SYNOPSIS
This study describes the design of a heterotetravalent allergen (HtTA) as a multi-component experimental system that enables an integrative approach to study mast cell degranulation. The HtTA design allows presentation of two distinct haptens, each with a valency of two, thereby better reflecting the complexity of natural allergens by displaying epitope heterogeneity and IgE antibody variability. Using the HtTA design, synthetic allergens HtTA-1 and HtTA-2 were synthesized to model a combination of epitope/IgE affinities. HtTA-1 presented DNP and dansyl haptens (Kd = 22 and 54 nM for IgEDNP and IgEdansyl respectively), and HtTA-2 presented dansyl and the weak affinity DNP-Pro haptens (Kd = 550 nM for IgEDNP). Both HtTAs effectively induced degranulation when mast cells were primed with both IgEDNP and IgEdansyl antibodies. Interestingly, tetravalent DNP-Pro or bivalent dansyl were insufficient in stimulating a degranulation response, illustrating the significance of valency, affinity, and synergy in allergen-IgE interactions. Importantly, maximum degranulation with both HtTA-1 and HtTA-2 was observed when only 50% of the mast cell-bound IgEs were hapten specific (25% IgEdansyl + 25% IgEDNP). Taken together, this study establishes the HtTA system as a physiologically relevant experimental model and demonstrates its utility in elucidating critical mechanisms of mast cell degranulation.
Keywords: Mast cell degranulation, synthetic allergen, allergy, IgE antibody, heterotetravalent, multivalency
INTRODUCTION
Type–1 hypersensitivity (allergic reactions) is an abnormal response of the adaptive immune system directed against otherwise harmless, non-infectious substances. It is caused by the crosslinking of IgE antibodies that are bound to their high-affinity receptor (FcεRI) on the surface of mast cells by multivalent allergens, which initiates a mast cell degranulation response resulting in the release of mediators such as vasoactive amines, neutral proteases, chemokines, and cytokines [1, 2]. Naturally occurring allergens are typically complex, structurally heterogeneous proteins, with multiple allergy-inducing epitopes. Consequently, the IgE antibodies that are generated against these proteins are polyclonal in nature, and bind to the various allergy-inducing epitopes with different affinities [3, 4]. Typical allergens can have 2 to 12 epitopes recognized by polyclonal IgE antibodies [5–8]. Recent evidence suggests that among the identified epitopes on a given allergen, 1 to 5 are immunodominant, meaning they are recognized in the majority of patients with that particular allergy [6, 7, 9–11]. For example, there are 4 epitopes on the peanut protein Ara h 3, which is recognized by 80–90% of patients with peanut allergies and play a significant role in triggering the allergic reaction [6].
As a result of the complexity of natural allergens, it has been a challenge to develop experimental models that mimic natural allergic responses. Consequently, in studies to date, simplified models have been utilized to study mast cell degranulation and type-I hypersensitivity. An example of a common and ubiquitously used model system involves the use of the Dinitrophenyl/anti-DNP IgE (DNP/IgEDNP) hapten/antibody pair [12]. Typically, in the experiments that utilize this system, rat basophilic leukemia (RBL) cells are first primed with monoclonal IgEDNP,and are then stimulated with a synthetic allergen prepared by conjugating multiple copies of DNP to a scaffold such as BSA [13–15]. Although this model has provided important insight into mast cell signaling, it falls short of being a realistic representation of natural allergy systems (perhaps with the exception of certain drug allergies). One shortcoming of this model is that DNP binds to IgEDNP with an atypically high monovalent affinity (Kd in the range of high picomolar to low nanomolar depending on the IgE clone), which is not representative of the broad range of affinities IgEs have for allergy epitopes present in nature [10, 16, 17]. Additionally, multivalent presentation of the same hapten on a scaffold does not accurately represent the multiple distinct epitopes on natural allergens. Given the heterogeneity of natural allergens, which possess a combination of epitopes with high and low affinities for the various polyclonal IgEs, better designed experimental model systems reflecting such epitope variability and incorporating multiple IgE clones that target each of these epitopes are needed to elucidate the critical and unrevealed aspects of mast cell activation.
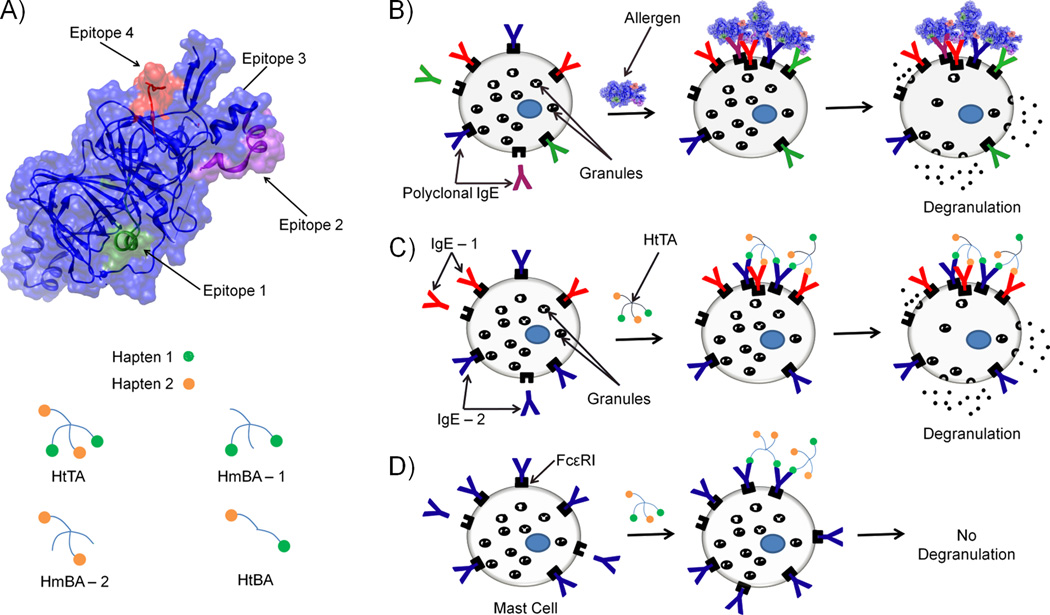
Here, we describe the design of a multi-component experimental model system of mast cell degranulation that incorporates epitope heterogeneity and IgE antibody variability to better reflect the complexity of natural allergens. In our design, we sought after the following two criteria: i) to mimic the presence of multiple epitopes on a natural allergen, the synthetic allergen must incorporate more than one type of hapten; and ii) to mimic the involvement of polyclonal antibodies in natural allergy systems, crosslinking of more than one IgE clone, each with a different hapten specificity, must be required to initiate an allergic response. To meet these criteria, we designed a heterotetravalent synthetic allergen (HtTA) scaffold that can present two distinct haptens, each with a valency of two (Figure 1). HtTA provides a realistic representation of a natural allergen since recent studies report that there are typically 1 to 5 immunodominant epitopes on an allergen [6, 7, 9–11]. For example various common allergens such as Ara h 3 of peanut, Tri a 14 of wheat, and Cuc m 2 of melon all have four immunodominant epitopes [6, 16, 18]. Therefore, the HtTA design, with its four haptens, is a close approximation of many natural allergens. Importantly, the HtTA design provides that if only one of the respective IgEs is present on the mast cell surface, the HtTA will essentially behave as a bivalent ligand, which, according to literature reports, is insufficient for triggering degranulation [19, 20]. Accordingly, for HtTA to trigger degranulation, the presence of both hapten-specific IgE antibodies on the mast cell surface is necessary. Therefore, the HtTA design better reflects the complexity of natural allergy systems by incorporating two distinct haptenic moieties that require the involvement of both respective IgE antibodies for a degranulation response. These concepts are summarized in Figure 1.
Figure 1. Design of a heterotetravalant synthetic allergen as a model system to study mast cell degranulation.
A) Crystal structure of the peanut allergy protein Ara h 3 (PDB 3C3V) with the 4 immunodominant epitopes shown. To mimic the multiple different epitopes present on a natural allergen such as Ara h 3, we synthesized the heterotetravalent synthetic allergen (HtTA) made of two distinct haptens, each with a valency of 2. Bivalent ligands composed of 2 copies of one hapten (HmBA-1, HmBA-2), or composed of 1 copy of each of the two haptens (HtBA) were also synthesized as controls. B) In an allergic reaction, the allergen binds to polyclonal IgE antibodies found on the surface of mast cells. The cross-linking of the IgE receptor, FcεRl, initiates a signaling cascade that results in mast cell degranulation. C) In the HtTA design, multiple different haptens of HtTA simultaneously bind to their respective IgE antibodies, mimicking the involvement of polyclonal antibodies in a natural allergic response. D) When the mast cells have only a single type of IgE on their surface that is specific for only one of the haptens, the HtTA can only bind bivalently to the IgE, which does not provide sufficient cross-linking to stimulate a degranulation response.
EXPERIMENTAL
Materials
We purchased N-Fmoc-amido-dPEG8-acid from Quanta BioDesign, Fmoc-NH-(PEG)4-COOH, N-Fmoc-amino acids, Fmoc-lys(ivDde)-OH, NovaPEG Rink Amide resin, 2-(1H-Benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU), and BSA from EMD Biosciences. Dansyl chloride, 1-Fluoro-2,4-dinitrobenzene (DNP), N,N-diisopropylethylamine (DIEA), Trifluoroacetic acid (TFA), and Piperidine were from Sigma and N,N-dimethylformamide (DMF) (>99.8%) was purchased from Thermo Fisher. IgEDansyl (clone 27–74) was purchased from BD Biosciences. IgEDNP, was isolated from ascites and purified using a trinitrophenyl-lysine affinity column, was a gift from Dr. Bridget Wilson.
Synthesis of the tetravalent and bivalent synthetic allergens
All molecules were synthesized using standard Fmoc chemistry on a solid support. Fmoc protected amino acids were activated with HBTU and DIEA in DMF for 3 minutes and coupling was monitored with Kaiser tests [21]. Fmoc protecting groups were removed by exposure to 20% piperidine in DMF. The synthetic allergens were synthesized using multiple lysine derivatives to achieve branching while N-Fmoc-amido-dPEG8-acid was used to provide the EG8 linkers [22, 23]. A detailed synthetic protocol is provided in the Supplementary Materials section.
Determination of IgEDNP/HtTA complex formation
The sizes of cyclic IgE complexes that formed upon addition of HtTA were measured using a Brookhaven dynamic light scattering system at 25 °C. The size of the IgE antibodies was measured by preparing a 3 µM IgE solution in PBS pH 7.4 and using an estimated refractive index of 1.45. IgE/HtTA complex formation was measured using a stoichiometric amount of HtTA to total IgE (1:2). The solution contained an equimolar solution of IgEDNP and IgEdansyl. In order to remove dust particles, the solution was centrifuged using an Eppendorf centrifuge model 5224 at 16,000 xg for 30 minutes prior to DLS measurement.
Fluorescence quenching assay for determination of IgE/hapten binding affinities
The binding constants of the monovalent haptens to IgE were determined as previously described in detail [14]. Briefly, DNP and dansyl quench the fluorescence from the IgE tryptophan residues, occurring at 335 nm, only when the two molecules are in proximity to each other (<10 nm). The monovalent haptens were titrated into a 96 well plate containing a 200 μL solution of 10 nM IgEDNP in PBS. All experiments were done in at least triplicates.
Synthesis of hapten conjugated BSA molecules as synthetic allergens
BSA (10 mg) was dissolved in 1 mL of bicarbonate buffer (0.1 M pH 9.0), 10 mg of dansyl chloride was dissolved in 1 mL of DMF and 100 uL of the dansyl chloride solution was added to the BSA solution. The conjugated BSA was purified using a 0.5 mL 10 kDa molecular weight cut off spin concentrator (Millipore). The purity of the dansyl-BSA was determined using SE-HPLC on an Agilent 1200 series system with a Tosoh Bioscience TSKgel Super SW3000 column (4.6mm × 300mm) at 0.35 mL/min PBS (pH 6.8). The purity of dansyl-BSA (elution time 8.7 minutes) was estimated to be >97% and the only contaminant detected was un-conjugated dansyl (elution time 33 minutes). On average there were 14 dansyl molecules per BSA as determined by the absorbance ratio of 335 nm to 280 nm.
RBL degranulation assay
RBL cells and IgEDNP were kindly provided by Dr. Wilson (University of New Mexico). RBL cells were maintained as described previously [13]. For the degranulation assays, cells were plated at 0.5 × 106 cells/ml in a 96 well plate, and were incubated for 24 hours followed by a two hour incubation with the indicated IgE antibodies. Cells were washed immediately before experiments and were stimulated with the indicated concentrations of ligand. Degranulation was detected spectroscopically by measuring the activity of the granule stored enzyme β-hexosaminidase secreted into the supernatant on the substrate p-nitrophenyl-N-actyl-β-O-glucosamine. All degranulation assays were repeated in at least triplicates. In all experiments the total IgE concentration was kept constant at 1 µg/ml.
RESULTS AND DISCUSSION
Design of the heterotetravalent model system
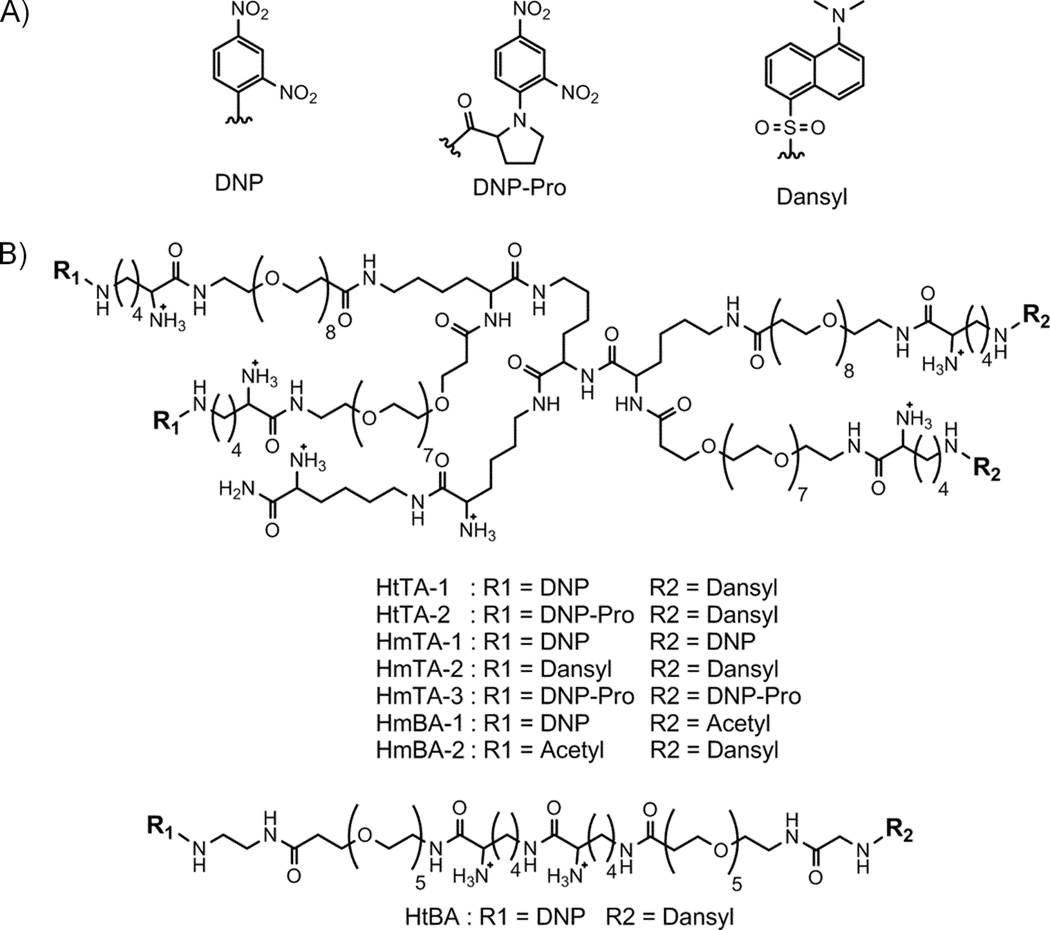
The HtTA model requires incorporation of multiple hapten/antibody pairs with a broad range of affinities. DNP/IgEDNP is the most commonly utilized hapten/antibody pair in allergy research and DNP has a high affinity for IgEDNP. Therefore, we selected DNP/IgEDNP as the high affinity pair in our design. The hapten DNP can be chemically modified to alter the affinity of this interaction to generate weaker affinity pairs [17, 24]. In order to create a second hapten/IgE pair with weaker affinity, we synthesized a DNP variant, DNP-proline (DNP-Pro). As the third pair, we selected 5-(dimethylamino)naphthalene-1-sulfonyl/anti-dansyl IgE (dansyl/IgEdansyl). Dansyl, similar to DNP, is a small molecule that is easily incorporated into multivalent synthetic schemes. The structures of the haptens used in the HtTA design are shown in Figure 2A.
Figure 2. Chemical structures of the haptens and tetravalent synthetic allergens.
A) Structures of the haptens used to synthesize the tetravalent allergens are shown. B) Heterotetravalent (HtTA-1, HtTA-2), homotetravalent (HmTA-1, HmTA-2, HmTA-3), homobivalent (HmBA-1, HmBA-2), and heterobivalent (HtBA) synthetic allergens are shown.
Next we designed the tetravalent scaffold that was used to synthesize the HtTAs (Figure 2B). The scaffold should space the four haptens sufficiently far apart such that the assembly of four antibodies to the tetravalent allergen would not be sterically hindered. Yet, the haptens need to be positioned close enough to make it sterically unfavorable for one HtTA molecule to bridge the two Fab arms of a single IgE. Previously, we identified that a separation distance of 6 nm is optimal for haptens to bind to multiple antibodies without bridging two antigen binding sites on a single antibody [25–27]. Another important parameter in the design of HtTA is the selection of the linker molecule used to conjugate the four haptens. In earlier studies, we identified ethylene glycol to be the preferred linker when designing multivalent molecules that bind antibodies [14]. Ethylene glycol does not form non-specific interactions with proteins, is flexible enough to minimize steric constraints for hapten binding, and enhances the solubility of the hydrophobic haptens [28–30]. With these design considerations in mind, we synthesized homotetravalent (HmTA-1, HmTA-2, and HmTA-3), and heterotetravalent (HtTA-1 and HtTA-2) allergens by conjugating the respective hapten molecules to each other with lysine containing ethylene glycol linkers (Figure 2B). The ethylene glycol linkers connecting the four hapten molecules to the tetravalent molecule are each 3.2 nm long (when fully extended), providing a maximum separation distance of ~6.4 nm, while the lysine residues provide a charged group to further enhance solubility. This design provided the HtTA model system with epitope heterogeneity and IgE antibody variability to better reflect the complexity of natural allergens.
Determination of the binding affinity for the IgE-hapten interactions
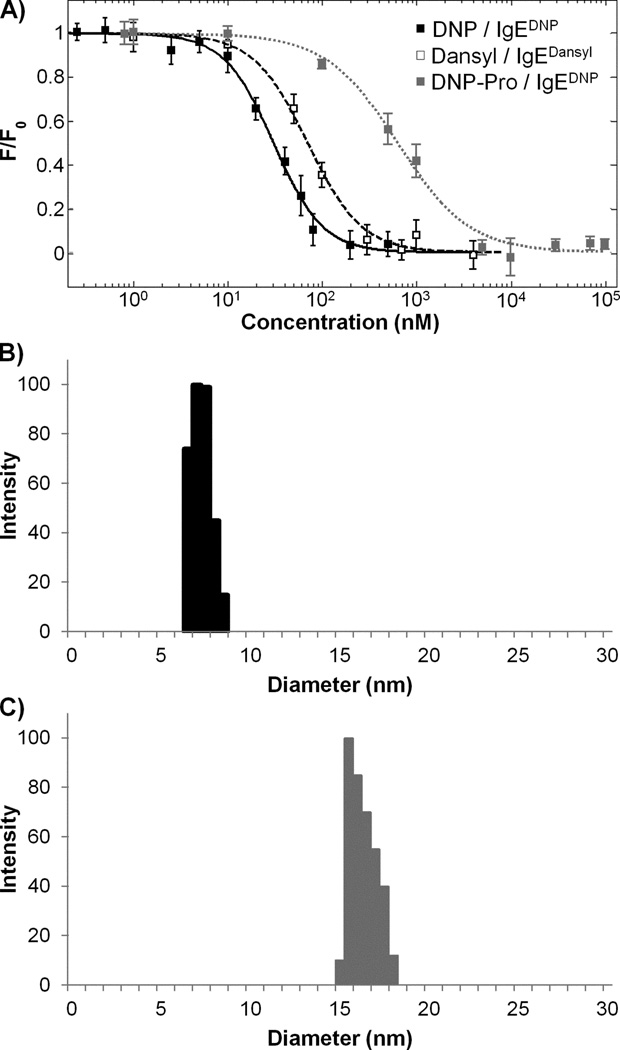
Since the affinity of the haptens DNP, DNP-Pro, and Dansyl are specific to the monoclonal antibody used, we first determined the monovalent binding affinities of the haptens for their corresponding IgEs. DNP and dansyl have significant absorbance at 335 nm. This overlaps with the tryptophan emission from the antibody and enables us to measure binding affinities using a fluorescence quenching method we described earlier [14]. We determined the monovalent dissociation constants for dansyl (Kddansyl = 54 ± 4 nM), DNP (KdDNP = 22 ± 2 nM) and DNP-Pro (KdDNP-pro = 550 ± 40 nM) for the respective antibodies (Figure 3A). Next, we tested cross-reactivity between hapten/antibody pairs. We confirmed that DNP and DNP-Pro did not cross-interact with IgEdansyl and likewise dansyl did not cross-react with IgEDNP (Supplementary Data, Figure S1). These results demonstrated that the affinity of the dansyl/IgEdansyl interaction is 2.5 times lower compared to the affinity of DNP/IgEDNP interaction. Furthermore, there is a 25 fold difference between affinities of DNP and DNP-Pro for IgEDNP. The binding affinities achieved with these hapten/antibody pairs cover the range necessary for simulating a realistic representation of the heterogeneity seen in natural allergens.
Figure 3. Characterization of the hapten/IgE binding interactions.
A) Binding curves for monovalent DNP/IgEDNP (■), monovalent DNP-Pro/IgEDNP ( ), and monovalent dansyl/IgEdansyl (□) interactions are shown. Binding was observed by monitoring the fluorescence quenching of tryptophan. Values for the binding constants are KdDNP = 22 ± 2 nM, KdDNP-Pro = 550 ± 40 nM and Kddansyl = 54 ± 4 nM. Control experiments done with DNP/IgEdansyl, DNP-Pro/IgEdansyl, or dansyl/IgEDNP did not show any cross-reactivity, Figure S1. Data represents means ± SD of triplicate experiments. B) DLS data demonstrates the aggregation of IgEDNP and IgEdansyl in response to addition of a stoichiometric concentration of HtTA. The size of the monomeric IgE antibodies was 7.5 nm and increased to 16 nm upon the addition of the HtTA.
), and monovalent dansyl/IgEdansyl (□) interactions are shown. Binding was observed by monitoring the fluorescence quenching of tryptophan. Values for the binding constants are KdDNP = 22 ± 2 nM, KdDNP-Pro = 550 ± 40 nM and Kddansyl = 54 ± 4 nM. Control experiments done with DNP/IgEdansyl, DNP-Pro/IgEdansyl, or dansyl/IgEDNP did not show any cross-reactivity, Figure S1. Data represents means ± SD of triplicate experiments. B) DLS data demonstrates the aggregation of IgEDNP and IgEdansyl in response to addition of a stoichiometric concentration of HtTA. The size of the monomeric IgE antibodies was 7.5 nm and increased to 16 nm upon the addition of the HtTA.
The IgEDNP and IgEdansyl antibodies simultaneously bind to HtTA molecules
As reported previously, the optimal separation distance between haptens for efficient bivalent binding to both Fabs on a single IgE antibody is 10 nm [31, 32]. In the HtTA design, we used eight repeating units of ethylene glycol as the linker that provides a maximum separation distance of 6.4 nm between haptens. This length is not long enough to effectively crosslink the two Fab arms on a single antibody, but provides enough space for the binding of multiple antibody molecules simultaneously [25–27]. Therefore, to verify that simultaneous binding of four antibodies (2 of each IgEDNP and IgEdansyl) to HtTA-1 was not sterically hindered, we determined HtTA-1’s ability to associate simultaneously with IgEDNP and IgEdansyl antibodies in solution using dynamic light scattering (DLS). A solution containing equimolar amounts of IgEDNP and IgEdansyl was analyzed using DLS, which established that the antibodies had an average hydrodynamic radius of 7.5 nm (Figure 3B). Upon the addition of a stoichiometric amount of HtTA-1 (HtTA-1: total IgE, 1:2) the peak at 7.5 nm disappeared and a new peak at 16 nm appeared indicating the formation of IgE/HtTA-1 complexes. No larger particles were detected and the signal at 7.5 nm was no longer present indicating the depletion of monomeric IgE (Figure 3C). Thermodynamic equilibrium was reached in less than 1 minute and complexes were stable over several hours. Since, HtTA-1 is incapable of binding bivalently to a single antibody (when mixed at a stoichiometric ratio of haptens to IgE binding sites), the only possible complex that can form is a bicyclic antibody tetramer (IgE4HtTA2) at this size (see Supplementary Section for further discussion on the complex formation). These results indicated that HtTAs were able to bind tetravalently to IgEDNP and IgEdansyl antibodies and form complexes, indicating the potential to trigger degranulation of mast cells primed with both antibodies.
Optimization of the rat basophilic leukemia (RBL) cell degranulation assay using BSA conjugated DNP and dansyl as synthetic allergens
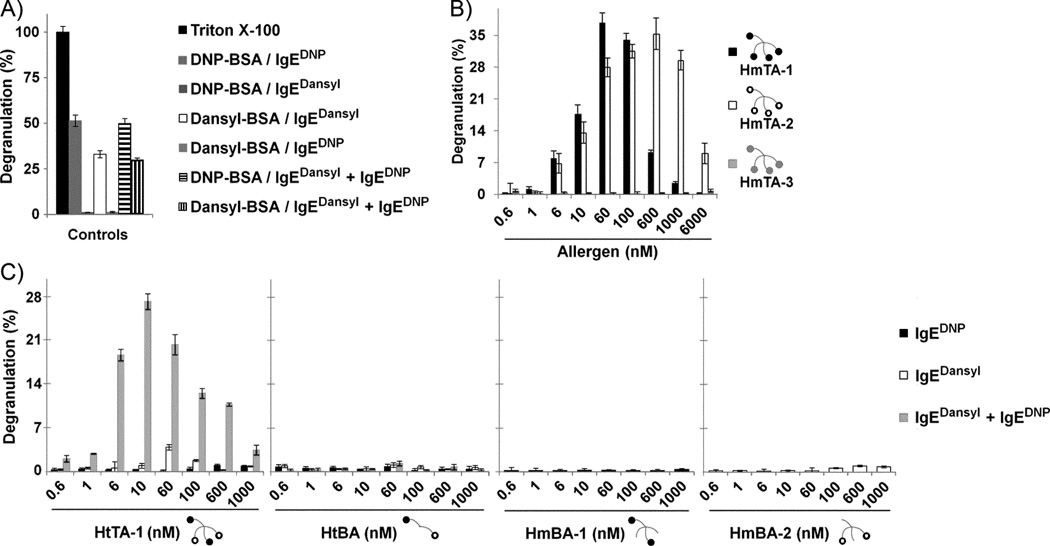
To evaluate if the designed HtTA molecules can stimulate mast cell degranulation, we used the well-established rat basophilic leukemia (RBL) cell line. The IgE receptor, FcεRl, is expressed on RBL cell surface and has a very high affinity for the Fc domain of IgE antibodies. BSA-conjugated haptens, owing to their high valency, have been shown to be potent stimulators of allergic responses. Therefore, we first optimized the RBL assay by using the synthetic allergens: DNP25-BSA and dansyl14-BSA, as positive controls. DNP25-BSA and dansyl14-BSA were synthesized and characterized as described in the experimental section. RBL cells were first primed with either monoclonal IgEDNP or monoclonal IgEdansyl to bind to FcεRl, and were then exposed to increasing concentrations of the corresponding BSA conjugate to crosslink the cell-bound IgE to trigger degranulation. Our results demonstrated both dansyl14-BSA and DNP25-BSA to be potent stimulators of degranulation with DNP25-BSA triggering a slightly stronger response (Figure 4A). This was expected as the hapten DNP has a higher affinity for IgEDNP than dansyl has for IgEdansyl and DNP25-BSA has more hapten moieties per BSA molecule than dansyl14-BSA. An increase in either the valency of an allergen or in the affinity between the haptens and the IgEs both correlate with an increase in the cellular response.
Figure 4. Mast cell degranulation response induced by homotetravalent and heterotetravalent synthetic allergens.
A) RBL cell assay was optimized using DNP25-BSA and dansyl14-BSA. RBL cells were primed with IgEDNP, IgEdansyl, or an equimolar mixture of both antibodies. DNP25-BSA and dansyl14-BSA both stimulated degranulation when the hapten specific antibody was present on the cell surface, but did not stimulate degranulation if it was absent. DNP25-BSA and dansyl14-BSA were used at 10 ng/mL. B) RBL cell degranulation in response to increasing concentrations of HmTA allergens are shown. In all experiments, RBL cells were primed with an equimolar mixture of IgEDNP and IgEdansyl. C) RBL cell degranulation in response to increasing concentrations of HtTA-1 is shown. HtBA, HmBA-1, and HmBA-2 were used as controls. HtTA-1 and HtBA were assayed with RBL cells primed with IgEDNP, IgEdansyl, or an equimolar mixture of both antibodies. HmBA-1, and HmBA-2 were assayed with hapten specific antibody, IgEDNP and IgEdansyl, respectively. The bivalent molecules did not stimulate degranulation under any of the conditions assayed. HtTA-1 was a potent stimulator of degranulation only when both IgE antibodies were present on the cell surface. HtTA-1 triggered degranulation from 0.6 nM to 1000 nM with a maximum response at 10 nM. Triton X (1%) was used to determine the percent degranulation. Data represents means ± SD of triplicate experiments.
Once we confirmed that DNP25-BSA and dansyl14-BSA were potent synthetic allergens, we verified that there was no cross-reactivity between haptens in the system. As expected, DNP25-BSA did not initiate a response from the mast cells primed only with IgEdansyl. Similarly, no response was observed when dansyl14-BSA was added to the RBL cells primed with IgEDNP (Figure 4A), which was consistent with the monovalent binding assay results.
Next, to confirm that both antibodies could simultaneously bind to the surface of the mast cells, we prepared a solution of equimolar IgEDNP and IgEdansyl to prime the cells, and used either DNP25-BSA or dansyl14-BSA as the synthetic allergen. Under these conditions the responses observed from the BSA conjugates were almost identical to the responses observed when the cells were primed with only one of the antibodies. This indicated that both antibodies were able to bind to the FcεRI receptors on cell surface, and that the presence of either antibody did not inhibit the other from binding to its hapten (Figure 4A).
Evaluation of Homotetravalent Synthetic Allergens as Stimulators of Mast Cell Degranulation
Next, we investigated if the homotetravalent molecules, which were synthesized by conjugating DNP, DNP-Pro, or dansyl to the tetravalent scaffold, were potent stimulators of mast cell degranulation by using the RBL cell assay. Homotetravalent DNP (HmTA-1) and dansyl (HmTA-2) demonstrated strong activity in triggering degranulation, while homotetravalent DNP-Pro (HmTA-3) was unable to initiate a degranulation response (Figure 4B). This result suggested that HmTA-3 was incapable of aggregating FcεRI receptors by crosslinking IgEDNP due to its weaker affinity for this antibody. Potentially a higher valency presentation of this hapten could induce degranulation, however, such a high valency synthetic allergen would not provide a good model of natural systems.
Evaluation of Heterotetravalent Synthetic Allergens as Stimulators of Mast Cell Degranulation
Due to the presence of a single hapten, the homotetravalent molecules provide only a marginally better model over DNP25-BSA and dansyl14-BSA synthetic allergens. Therefore, we synthesized a heterotetravalent synthetic allergen composed of two DNP and two dansyl moieties (HtTA-1). To evaluate the potency of HtTA-1 in initiating a degranulation response, RBL cells were sensitized with equal concentrations of IgEdansyl and IgEDNP, and then exposed to increasing concentrations of HtTA-1. HtTA-1 induced degranulation over a wide concentration range from 0.6 nM to 1 µM with a maximum response at 10 nM (Figure 4C). As expected, the degranulation response followed a bell-shape curve and decreased at elevated allergen concentrations. The decrease at high concentrations is presumably caused by the presence of excess HtTA-1 in solution, competitively inhibiting multivalent binding of HtTA-1 to cell bound IgE preventing FcεRl clustering, and therefore reducing the degranulation response. Under conditions where only one of the two IgEs were present on the RBL cell surface, HtTA-1 behaved as a bivalent molecule and was not capable of inducing degranulation. This result confirms that both IgE antibodies are required for the HtTA to successfully stimulate degranulation (Figure 4C). Control experiments were also performed on RBL cells primed with both IgEs by using the synthetic bivalent molecules: homobivalent DNP (HmBA-1), homobivalent dansyl (HmBA-2), and heterobivalent DNP-dansyl (HtBA). None of the bivalent molecules induced degranulation (Figure 4C). These results are in line with literature reports, which demonstrated that bivalent ligands are not potent stimulators of mast cell degranulation [19, 20]. It should be noted that in a few reports rigid bivalent ligands have been shown to stimulate mast cell degranulation [33–36]. However, these bivalent allergens were still poor stimulators of degranulation compared to higher valency allergens and required enhancing reagents such as cytochalasin D or D2O to detect a response. Combined, our results demonstrate that the HtTA system provides us with a multicomponent experimental model that better represents natural allergens by incorporating hapten and IgE antibody variability.
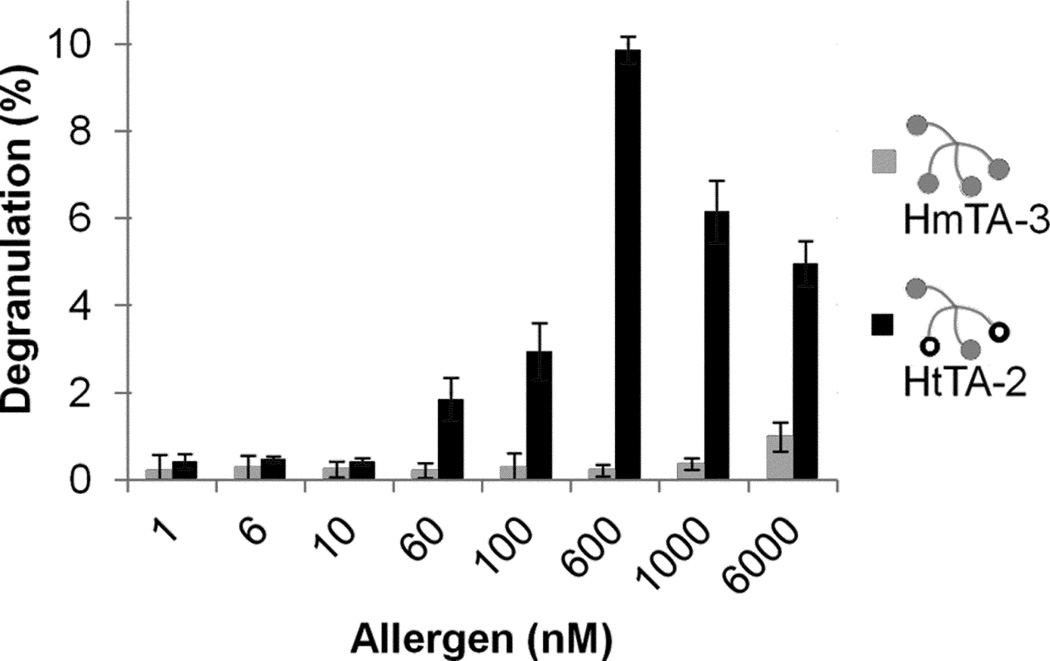
The HtTA system also provides us with a model to evaluate the role that low affinity epitopes play in mast cell degranulation. In order to evaluate the significance of low affinity epitopes, particularly when they are presented in combination with higher affinity epitopes, we synthesized yet another heterotetravalent allergen composed of two DNP-Pro and two dansyl molecules (HtTA-2). It is noteworthy that, in our earlier experiments, we have shown that a tetravalent presentation of the low affinity DNP-Pro hapten (HmTA-3), or a bivalent presentation of dansyl (HmBA-2) did not induce any degranulation response (Figure 4B, 4C, respectively). Interestingly, HtTA-2 effectively stimulated RBL cell degranulation (Figure 5). The synergistic activity of these two components, each of which are insufficient to initiate a response on their own, demonstrates the significant role that low-binding epitopes can play in mast cell degranulation, particularly when they are presented in combination with higher-affinity epitopes.
Figure 5. Mast cell degranulation response induced by low-affinity epitopes.
Degranulation response of RBL cells in response to increasing concentrations of HmTA-3 and HtTA-2. HmTA-3 was unable to stimulate a response at any concentration, while HtTA-2 was a potent stimulator with a maximum response observed at 600 nM. Triton X (1%) was used to determine the percent degranulation. Data represents means ± SD of triplicate experiments.
Effect of allergen specific IgE density on mast cell degranulation
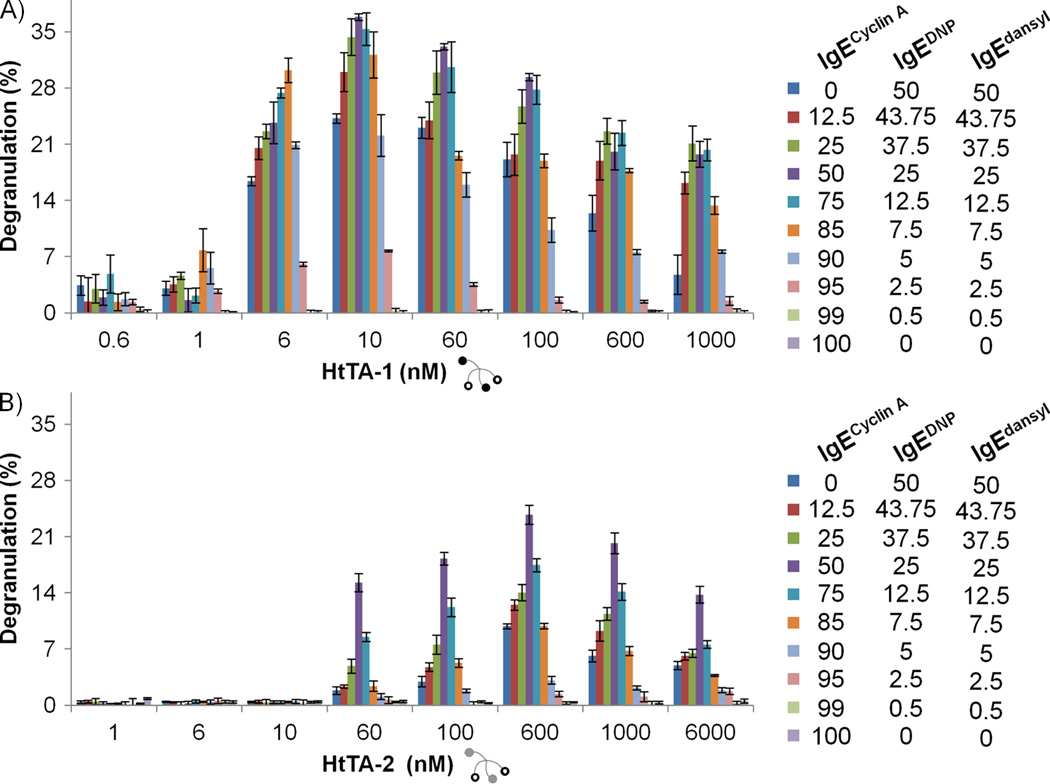
It has previously been demonstrated that, in a patient’s serum, the level of IgE that is specific for a given allergen can range from 0.01% to 82%, however, is typically less than 25% of all IgEs present [37]. As a result, mast cells present multiple clones of IgEs on their surface, with each particular clone of IgE occupying only a certain fraction of the total FcεRI receptors. Therefore, by using the heterotetravalent model system, we next investigated the effect of allergen specific IgE density on mast cell degranulation. In order to mimic physiologically relevant conditions, we assayed the HtTAs for their efficiency in RBL degranulation while varying relative IgEDNP and IgEdansyl ratios as well as their total percentages on RBL cell surface. This was accomplished by changing their relative stoichiometries used to prime the RBL cells, while reducing their total amount with the addition of an orthogonal IgE (IgECyclin A). Since all 3 IgE antibodies are murine, they have the same affinity for the FcεRI receptor and therefore their relative ratios in solution are representative of the surface bound IgE ratios on the RBL cells.
The results of the experiments that measured the degranulation response induced by HtTA-1 and HtTA-2, with decreasing specific IgE ratios on the cell surface are summarized in Figure 6. During these experiments, the relative ratios of IgEdansyl and IgEDNP were kept constant at 1:1, while the orthogonal IgE’s (IgEcyclin A) relative abundance was increased from 0 to 100%. According to the results, HtTA-1 generated the strongest degranulation response at 10 nM concentration with cells primed with 25% IgEdansyl, 25% IgEDNP and 50% orthogonal IgE (Figure 6A). Meanwhile, the strongest response from HtTA-2 stimulation was achieved at 600 nM concentration again with cells primed with the same IgE ratio: 25% IgEdansyl, 25% IgEDNP and 50% orthogonal IgE (Figure 6B). Both results establish that the ratio of the IgE antibody present on the cell surface is a significant factor that affects the degranulation response. Furthermore, for both the stronger stimulant HtTA-1 and the weaker stimulant HtTA-2, the ratios of the antibodies that generated the most intense response were identical. A closer analysis of the data reveals that the IgE ratio is more critical for the weaker of the synthetic allergens, HtTA-2, where the degranulation response is increased by 140% when compared to conditions where the cells were only primed with specific IgEs. The increase was still significant for the stronger synthetic allergen, HtTA-1, where we observed an increase of 54% in degranulation. While these results may appear surprising, they were not completely unexpected. Earlier studies reported that maximal degranulation could occur at 5% IgE crosslinking on mast cells [33]. Furthermore, an inverse correlation between FcεRl aggregate size and degranulation response has also been reported [13, 38]. We predict that reducing the ratio of allergen specific IgEs on the mast cell surface reduces the sizes of the FcεRl clusters due to decreased crosslinking by the synthetic allergen, resulting in a stronger degranulation signaling by the receptors.
Figure 6. Effect of allergen specific IgE density on mast cell degranulation.
Degranulation response of RBL cells primed with a decreasing fraction of allergen specific IgE after exposure to A) HtTA-1 or B) HtTA-2. Maximum degranulation occurred with 50% allergen specific IgE (25% IgEDNP and 25% IgEdansyl). Triton X (1%) was used to determine the percent degranulation. Data represents means ± SD of triplicate experiments.
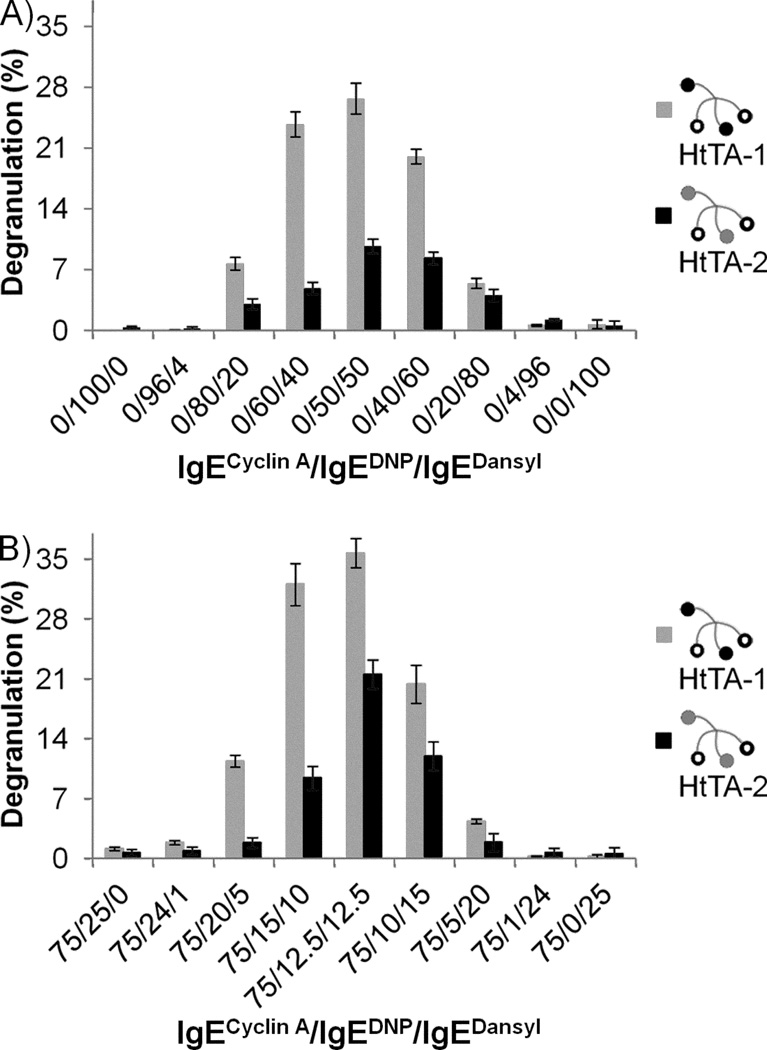
Finally, we investigated the effect of the relative ratios of specific IgEs on cellular degranulation. We performed two experiments with each synthetic heterotetravalent allergen. In the first experiment the RBL cells were primed only using specific IgEs at different ratios. In the second experiment 75% of the antibody used to prime the RBL cells was the orthogonal IgE, and the relative ratios of the specific IgEs was varied only with the remaining 25%. In both experiments the allergens were used at the concentration that elicited maximum degranulation, HtTA-1 at 10 nM, and HtTA-2 at 600 nM. The results of these experiments established that regardless of the amount of orthogonal IgE used, maximum degranulation was consistently obtained at a 1:1 ratio of IgEdansyl to IgEDNP for both HtTA-1 and HtTA-2 (Figure 7). This result is likely a reflection of the symmetry of the HtTAs as both HtTAs present the same number of haptens specific for each IgE. Additionally, as the maximum response occurred at a 1:1 ratio of IgEdansyl to IgEDNP, this indicates equal binding of both haptens to their respective IgEs, which can only occur if the HtTAs are binding tetravalently to cell bound IgE. Furthermore, these results illustrate the importance of the presence of both IgE antibodies in eliciting degranulation. Prior to these results, it was assumed that higher affinity IgEs (IgEdansyl in the case of HtTA-2) are more important in promoting degranulation than lower affinity IgEs. These results demonstrate, however, that each IgE was equally important, further highlighting the significance of weak binding epitopes in allergies.
Figure 7. Effect of the relative IgEDNP and IgEdansyl ratios on mast cell degranulation.
RBL cells were primed with A) 100% hapten specific IgE or B) 25% hapten specific IgE. In both experiments the relative ratios of IgEDNP to IgEdansyl were varied. HtTA-1 and HtTA-2 both elicited the strongest response when the mast cells were primed with equal relative ratios of IgEDNP and IgEdansyl. Triton X (1%) was used to determine the percent degranulation. Data represents means ± SD of triplicate experiments.
CONCLUSION
This study describes the development of a well-defined, multi-component experimental system that enables an integrative approach to study mast cell degranulation. The HtTA design allows for the multivalent presentation of two different haptens with varying affinities on the same scaffold, and requires the use of two distinct IgE antibodies to elicit mast cell degranulation, while the overall fraction of allergen specific IgE can be controlled with the use of a third orthogonal IgE. As such, the model replicates epitope heterogeneity, as well as the variations in epitope-IgE affinity, and thereby better reflects the complexity of natural allergy systems.
In this study we also demonstrate the utility of the heterotetravalent model system for a more complete elucidation of the mechanism of mast-cell degranulation. By using the HtTA design, we synthesized two synthetic allergens, HtTA-1 and HtTA-2, which covered a range of 2.5 and 10 fold variation in epitope affinity on the same scaffold, respectively. Both synthetic allergens required the presence of both IgEdansyl and IgEDNP antibodies on the mast cell surface to trigger degranulation. In each case, the degranulation response demonstrated a bell shaped curve – where degranulation first increased, reached a maximum, and then decreased with increasing synthetic allergen concentration. As expected, the relative potency of the HtTAs, both in terms of the concentration required for stimulating a response and intensity of degranulation, correlated with hapten affinity. Therefore, HtTA-1, being composed of higher affinity haptens than HtTA-2, proved to be the more potent synthetic allergen. Interestingly, the maximum degranulation response for both HtTA-1 and HtTA-2 occurred when only 50% of the total IgE on the mast cell surface were hapten specific (25% IgEdansyl + 25% IgEDNP). We predict that reducing the ratio of allergen specific IgEs on the mast cell surface reduces the sizes of the FcεRl clusters due to decreased crosslinking, resulting in a stronger degranulation signaling by the receptors.
Another interesting outcome of this study that was revealed by utilizing the HtTA model is that the individual components of HtTA-2, tetravalent DNP-Pro (HmTA-3) and bivalent dansyl (HmBA-2), were unable to stimulate degranulation. Yet, when DNP-Pro and dansyl were combined each with a valency of two in the same scaffold to create HtTA-2, the molecule proved to be a potent allergen. We believe that HtTA-2’s synergistic activity originates from a sequential order of events: First, the tighter binding dansyl on the allergen binds to IgEdansyl on the RBL surface, attaching HtTA-2 to the cell surface and preventing it from dissociating and diffusing back into the bulk solution. Next, the HtTA-2/IgEdansyl/FcεRI complex diffuses laterally on the cell surface until it encounters an IgEDNP/FcεRI complex, upon which the weaker affinity DNP-Pro moiety binds and causes clustering. In the absence of a tighter binding hapten, the weak affinity DNP-Pro is insufficient to bind to multiple IgEDNP antibodies simultaneously because the rate of dissociation of HtTA-2 from IgEDNP is too rapid to allow the formation of signaling competent clusters of IgEDNP/FcεRl. In the absence of the IgEDNP antibody, the tighter binding hapten dansyl is unable to form large enough clusters due to its insufficient valency, as there are only two copies of dansyl on each HtTA-2. These experiments illustrate the importance of valency, affinity, and cooperativity in allergen-IgE binding interactions in mast cell degranulation. Moreover, these results demonstrate the significance of weak affinity epitope/IgE interactions in mast cell degranulation, especially when they are presented simultaneously with higher affinity epitopes on the same allergen.
Finally, the architectural elements of the synthetic allergen, HtTA, can be easily modified to expand the platform to include various functionalities such as fluorescent labels, drug conjugates, or ligands that target other receptors to address fundamental questions in allergy research. Importantly, variations of HtTA can be synthesized to further explore the relationship between epitope affinity and stimulation of mast cell degranulation, as well as its inhibition. For example, in this study we have shown that HtTA did not stimulate degranulation, unless both types of haptens were interacting with their respective IgEs on the mast cells. In other words, the interaction of either hapten alone with its respective IgE was insufficient to trigger degranulation. Given that there are 1–5 immunodominant allergy epitopes on a natural allergen, our result suggests that inhibiting as little as a single allergy epitope on an allergen may be sufficient to inhibit the mast cell degranulation response completely. Therefore the HtTA design also provides a model system that can be utilized to help in the design of selective inhibitors of allergic responses.
The results presented in this study emphasize the significance of more advanced, physiologically relevant, experimental models in allergy research. It would not have been possible to undertake these types of analysis using the previously established, simplified models of mast cell degranulation. Altogether, this study establishes the HtTA design as a well-characterized, multi-component experimental model system to address fundamental questions regarding mast cell stimulation and its inhibition by bringing an integrative approach to allergy research.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Wilson (University of New Mexico) for generously providing us with IgEDNP and the RBL cells. We thank Dr. Bill Boggess at the Mass Spectrometry and Proteomics Facility in the University of Notre Dame for the use of mass spec instrumentation.
FUNDING
This research is supported by NIH-NIAID R03 AI085485.
Abbreviations used
- HtTA
Heterotetravalent Allergen
- HmTA
Homotetravalent Allergen
- HmBA
Homobivalent Allergen
- HtBA
Heterobivalent Allergen
- BSA
Bovine Serum Albumin
- IgE
Immunoglobulin E
- RBL
Rat Basophilic Leukemia Cells
- DNP
Dinitrophenyl
- PBS
Phosphate buffered saline
- DLS
Dynamic Light Scattering
Footnotes
AUTHOR CONTRIBUTION
Michael Handlogten researched data, designed and performed the experiments and wrote the paper. Tanyel Kiziltepe and Basar Bilgicer researched data, designed experiments, and reviewed/edited the paper prior to submission.
REFERENCES
- 1.Metzger H. Transmembrane signaling - the joy of aggregation. J. Immunol. 1992;149:1477–1487. [PubMed] [Google Scholar]
- 2.Blank U, Rivera J. Assays for regulated exocytosis of mast cell granules. Curr Protoc Cell Biol. 2006;Chapter 15 doi: 10.1002/0471143030.cb1511s32. Unit 15.11. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Lin J, Bardina L, Goldis M, Nowak-Wegrzyn A, Shreffler WG, Sampson HA. Correlation of IgE/IgG4 milk epitopes and affinity of milk-specific IgE antibodies with different phenotypes of clinical milk allergy. J. Allergy Clin. Immunol. 2010;125:695–702. doi: 10.1016/j.jaci.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez-Torrejon G, Diaz-Perales A, Rodriguez J, Sanchez-Monge R, Crespo JF, Salcedo G, Pacios LF. An experimental and modeling-based approach to locate IgE epitopes of plant profilin allergens. J. Allergy Clin. Immunol. 2007;119:1481–1488. doi: 10.1016/j.jaci.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Christensen LH, Holm J, Lund G, Riise E, Lund K. Several distinct properties of the IgE repertoire determine effector cell degranulation in response to allergen challenge. J. Allergy Clin. Immunol. 2008;122:298–304. doi: 10.1016/j.jaci.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 6.Rouge P, Culerrier R, Sabatiera V, Granier C, Rance F, Barre A. Mapping and conformational analysis of IgE-binding epitopic regions on the molecular surface of the major ara h 3 legumin allergen of peanut (arachis hypogaea) Mol. Immunol. 2009;46:1067–1075. doi: 10.1016/j.molimm.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 7.Pacios LF, Tordesillas L, Cuesta-Herranz J, Compes E, Sanchez-Monge R, Palacin A, Salcedo G, Diaz-Perales A. Mimotope mapping as a complementary strategy to define allergen IgE-epitopes: Peach pru p 3 allergen as a model. Mol. Immunol. 2008;45:2269–2276. doi: 10.1016/j.molimm.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 8.Stanley J, King N, Burks A, Huang S, Sampson H, Cockrell G, Helm R, West C, Bannon G. Identification and mutational analysis of the immunodominant IgE binding epitopes of the major peanut allergen ara h 2. Arch. Biochem. Biophys. 1997;342:244–253. doi: 10.1006/abbi.1997.9998. [DOI] [PubMed] [Google Scholar]
- 9.Tanabe S. Epitope peptides and immunotherapy. Curr. Protein Peptide Sci. 2007;8:109–118. doi: 10.2174/138920307779941569. [DOI] [PubMed] [Google Scholar]
- 10.Cerecedo I, Zamora J, Shreffler WG, Lin J, Bardina L, Dieguez MC, Wang J, Muriel A, de la Hoz B, Sampson HA. Mapping of the IgE and IgG4 sequential epitopes of milk allergens with a peptide microarray-based immunoassay. J. Allergy Clin. Immunol. 2008;122:589–594. doi: 10.1016/j.jaci.2008.06.040. [DOI] [PubMed] [Google Scholar]
- 11.Cong Y, Lou F, Xue W, Li L, Chen M. Characterisation of the IgE-binding immunodominant epitopes on ara h1. Food Agric. Immunol. 2008;19:175–185. [Google Scholar]
- 12.Passante E, Frankish N. The RBL-2H3 cell line: Its provenance and suitability as a model for the mast cell. Inflammation Res. 2009;58:737–745. doi: 10.1007/s00011-009-0074-y. [DOI] [PubMed] [Google Scholar]
- 13.Andrews NL, Pfeiffer JR, Martinez AM, Haaland DM, Davis RW, Kawakami T, Oliver JM, Wilson BS, Lidke DS. Small, mobile Fc epsilon R1 receptor aggregates are signaling competent. Immunity. 2009;31:469–479. doi: 10.1016/j.immuni.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Handlogten MW, Kiziltepe T, Moustakas DT, Bilgicer B. Design of a heterobivalent ligand to inhibit IgE clustering on mast cells. Chem. Biol. 2011;18:1179–1188. doi: 10.1016/j.chembiol.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Collins AM, Basil M, Nguyen K, Thelian D. Rat basophil leukaemia (RBL) cells sensitized with low affinity IgE respond to high valency antigen. Clinical and Experimental Allergy. 1996;26:964–970. [PubMed] [Google Scholar]
- 16.Tordesillas L, Pacios LF, Palacin A, Cuesta-Herranz J, Madero M, Diaz-Perales A. Characterization of IgE epitopes of cuc m 2, the major melon allergen, and their role in cross-reactivity with pollen profilins. Clin. Exp. Allergy. 2010;40:174–181. doi: 10.1111/j.1365-2222.2009.03401.x. [DOI] [PubMed] [Google Scholar]
- 17.James LC, Tawfik DS. The specificity of cross-reactivity: Promiscuous antibody binding involves specific hydrogen bonds rather than nonspecific hydrophobic stickiness. Protein Science. 2003;12:2183–2193. doi: 10.1110/ps.03172703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denery-Papini S, Bodinier M, Pineau F, Triballeau S, Tranquet O, Adel-Patient K, Moneret-Vautrin DA, Bakan B, Marion D, Mothes T, Mameri H, Kasarda D. Immunoglobulin-E-binding epitopes of wheat allergens in patients with food allergy to wheat and in mice experimentally sensitized to wheat proteins. Clinical and Experimental Allergy. 2011;41:1478–1492. doi: 10.1111/j.1365-2222.2011.03808.x. [DOI] [PubMed] [Google Scholar]
- 19.Posner RG, Geng D, Haymore S, Bogert J, Pecht I, Licht A, Savage PB. Trivalent antigens for degranulation of mast cells. Org. Lett. 2007;9:3551–3554. doi: 10.1021/ol071175h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sil D, Lee JB, Luo D, Holowka D, Baird B. Trivalent ligands with rigid DNA spacers reveal structural requirements for IgE receptor signaling in RBL mast cells. ACS Chem. Biol. 2007;2:674–684. doi: 10.1021/cb7001472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaiser E, Colescott RL, Bossinger CD, Cook P. Color test for detection of free terminal amino groups in solid-phase synthesis of peptides. Anal. Biochem. 1970;34:595–598. doi: 10.1016/0003-2697(70)90146-6. [DOI] [PubMed] [Google Scholar]
- 22.Posnett D, Mcgrath H, Tam J. A novel method for producing anti-peptide antibodies - production of site-specific antibodies to the T-cell antigen receptor beta-chain. J. Biol. Chem. 1988;263:1719–1725. [PubMed] [Google Scholar]
- 23.Chhabra S, Hothi B, Evans D, White P, Bycroft B, Chan W. An appraisal of new variants of dde amine protecting group for solid phase peptide synthesis. Tetrahedron Lett. 1998;39:1603–1606. [Google Scholar]
- 24.Handlogten MW, Kiziltepe T, Alves NJ, Bilgicer B. Synthetic allergen design reveals the significance of moderate affinity epitopes in mast cell degranulation. ACS Chem. Biol. 2012 doi: 10.1021/cb300193f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bilgicer B, Thomas SW, Shaw BF, Kaufman GK, Krishnamurthy VM, Estroff LA, Yang J, Whitesides GM. A non-chromatographic method for the purification of a bivalently active monoclonal IgG antibody from biological fluids. J. Am. Chem. Soc. 2009;131:9361–9367. doi: 10.1021/ja9023836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bilgicer B, Moustakas DT, Whitesides GM. A synthetic trivalent hapten that aggregates anti-2,4-DNP IgG into bicyclic trimers. J. Am. Chem. Soc. 2007;129:3722–3728. doi: 10.1021/ja067159h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stefanick JF, Kiziltepe T, Handlogten MW, Alves NJ, Bilgicer B. Enhancement of antibody selectivity via bicyclic complex formation. J. Phys. Chem. Lett. 2012;3:598–602. [Google Scholar]
- 28.Delgado C, Francis GE, Fisher D. The uses and properties of peg-linked proteins. Crit. Rev. Ther. Drug Carr. Syst. 1992;9:249–304. [PubMed] [Google Scholar]
- 29.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 30.Roberts MJ, Bentley MD, Harris JM. Chemistry for peptide and protein PEGylation. Adv. Drug Deliv. Rev. 2002;54:459–476. doi: 10.1016/s0169-409x(02)00022-4. [DOI] [PubMed] [Google Scholar]
- 31.Baird B, Zheng Y, Holowka D. Structural mapping of IgE-Fc-epsilon-R1, an immunoreceptor complex. Acc. Chem. Res. 1993;26:428–434. [Google Scholar]
- 32.Baird EJ, Holowka D, Coates GW, Baird B. Highly effective poly(ethylene glycol) architectures for specific inhibition of immune receptor activation. Biochemistry. 2003;42:12739–12748. doi: 10.1021/bi034884l. [DOI] [PubMed] [Google Scholar]
- 33.Ortega E, Schweitzerstenner R, Pecht I. Possible orientational constraints determine secretory signals induced by aggregation of IgE receptors on mast-cells. EMBO J. 1988;7:4101–4109. doi: 10.1002/j.1460-2075.1988.tb03304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schweitzerstenner R, Licht A, Luscher I, Pecht I. Oligomerization and ring-closure of immunoglobulin-E class antibodies by divalent haptens. Biochemistry. 1987;26:3602–3612. doi: 10.1021/bi00386a053. [DOI] [PubMed] [Google Scholar]
- 35.Kane PM, Holowka D, Baird B. Cross-linking of IgE-receptor complexes by rigid bivalent antigens greater than 200 A in length triggers cellular degranulation. J. Cell Biol. 1988;107:969–980. doi: 10.1083/jcb.107.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holowka D, Sil D, Torigoe C, Baird B. Insights into immunoglobulin E receptor signaling from structurally defined ligands. Immunol. Rev. 2007;217:269–279. doi: 10.1111/j.1600-065X.2007.00517.x. [DOI] [PubMed] [Google Scholar]
- 37.Hamilton RG, MacGlashan DW, Jr, Saini SS. IgE antibody-specific activity in human allergic disease. Immunol. Res. 2010;47:273–284. doi: 10.1007/s12026-009-8160-3. [DOI] [PubMed] [Google Scholar]
- 38.Seagrave J, Oliver JM. Antigen-dependent transition of IgE to a detergent-insoluble form is associated with reduced IgE receptor-dependent secretion from RBL-2H3 mast-cells. J. Cell. Physiol. 1990;144:128–136. doi: 10.1002/jcp.1041440117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.