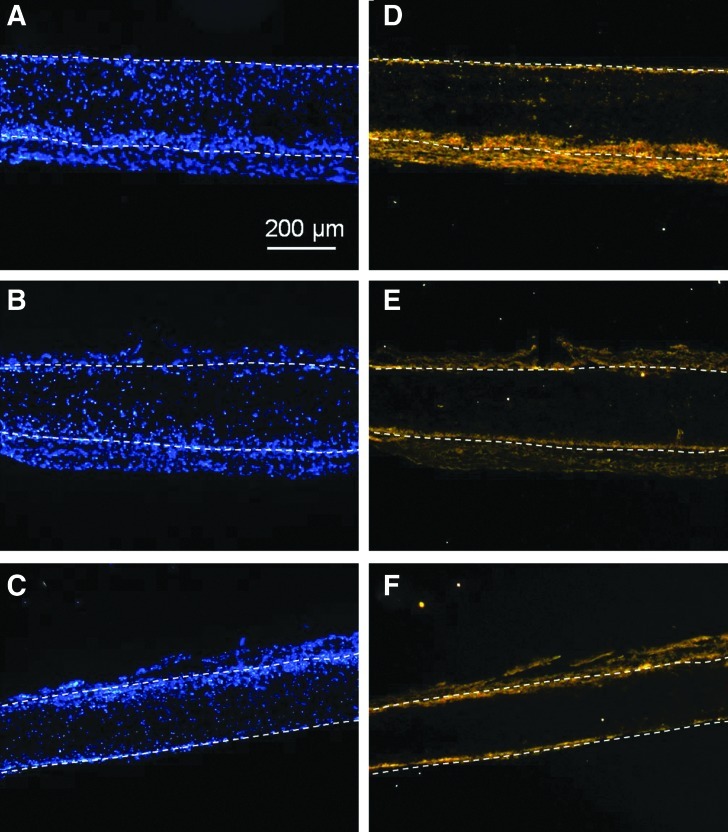
Staining the 14-day samples for markers of endothelial and smooth muscle cells (CD31 and smooth muscle α-actin, respectively) was performed not only to further identify the types of infiltrated cells but also to assess the extent of angiogenesis and vessel ingrowth into the implanted scaffolds. After in vivo implantation for 14 days, positive CD31 cells could not be detected (data not shown) and positive α-actin cells were primarily distributed at the interface between the scaffold surface and the host tissue in all samples, as shown in Figure 10. These results suggest that angiogenic factors may need to be included in the scaffolds to stimulate angiogenesis and that a general long-term response of tissues to implanted scaffolds is to recruit myofibroblasts/smooth muscle progenitors to form a capsule layer on the scaffold surface. Therefore, further modification of the scaffolds with growth factors is desirable to customize the application of these scaffolds for the repair of various tissues. Nevertheless, the above results demonstrate that surface chemical modification of PLLA microfibrous scaffolds with Ar and Ar-NH3/H2 plasma treatments not only increases the cell affinity and ingrowth in vitro, but also improves the scaffold bioactivity in vivo to enhance cell infiltration, which is critical in tissue engineering.
FIG. 10.
Cross-sectional images of (A, D) untreated, (B, E) Ar plasma-treated, and (C, F) Ar-NH3/H2 plasma-treated scaffolds obtained after in vivo implantation under the skin of Sprague-Dawley rats for 14 days. Cells in (A–C) were stained with DAPI, whereas cells in (D–F) were stained for smooth muscle α-actin. Top and bottom scaffold surfaces are distinguished by dashed lines.
Conclusions
The effect of surface chemical modification of electrospun PLLA microfibrous scaffolds induced by the treatment with inert (Ar) and reactive (Ar-NH3/H2) plasmas on cell attachment, growth, and infiltration was examined in this study. Surface morphology and chemical composition measurements demonstrated that the scaffold surface chemistry was successfully modified without affecting the fiber surface morphology and structure integrity. Cell culture studies showed that both plasma treatments effectively improve cell spreading and growth, with Ar-NH3/H2 plasma treatment enhancing more cell spreading and Ar plasma treatment increasing more cell proliferation. In vitro and in vivo cell infiltration experiments showed that, in addition to increasing cell adhesion and growth, both plasma treatments promote cell ingrowth into the microfibrous scaffold structure and result in higher cell proliferation rates. Further scaffold modification with bioactive factors is needed to promote angiogenesis and tissue-specific responses and regeneration.
Acknowledgments
This research was partially supported by the National Institute of Health (HL083900 and EB11240). The XPS measurements were carried out at the Molecular Foundry of the Lawrence Berkeley National Laboratory.
Disclosure Statement
No competing financial interests exist.
References
- 1.Yoo H.S. Kim T.G. Park T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv Drug Deliv Rev. 2009;61:1033. doi: 10.1016/j.addr.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Gugala Z. Gogolewski S. Attachment, growth, and activity of rat osteoblasts on polylactide membranes treated with various low-temperature radiofrequency plasmas. J Biomed Mater Res. 2006;76A:288. doi: 10.1002/jbm.a.30462. [DOI] [PubMed] [Google Scholar]
- 3.Latkany R. Tsuk A. Sheu M.-S. Loh I.-H. Trinkaus–Randall V. Plasma surface modification of artificial corneas for optimal epithelialization. J Biomed Mater Res. 1997;36:29. doi: 10.1002/(sici)1097-4636(199707)36:1<29::aid-jbm4>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 4.Chim H. Ong J.L. Schantz J.-T. Hutmacher D.W. Agrawal C.M. Efficacy of glow discharge gas plasma treatment as a surface modification process for three-dimensional poly (d,l-lactide) scaffolds. J Biomed Mater Res. 2003;65A:327. doi: 10.1002/jbm.a.10478. [DOI] [PubMed] [Google Scholar]
- 5.Baek H.S. Park Y.H. Ki C.S. Park J.-C. Rah D.K. Enhanced chondrogenic responses of articular chondrocytes onto porous silk fibroin scaffolds treated with microwave-induced argon plasma. Surf Coat Technol. 2008;202:5794. [Google Scholar]
- 6.Prabhakaran M.P. Venugopal J. Chan C.K. Ramakrishna S. Surface modified electrospun nanofibrous scaffolds for nerve tissue engineering. Nanotechnology. 2008;19:455102. doi: 10.1088/0957-4484/19/45/455102. [DOI] [PubMed] [Google Scholar]
- 7.Martins A. Pinho E.D. Faria S. Pashkuleva I. Marques A.P. Reis R.L. Neves N.M. Surface modification of electrospun polycaprolactone nanofiber meshes by plasma treatment to enhance biological performance. Small. 2009;5:1195. doi: 10.1002/smll.200801648. [DOI] [PubMed] [Google Scholar]
- 8.Park H. Lee K.Y. Lee S.J. Park K.E. Park W.H. Plasma-treated poly(lactic-co-glycolic acid) nanofibers for tissue engineering. Macromolecular Res. 2007;15:238. [Google Scholar]
- 9.Barry J.J.A. Silva M.M.C.G. Shakesheff K.M. Howdle S.M. Alexander M.R. Using plasma depostits to promote cell population of the porous interior of the three-dimensional poly(d,l-lactic acid) tissue-engineering scaffolds. Adv Funct Mater. 2005;15:1134. [Google Scholar]
- 10.Park K. Ju Y.M. Son J.S. Ahn K.-D. Han D.K. Surface modification of biodegradable electrospun nanofiber scaffolds and their interaction with fibroblasts. J Biomater Sci Polym Edn. 2007;18:369. doi: 10.1163/156856207780424997. [DOI] [PubMed] [Google Scholar]
- 11.Gupta B. Plummer C. Bisson I. Frey P. Hilborn J. Plasma-induced graft polymerization of acrylic acid onto poly(ethylene terephthalate) films: characterization and human smooth muscle cell growth on grafted films. Biomaterials. 2002;23:863. doi: 10.1016/s0142-9612(01)00195-8. [DOI] [PubMed] [Google Scholar]
- 12.Koh H.S. Yong T. Chan C.K. Ramakrishna S. Enhancement of neurite outgrowth using nano-structured scaffolds coupled with laminin. Biomaterials. 2008;29:3574. doi: 10.1016/j.biomaterials.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 13.He W. Ma Z. Yong T. Teo W.E. Ramakrishna S. Fabrication of collagen-coated biodegradable polymer nanofiber mesh and its potential for endothelial cells growth. Biomaterials. 2005;26:7606. doi: 10.1016/j.biomaterials.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 14.Ma Z. He W. Yong T. Ramakrishna S. Grafting of gelatin on electrospun poly(caprolactone) nanofibers to improve endothelial cell spreading and proliferation and to control cell orientation. Tissue Eng. 2005;11:1149. doi: 10.1089/ten.2005.11.1149. [DOI] [PubMed] [Google Scholar]
- 15.Chen J.-P. Su C.-H. Surface modification of electrospun PLLA nanofibers by plasma treatment and cationized gelatin immobilization for cartilage tissue engineering. Acta Biomater. 2011;7:234. doi: 10.1016/j.actbio.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Feng Z.-Q. Lu H.-J. Leach M.K. Huang N.-P. Wang Y.-C. Liu C.-J. Gu Z.-Z. The influence of type-I collagen-coated PLLA aligned nanofibers on growth of blood outgrowth endothelial cells. Biomed Mater. 2010;5:065011. doi: 10.1088/1748-6041/5/6/065011. [DOI] [PubMed] [Google Scholar]
- 17.Shen H. Hu X. Yang F. Bei J. Wang S. Combining oxygen plasma treatment with anchorage of cationized gelatin for enhancing cell affinity of poly(lactide-co-glycolide) Biomaterials. 2007;28:4219. doi: 10.1016/j.biomaterials.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Yang J. Bei J. Wang S. Enhanced cell affinity of poly (d,l-lactide) by combining plasma treatment with collagen anchorage. Biomaterials. 2002;23:2607. doi: 10.1016/s0142-9612(01)00400-8. [DOI] [PubMed] [Google Scholar]
- 19.Paletta J.R.J. Bockelmann S. Walz A. Theisen C. Wendorff J.H. Greiner A. Fuchs-Winkelmann S. Schofer M.D. RGD-functionalisation of PLLA nanofibers by surface coupling using plasma treatment: influence on stem cell differentiation. J Mater Sci Mater Med. 2010;21:1363. doi: 10.1007/s10856-009-3947-2. [DOI] [PubMed] [Google Scholar]
- 20.Jia J. Duan Y.-Y. Yu J. Lu J.-W. Preparation and immobilization of soluble eggshell membrane protein on the electrospun nanofibers to enhance cell adhesion and growth. J Biomed Mater Res. 2008;86A:364. doi: 10.1002/jbm.a.31606. [DOI] [PubMed] [Google Scholar]
- 21.Tajima S. Komvopoulos K. Surface modification of low-density polyethylene by inductively coupled argon plasma. J Phys Chem B. 2005;109:17623. doi: 10.1021/jp052121x. [DOI] [PubMed] [Google Scholar]
- 22.Favia P. Stendardo M.V. d'Agostino R. Selective grafting of amine groups on polyethylene by means of NH3-H2 RF glow discharges. Plasmas Polym. 1996;1:91. [Google Scholar]
- 23.Kurpinski K.T. Stephenson J.T. Janairo R.R.R. Lee H. Li S. The effect of fiber alignment and heparin coating on cell infiltration into nanofibrous PLLA scaffolds. Biomaterials. 2010;31:3536. doi: 10.1016/j.biomaterials.2010.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li S. Butler P. Wang Y. Hu Y. Han D.C. Usami S. Guan J.-L. Chien S. The role of the dynamics of focal adhesion kinase in the mechanotaxis of endothelial cells. Proc Natl Acad Sci U S A. 2002;99:3546. doi: 10.1073/pnas.052018099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li S. Moon J.J. Miao H. Jin G. Chen B.P.C. Yuan S. Hu Y. Usami S. Chien S. Signal transduction in matrix contraction and the migration of vascular smooth muscle cells in three-dimensional matrix. J Vasc Res. 2003;40:378. doi: 10.1159/000072702. [DOI] [PubMed] [Google Scholar]
- 26.Cheng Q. Komvopoulos K. Synthesis of polyethylene glycol-like films from capacitively coupled plasma of diethylene glycol dimethyl ether monomer. J Phys Chem C. 2009;113:213. [Google Scholar]
- 27.Messina G.M.L. Satriano C. Marletta G. A multitechnique study of preferential protein adsorption on hydrophobic and hydrophilic plasma-modified polymer surfaces. Colloids Surf B: Biointerfaces. 2009;70:76. doi: 10.1016/j.colsurfb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 28.Lim C.T. Tan E.P.S. Ng S.Y. Effects of crystalline morphology on the tensile properties of electrospun polymer nanofibers. Appl Phys Lett. 2008;92:141908. [Google Scholar]
- 29.Wan Y. Yang J. Yang J. Bei J. Wang S. Cell adhesion on gaseous plasma modified poly-(l-lactide) surface under shear stress field. Biomaterials. 2003;24:3757. doi: 10.1016/s0142-9612(03)00251-5. [DOI] [PubMed] [Google Scholar]