Abstract
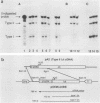
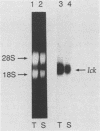
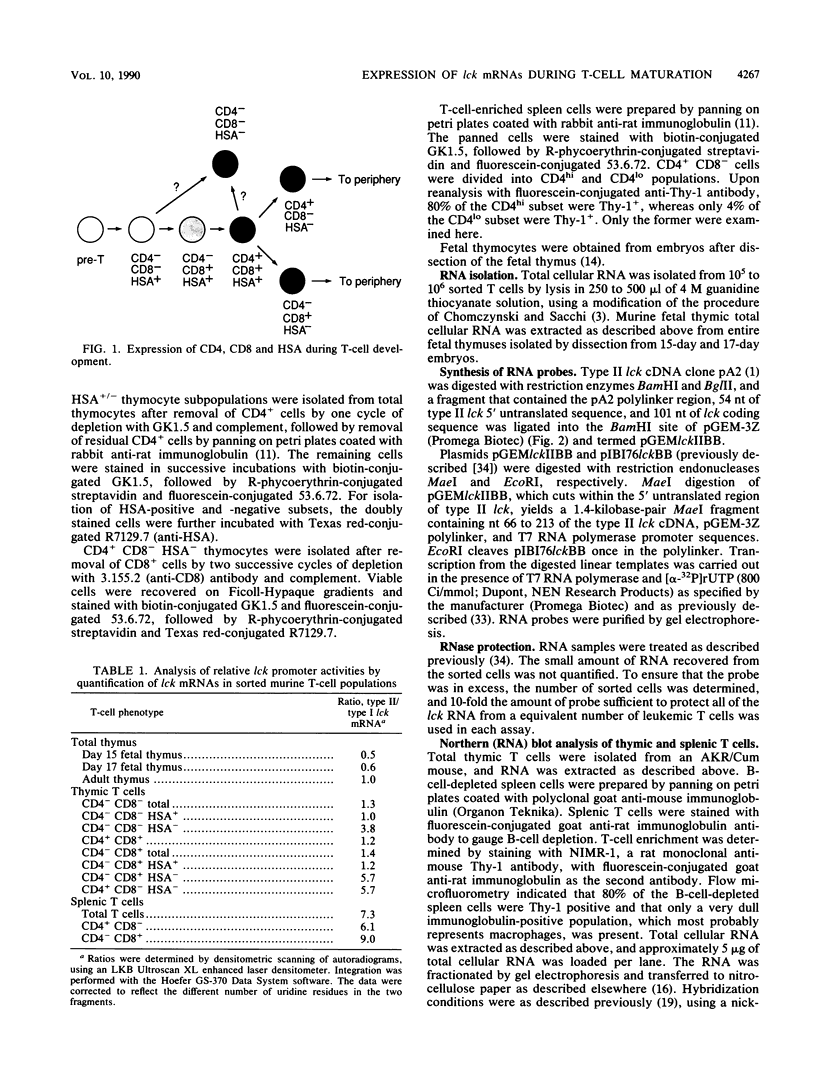
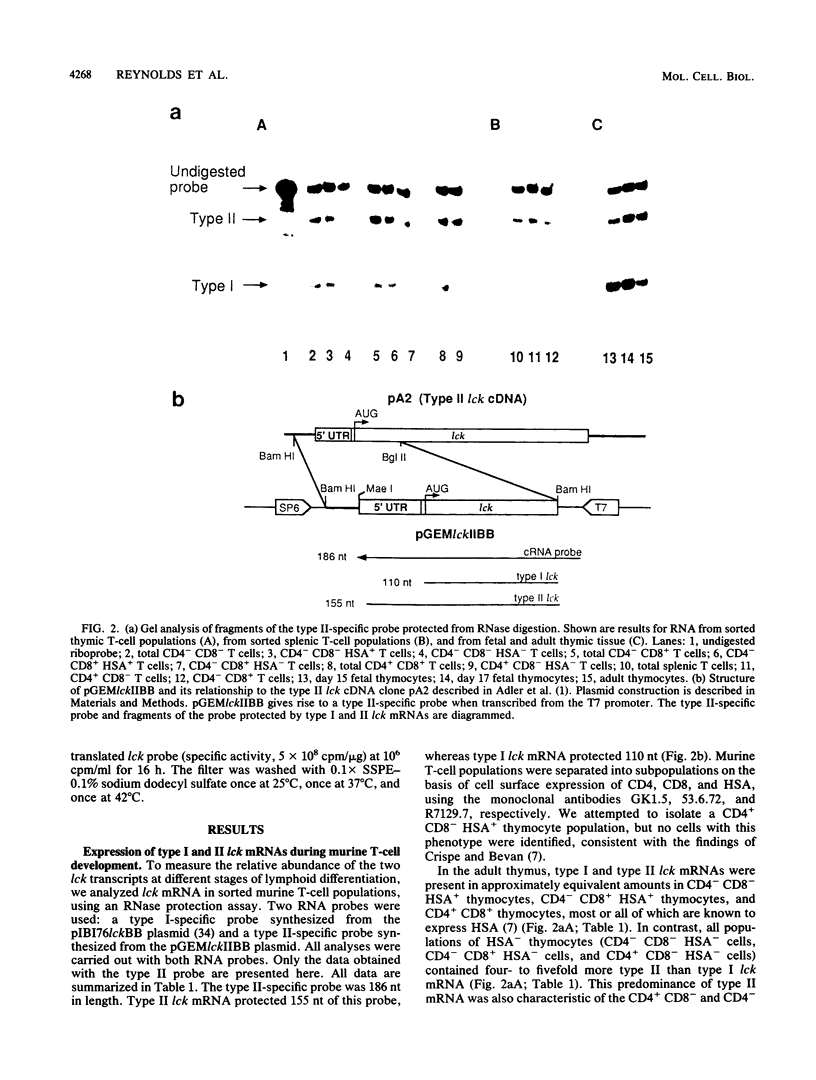
The lck gene, which encodes the lymphoid cell-specific tyrosine protein kinase p56lck, is expressed from two widely separated promoters. The proximal promoter gives rise to a type I lck transcript, and the distal promoter gives rise to a type II transcript. We found that the ratio of the two transcripts changed during T-cell maturation. Type I lck mRNA was twofold more abundant than the type II transcript in early fetal thymocytes. In the adult, the type I and type II lck mRNAs were present in approximately equal amounts in immature thymocytes expressing the heat-stable antigen. In contrast, there was five- to ninefold more type II lck than type I lck mRNA in more mature thymocytes that did not express the heat-stable antigen and in splenic T cells. This change in relative transcript abundance probably reflects activation of the distal promoter and inactivation of the proximal promoter during T-cell maturation in the thymus. It is possible that the two promoters are regulated by different trans-acting factors whose expression is regulated during T-cell maturation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. T., Reynolds P. J., Kelley C. M., Sefton B. M. Transcriptional activation of lck by retrovirus promoter insertion between two lymphoid-specific promoters. J Virol. 1988 Nov;62(11):4113–4122. doi: 10.1128/jvi.62.11.4113-4122.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce J., Symington F. W., McKearn T. J., Sprent J. A monoclonal antibody discriminating between subsets of T and B cells. J Immunol. 1981 Dec;127(6):2496–2501. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Corbin V., Maniatis T. Role of transcriptional interference in the Drosophila melanogaster Adh promoter switch. Nature. 1989 Jan 19;337(6204):279–282. doi: 10.1038/337279a0. [DOI] [PubMed] [Google Scholar]
- Corbin V., Maniatis T. The role of specific enhancer-promoter interactions in the Drosophila Adh promoter switch. Genes Dev. 1989 Dec;3(12B):2191–2120. doi: 10.1101/gad.3.12b.2191. [DOI] [PubMed] [Google Scholar]
- Crispe I. N., Bevan M. J. Expression and functional significance of the J11d marker on mouse thymocytes. J Immunol. 1987 Apr 1;138(7):2013–2018. [PubMed] [Google Scholar]
- Crispe I. N., Moore M. W., Husmann L. A., Smith L., Bevan M. J., Shimonkevitz R. P. Differentiation potential of subsets of CD4-8- thymocytes. Nature. 1987 Sep 24;329(6137):336–339. doi: 10.1038/329336a0. [DOI] [PubMed] [Google Scholar]
- Fowlkes B. J., Edison L., Mathieson B. J., Chused T. M. Early T lymphocytes. Differentiation in vivo of adult intrathymic precursor cells. J Exp Med. 1985 Sep 1;162(3):802–822. doi: 10.1084/jem.162.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidos C. J., Weissman I. L., Adkins B. Intrathymic maturation of murine T lymphocytes from CD8+ precursors. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7542–7546. doi: 10.1073/pnas.86.19.7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman R., Lesley J., Schulte R., Trotter J. Progenitor cells in the thymus: most thymus-homing progenitor cells in the adult mouse thymus bear Pgp-1 glycoprotein but not interleukin-2 receptor on their cell surface. Cell Immunol. 1986 Sep;101(2):320–327. doi: 10.1016/0008-8749(86)90145-0. [DOI] [PubMed] [Google Scholar]
- Koga Y., Kimura N., Minowada J., Mak T. W. Expression of the human T-cell-specific tyrosine kinase YT16 (lck) message in leukemic T-cell lines. Cancer Res. 1988 Feb 15;48(4):856–859. [PubMed] [Google Scholar]
- Lesley J., Schulte R., Trotter J., Hyman R. Qualitative and quantitative heterogeneity in Pgp-1 expression among murine thymocytes. Cell Immunol. 1988 Mar;112(1):40–54. doi: 10.1016/0008-8749(88)90274-2. [DOI] [PubMed] [Google Scholar]
- Lesley J., Trotter J., Hyman R. The Pgp-1 antigen is expressed on early fetal thymocytes. Immunogenetics. 1985;22(2):149–157. doi: 10.1007/BF00563512. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Howe R. C., Pedrazzini T., Lees R. K., Budd R. C., Schneider R., Liao N. S., Zinkernagel R. M., Louis J. A., Raulet D. H. T-cell lineages, repertoire selection and tolerance induction. Immunol Rev. 1988 Aug;104:157–182. doi: 10.1111/j.1600-065x.1988.tb00762.x. [DOI] [PubMed] [Google Scholar]
- Marth J. D., Overell R. W., Meier K. E., Krebs E. G., Perlmutter R. M. Translational activation of the lck proto-oncogene. Nature. 1988 Mar 10;332(6160):171–173. doi: 10.1038/332171a0. [DOI] [PubMed] [Google Scholar]
- Marth J. D., Peet R., Krebs E. G., Perlmutter R. M. A lymphocyte-specific protein-tyrosine kinase gene is rearranged and overexpressed in the murine T cell lymphoma LSTRA. Cell. 1985 Dec;43(2 Pt 1):393–404. doi: 10.1016/0092-8674(85)90169-2. [DOI] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Nikolić-Zugić J., Bevan M. J. Thymocytes expressing CD8 differentiate into CD4+ cells following intrathymic injection. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8633–8637. doi: 10.1073/pnas.85.22.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolić-Zugić J., Moore M. W., Bevan M. J. Characterization of the subset of immature thymocytes which can undergo rapid in vitro differentiation. Eur J Immunol. 1989 Apr;19(4):649–653. doi: 10.1002/eji.1830190412. [DOI] [PubMed] [Google Scholar]
- Rudd C. E., Trevillyan J. M., Dasgupta J. D., Wong L. L., Schlossman S. F. The CD4 receptor is complexed in detergent lysates to a protein-tyrosine kinase (pp58) from human T lymphocytes. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5190–5194. doi: 10.1073/pnas.85.14.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. H. Acquisition of immunologic self-tolerance. Cell. 1989 Jun 30;57(7):1073–1081. doi: 10.1016/0092-8674(89)90044-5. [DOI] [PubMed] [Google Scholar]
- Scollay R., Bartlett P., Shortman K. T cell development in the adult murine thymus: changes in the expression of the surface antigens Ly2, L3T4 and B2A2 during development from early precursor cells to emigrants. Immunol Rev. 1984 Dec;82:79–103. doi: 10.1111/j.1600-065x.1984.tb01118.x. [DOI] [PubMed] [Google Scholar]
- Scollay R., Wilson A., D'Amico A., Kelly K., Egerton M., Pearse M., Wu L., Shortman K. Developmental status and reconstitution potential of subpopulations of murine thymocytes. Immunol Rev. 1988 Aug;104:81–120. doi: 10.1111/j.1600-065x.1988.tb00760.x. [DOI] [PubMed] [Google Scholar]
- Shaw P., Sordat B., Schibler U. The two promoters of the mouse alpha-amylase gene Amy-1a are differentially activated during parotid gland differentiation. Cell. 1985 Apr;40(4):907–912. doi: 10.1016/0092-8674(85)90350-2. [DOI] [PubMed] [Google Scholar]
- Shortman K., Wilson A., Egerton M., Pearse M., Scollay R. Immature CD4- CD8+ murine thymocytes. Cell Immunol. 1988 May;113(2):462–479. doi: 10.1016/0008-8749(88)90042-1. [DOI] [PubMed] [Google Scholar]
- Smith L. CD4+ murine T cells develop from CD8+ precursors in vivo. Nature. 1987 Apr 23;326(6115):798–800. doi: 10.1038/326798a0. [DOI] [PubMed] [Google Scholar]
- Tamauchi H., Tamaoki N., Habu S. CD4+CD8+ thymocytes develop into CD4 or CD8 single-positive cells in athymic nude mice. Eur J Immunol. 1988 Nov;18(11):1859–1862. doi: 10.1002/eji.1830181134. [DOI] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Bolen J. B. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988 Oct 21;55(2):301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- Voronova A. F., Adler H. T., Sefton B. M. Two lck transcripts containing different 5' untranslated regions are present in T cells. Mol Cell Biol. 1987 Dec;7(12):4407–4413. doi: 10.1128/mcb.7.12.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronova A. F., Sefton B. M. Expression of a new tyrosine protein kinase is stimulated by retrovirus promoter insertion. Nature. 1986 Feb 20;319(6055):682–685. doi: 10.1038/319682a0. [DOI] [PubMed] [Google Scholar]
- Wilson A., Day L. M., Scollay R., Shortman K. Subpopulations of mature murine thymocytes: properties of CD4-CD8+ and CD4+CD8- thymocytes lacking the heat-stable antigen. Cell Immunol. 1988 Dec;117(2):312–326. doi: 10.1016/0008-8749(88)90121-9. [DOI] [PubMed] [Google Scholar]
- von Boehmer H. The developmental biology of T lymphocytes. Annu Rev Immunol. 1988;6:309–326. doi: 10.1146/annurev.iy.06.040188.001521. [DOI] [PubMed] [Google Scholar]