Abstract
Extracellular matrix remodelling is a prerequisite for plaque rupture in atherosclerotic lesion. Versican, an extracellular matrix proteoglycan present in normal and atherosclerotic arteries is a substrate for matrix metalloproteinases (MMPs) present in macrophage rich areas. The aim of the current study was to develop an immunoassay to detect a specific MMP-12 derived versican degradation fragment (VCANM) and assess its potential as a biomarker for extracellular matrix remodelling in atherosclerosis. A mouse monoclonal antibody raised against VCANM was used for the development of a competitive ELISA for detection of the fragment in plasma. VCANM was measured in plasma of patients with different levels of heart diseases. Patients experiencing I) acute coronary syndrome, II) stable ischemic heart disease and III) demonstrating high levels of coronary calcium deposits had significantly higher plasma levels of VCANM compared to a control group of individuals with no detectable coronary calcium deposits. VCANM was also detected by immunohistochemistry in coronary artery sections of patients with different degrees of atherosclerosis. VCANM ability to separate patients with atherosclerotic diseases from healthy individuals suggested VCANM as a potential biomarker for the pathological arterial matrix remodelling associated with atherosclerosis.
Keywords: Versican, acute coronary syndrome, atherosclerosis, biomarker, matrix, remodeling, neo-epitope
Introduction
Atherosclerosis is the underlying basis for cardiovascular disease, which is the leading cause of morbidity and mortality in the Western world. The hallmark of atherosclerosis is spotty thickening of the arterial intima, followed by narrowing of the arterial lumen, leading to local ischemia particularly in the case of coronary atherosclerotic plaque rupture and myocardial infarction [1].
Due to its asymptomatic nature in its early stages, atherosclerosis has proven difficult to reliably diagnose. Most candidates for novel circulatory markers for cardiovascular diseases are either related to specific risk components (lipids, diabetes) or to the inflammatory aspects of the atherogenic process (CRP). Very few candidates have so far been based on the composition of the atherosclerotic plaque.
Remodelling of the arterial extracellular matrix (ECM) is an important feature of atherosclerosis. The ECM is critically important for maintaining the physicochemical structure of the artery, providing tensile strength as well as viscoelastic properties [2]. The pathologically elevated levels of matrix metalloproteinases (MMPs) produced by macrophage foam cells and/or other inflammatory cells, as seen in atherosclerosis, may lead to excessive matrix remodelling and ultimately rupture of the fibrous cap of the atherosclerotic plaque [3-6]. MMP-12 is mainly released by macrophage foam cells and has a broad range of substrates, in particular collagens, elastin and proteoglycans.
Versican is a large chondroitin sulfate proteoglycan (CSPG) and one of the main CSPGs found in the ECM of normal blood vessels. It is present in the intima and adventitia of most arteries and veins, where it is synthesized by vascular smooth muscle cells (SMCs) as well as endothelial cells (ECs), myofibroblasts and even macrophages [7]. Dramatically increased levels of versican have been reported in atherosclerosis and restenosis, implicating this proteoglycan as a specific component of developing lesions and contributing to the progression of atherosclerotic and restenotic lesions [8]. Studies have shown that proteoglycans, in particular versican and biglycan, have a capacity for binding to and retaining low density lipoproteins (LDL) and thus to contribute to the pathological events leading to atherosclerotic tissue remodeling [9]. The atherosclerotic artery is continuously being remodelled, releasing in the process a range of degradation products of ECM proteins, the so-called neo-epitopes, into the circulation [10].
The aim of the current study was to develop a plasma assay for a MMP-derived versican neo-epitope present in atherosclerotic tissue. Our hypothesis was that the level of such epitope in plasma may reflect the presence of actively remodelling atherosclerotic plaques. To verify our hypothesis, the levels of VCANM were measured in I) asymptomatic individuals with no detectable coronary calcium (control group), II) asymptomatic patients with CT-verified presence of coronary calcium, III) patients with stable ischemic heart disease (IHD) and IV) patients with acute coronary syndrome (ACS).
Materials and methods
Reagents
All chemicals were purchased from either Sigma Aldrich or Merck unless otherwise stated. Cell culture reagents were obtained from Gibco, Sigma Aldrich or Roche. The ELISA streptavidin pre-coated plates were purchased from NUNC. Peptide conjugation reagents and buffers were obtained from Pierce.
In vitro cleavage
For in vitro digestion of versican by MMPs, we have used the commercially available extracted extracellular chondroitin sulfate proteoglycans from chicken brain purchased from Millipore, Denmark (product id: cc117). This proteoglycan mix composed of neurocan, versican, and aggrecan was subjected to proteolytic cleavage in vitro by different proteases, including MMP-8 and MMP-12 following a previously described methodology [11]. Briefly, the digestion was performed by mixing 100 μg of chicken brain extract and 10 μg of protease in MMP buffer (100 mM Tris-HCl, 100 mM NaCl, 10 mM CaCl2, 2 mM Zn acetate, pH 8.0). The solution was incubated for 7 days at 37°C. 100 μg of chicken brain extract was mixed with buffer alone as a control. The cleavage reaction was stopped using 50 μM ethylenediaminetetraacetic acid (EDTA) to a final concentration of 1 μM.
Peptide fragments so generated were identified by LC-MS/MS. Samples were ultrafiltrated to remove proteins above 10 kDa, the pH was adjusted to 2.0 using formic acid and the samples (4 μL) were separated on a nanoACQUITY UPLC BEH C18 Column (Waters, Milford, MA, USA) using a formic acid/acetonitrile gradient. MS and MS/MS were performed on a Synapt G1 High Definition Mass Spectrometer quadrupole time-offlight MS (QUAD-TOF; Waters) with an acquisition range of 350 to 1,600 m/z in MS and 50 to 2000 m/z in MS/MS. ProteinLynx Global SERVER software (Waters) was used to analyze spectra and generate peak lists. To identify peptides, MS and MS/MS data were searched against a versican protein database (FASTA) using Mascot 2.2 software (Matrix Science, Boston, MA, USA) with the ESI-QUAD-TOF settings and carbamidomethyl (C), oxidation of methionine (M), oxidation of lysine (K) and oxidation of proline (P) as variable modifications.
The six amino acids in the N- or C-terminal of the peptides identified by MS were regarded as a neo-epitope generated by the specific protease. All protease-generated sequences were analyzed for distance to other cleavage sites, for homology between different species and with other proteins using NPS@: network protein sequence analysis [12].
Peptide selection and monoclonal antibody production
A monoclonal antibody (mAb) specific for the selected versican neo-epitope (VCANM) was generated. The synthetic peptides used for mAb production and validation were:
a) Immunogenic peptide: KTFGKMKPRY-GGC. This peptide was selected for being unique to human versican and homologue across several species such as primates, rat and mouse. The immunogenic peptide conjugated to the carrier protein Ovalbumin (OVA) was used as immunogen.
b) Screening peptide: KTFGKMKPRY-K-Biotin. A biotinylated version of the peptide was generated to be used in the competitive ELISA setting as coating peptide.
c) Standard peptide: KTFGKMKPRY. This peptide was used in the competitive ELISA setting as standard.
d) Elongated peptide: AKTFGKMKPRY. The selected peptide was elongated with one amino acid at the site of the protease cleavage. In this case one alanine, the residual amino acid placed on the N-terminal end of the cleavage site, was added. Antibodies recognizing the elongated peptide were not selective for the neo-epitope and were therefore discarded.
All the peptides were purchased from the Chinese Peptide Company, Beijing, China.
The production of the mAb directed towards the VCANM neo-epitope was performed as previously detailed [11].
VCANM enzyme-linked immunosorbent assay (ELISA) methodology
A 96-well streptavidin pre-coated plate was coated with 2.5 ng/mL of the biotinylated synthetic peptide, KTFGKMKPRY-K-Biotin, dissolved in PBS-BTB buffer (2 mM KH2PO4, 9 mM Na2HPO4, 2H2O, 3 mM KCl, 137 mM NaCl, pH 7.4) and incubated for 30 minutes at 20°C. 20 μL of the peptide calibrator or sample were added to appropriate wells, followed by 100 μL of 20 ng/ml HRP-conjugated mAb, and incubated for 1 hour at 20°C. Finally, 100 μL tetramethyl benzidine (TMB) developer (Kem-En-Tec cat.438OH, Taastrup, Denmark) was added, and the plate was incubated for 15 minutes at 20°C in the dark. All the above incubation steps included shaking at 300 rpm. After each incubation step the plate was washed five times in washing buffer (20 mM Tris, 50 mM NaCl, pH 7.2). The TMB reaction was stopped by adding 100 μL of stopping solution (1% HCl) and measured at 450 nm with 650 nm as the reference. A standard calibration curve was plotted using a 4-parametric mathematical fit model with a starting concentration of 10 ng/mL of the standard peptide following a 2-fold dilution and the last standard was a zero standard.
The ELISA was technically validated according to the Nordic Bioscience standard operating procedures. Linearity, lower detection limit (LDL), intra- and inter-variation, spiking recovery and assay stability were assessed as previously described [11]. The specificity for the neo-epitope was tested using the extracted extracellular chondroitin sulfate proteoglycans from chicken brain cleaved for 7 days at 37°C with MMP-8, MMP-12, Cathepsin K and with MMP buffer only (intact VCAN) as sample.
Samples and procedures for immunohistochemistry
Coronary arteries were excised from hearts obtained at autopsy from patients with different degree of atherosclerosis aged 25-75 years of age, who all died within 18 month of each other at North Carolina Baptist Hospital, Winston-Salem, USA [13]. Hearts were flushed with normal saline and were perfusion fixed with 4% neutral buffered formaldehyde solution at a pressure of 100 mm Hg for 1 hour. Adjacent tissue blocks for histological sectioning (each 3 mm long) were cut perpendicular to the long axis (proximal 3 cm) of the left anterior descending (LAD), the left circumflex, and the right coronary arteries.
The standard Nordic Bioscience protocol was used for VCANM staining of the human LAD coronary arteries. Briefly, the paraffin embedded arteries were cut into 5 μm sections, deparaffinized, peroxidase blocked and rehydrated. The sections were subjected to pre-treatment in citrate buffer over night at 60°C. Hereafter, the sections were washed in TBS, blocked in 0.5% casein TBS and incubated with 2.25 μg/ml VCANM mAb diluted in TBS buffer over night at 4°C. Negative controls were performed incubating the tissue with mouse IgG1 (Dako) in TBS.
The sections were subsequently rinsed and incubated with Super Sensitive Polymer-HRP IHC Detection System combined with DAB substrate according to the supplier’s instructions (Biogenex, Taby, Sweden). Sections were counterstained with Mayer’s hematoxylin for 12 seconds, mounted and left to dry. Digital photographs were taken using an Olympus B 60 x microscope equipped with an Olympus C-5050 Zoom digital camera (Olympus, Tokyo, Japan).
Patients and sample collection
Four groups of 30 individuals each, with different degrees of atherosclerotic heart disease, were selected from three large studies (DanRisk, DEFAMI and Odense Artery Biobank). The DanRisk and DEFAMI protocols were approved by the Regional Scientific Ethical Committee for Southern Denmark (S-20080140 and S-20090082) and were conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from each participant. Participants in these studies were enrolled simultaneously by Odense University Hospital, Odense, Denmark. All samples were handled and tested by the Department of Clinical Biochemistry and Pharmacology at Odense University Hospital (Odense, Denmark).
The four groups consisted of: 1) 30 asymptomatic individuals with no detectable coronary calcium (CT-no Ca), 2) 30 asymptomatic subjects with subclinical CVD defined by the presence of coronary calcium (CT-plus Ca), 3) 30 patients with stable ischemic heart disease (IHD), bound for coronary by-pass surgery and 4) 30 patients with acute coronary syndrome (ACS) and symptom onset within the previous 24 hours.
The group 1 and 2 patients belonged to the DanRisk-cohort, a random recruitment of 1825 men and women aged 50 and 60 years old, who in 2009 were invited for screening for coronary disease. The screening included a non-contrast enhanced cardiac CT-scan, followed by an estimation of the amount of coronary calcium (CAC score). Complete details of the study have been described by Diederichsen and coauthors [14]. Among the individuals aged 60 years (n=647), 30 patients without detectable coronary calcium (CT-no Ca, group 1), and 30 with an Agatston score ≥400 (CT-plus Ca, group 2), were chosen. These two groups were matched for total cholesterol and blood pressure. The group of patients with stable IHD (group 3) belonged to The Odense Artery Biobank, which gathers spare arterial tissue and blood samples from patients undergoing coronary by-pass surgery. Blood samples were taken the day before the surgery. 30 patients with a mean age of approximately 60 years and a gender distribution similar to the above-mentioned populations were selected. The group of patients with ACS (group 4) was recruited from the DEFAMI-cohort. This cohort consisted of all patients admitted to Odense University Hospital between October 1 2009 and April 30 2010 who had troponin analysis performed on suspicion of ACS. In these patients, serial blood sampling was performed within the first 24 hours of symptom onset. Of the 822 patients in the DEFAMI cohort, 30 patients with non-ST segment elevation myocardial infarction (NSTEMI, n=24) or unstable angina pectoris (n=6) were selected to participate in the current study. Their age was approximately 60 years and their gender distribution matched the two DanRisk subgroups (group 1 and group 2). NSTEMI was defined as a typical rise and/or fall of TnI levels above 0.03 μg/l, in association with the presence of typical clinical symptoms. Unstable angina pectoris was defined as the presence of typical chest pain during rest or minimal physical exertion together with ST-segment depression or T-wave fluctuations, but with normal TnI levels (<0.03 μg/l). All patients with unstable angina pectoris also had to demonstrate at least one significant coronary artery stenosis (>50% diameter stenosis). Patients with ST segment elevation myocardial infarction (STEMI) were not included, because it would not be possible to obtain sufficient serial blood samples from these patients before medication with heparin and acute PCI were done. Both interventions might interfere in biochemical assays. The mean TnI level in the 30 ACS patients (group 4) was 0.62 μg/l (range 0.01-5.23 μg/l).
In all patients, group 1-4 (n=120), hypertension was considered if the patient used anti-hypertensive medical treatment and diabetes was defined if anti-diabetic medication was prescribed. Systolic and diastolic blood pressure was measured on the same day as blood sampling. Agatston score was calculated in group 1 and 2 patients. TnI in the ACS group was measured immediately on hospital admittance, prior to the current blood sampling. Total cholesterol, LDL, HDL and triglycerides were also measured prior to blood sampling. Blood samples were drawn in tubes with EDTA and centrifuged at 2000 x g for 10 min. Plasma was stored at -80°C until biochemical analysis.
Statistical analysis
One-way analysis of variance (ANOVA) was used for comparison among groups in both clinical cohorts. If ANOVA revealed a statistically significant difference between groups, each of the groups (CT-plus Ca, ACS and IHD) was compared with the control group (CT-no Ca) with the level of significance adjusted for multiple comparisons by the method of Dunnett. Differences were considered statistically significant if p<0.05. The diagnostic value of the assay was calculated by receiver operating characteristic (ROC) curve plots. GRAPH PAD PRISM 5 (Graph Pad Software, LaJolla, CA, USA) was used for calculations. The Statistical Analysis System (SAS Institute, Cary, NC, USA) was used for the analyses.
Results
Identification of VCANM neo-epitope
More than 100 peptides from chicken brain extract cleaved by the different proteases were identified with a statistically significant Mascot score (p<0.05) and tested for homology to other human proteins (data not shown). Antibodies were generated against six neo-epitope sequences, and based on the reactivity against the selection peptide, the specificity for the cleaved versican and the reactivity against native material (human plasma), the mAb recognizing one of the peptides identified by LC-MS/MS was selected for assay development. The sequence KTFGKMKPRY (VCANM), cleaved at the N-terminal in position 3306 by MMP-8 and MMP-12 was identified by LC-MS/MS and selected for immunization. The VCANM peptide was shown to be unique to versican with 100% homology across different species (Homo sapiens, Mus musculus, Rattus norvegicus, Equus caballus, Bos Taurus, Gallus gallus).
Technical performance of the VCANM assay
The antibody NB158-8B2 raised against VCANM antigen was chosen for ELISA development, on the basis of best native reactivity, affinity and stability. As illustrated in Figure 1, the VCANM antibody was specific to in vitro cleaved versican, with preference to MMP-12 digestion. Importantly, there was no inhibition of the signal in the samples containing either synthetic elongated peptide or intact versican. Furthermore, we demonstrated that VCANM is not generated by Cathepsin K.
Figure 1.
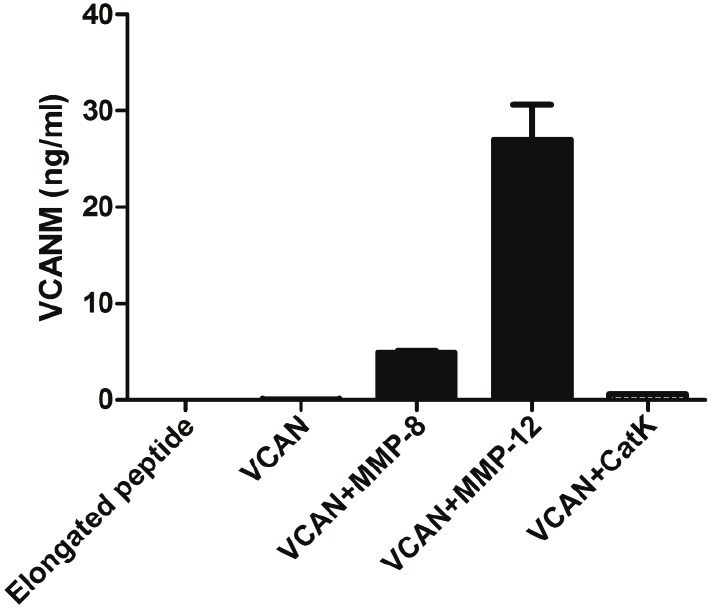
Test of antibody specificity towards VCANM neo-epitope. Samples added and tested in a competitive ELISA setting were: elongated peptide, uncleaved versican (VCAN), MMP-8 cleaved versican (VCAN+MMP-8), MMP-12 cleaved versican (VCAN+MMP-12) and Cathepsin K cleaved versican (VCAN+CatK).
The assay showed good technical performances: the lowest detection limit (LDL) was 0.38 ng/ml, dilution recovery for samples diluted 1:2 and 1:4 to undiluted sample was within 100%±10%, and the mean intra- and inter assay variations were 6.98% and 6.65% respectively, both below the recommended 10%.
Immunohistochemistry of VCANM
The human arterial left anterior descending (LAD) coronary artery from two deceased individuals with different severity of atherosclerosis, stained with monoclonal anti-VCANM antibody, is shown in Figure 2. The versican neo-epitope is present in the specimens of atherosclerotic patients, with specific localization at the plaque level, towards the edges of the atherosclerotic plaque.
Figure 2.

Immunohistochemical analysis of VCANM on LAD coronary arteries from patients with different degrees of atherosclerosis. The photomicrographs show a particular of the plaque region (original magnification, x40) with presence of VCANM localized at the plaque edges (arrows).
Clinical demographics
The demographics of the 120 patients included in this study are presented in Table 1. From the 30 patients in each of the four group, the group consisting of ACS patients had a small, but significant higher mean age (p=0.0001) and systolic blood pressure (p=0.004) compared to the other three groups. The IHD group had significantly lower total and LDL cholesterol levels (p=0.007 and p=0.02), probably due to a high prevalence of lipid-lowering medication. It is worth mentioning that 13% CT-no Ca, 23% of CT-plus Ca, 87% of subjects with stable IHD and 60% of patients with ACS were treated with statins and therefore the data regarding total and LDL cholesterol cannot be used for correlation with VCANM levels.
Table 1.
Demographics of each group included in the study with mean values and standard deviation (in [])
| CT-no Ca | CT-plus Ca | ACS | IHD | ANOVA | |
|---|---|---|---|---|---|
| n=30 | n=30 | n=30 | n=30 | p-value | |
| Age (years) | 60.2 | 60.3 | 64.5** | 59.7 | 0.0001 |
| [59.8; 60.7] | [60.0-60.6] | [56.0; 73.0] | [56.8; 62.6] | ||
| Triglycerides# (mmol/L) | 1.57 | 1.49 | 1.30 | 1.48 | 0.52 |
| [0.85; 2.93] | [1.00; 2.21] | [0.78; 2.19] | [0.92; 2.30] | ||
| HDL Cholesterol# (mmol/L) | 1.28 | 1.36 | 1.13 | 1.30 | 0.14 |
| [0.93; 1.75] | [0.98; 1.89] | [0.83; 1.52] | [0.90;1.87] | ||
| LDL Cholesterol (mmol/L) | 3.14 | 3.26 | 2.93 | 2.41* | 0.02 |
| [2.13; 4.16] | [2.29; 4.22] | [1.74; 4.11] | [1.27; 3.55] | ||
| Total Cholesterol (mmol/L) | 5.25 | 5.47 | 4.74 | 4.45* | 0.007 |
| [4.06; 6.43] | [4.29; 6.64] | [3.44; 6.04] | [3.20; 5.71] | ||
| Systolic Blood Pressure (mmHg) | 145 | 147 | 159* | 145 | 0.04 |
| [131; 159] | [128; 167] | [132; 187] | [123; 168] | ||
| Diastolic Blood Pressure (mmHg) | 86 | 85 | 88 | 83 | 0.56 |
| [76; 95] | [75; 96] | [72; 103] | [74; 92] | ||
| Agatston score# | 0 | 1299 | - | - | - |
| [717; 2352] | |||||
| Heart Score# | 6.33 | 8.30 | - | - | 0.3 |
| [2.23; 8.92] | [3.10;12.42] |
CT-no Ca: Asymptomatic individuals without detectable coronary calcium; CT-plus Ca: Asymptomatic individuals with high coronary calcium; ACS: Acute Coronary Syndrome; IHD: patients with stable Ischemic Heart Disease. Mean values [+/- STD];
Geometric mean values [+/- STD], and p-value from one-way of analysis of variance (ANOVA).
The level of significance of p-values from comparison of each group against the control group CT-no Ca was adjusted for multiple comparisons by the method of Dunnet.
p<0.05;
p<0.01.
Biomarker levels of circulating VCANM
Measurements of VCANM in plasma are shown in Figure 3. Levels of the VCANM neo-epitope were significantly increased in the groups of patients with high-coronary calcium, ACS, and IHD compared to the control group, with no detectable coronary calcium deposits. The average value of circulating VCANM in the control group was 1.52 ng/ml, while the patients in the ACS group had average levels of 1.98 ng/ml (p<0.0005), CT-plus Ca 1.92 ng/ml (p<0.0005) and IHD 1.85 ng/ml (p<0.05).
Figure 3.

Plasma levels of VCANM measured in patients diagnosed with acute coronary syndrome (ACS), high deposits of coronary calcium (CT-plus Ca), stable ischemic heart disease (IHD) and compared with age-matched controls comprising individuals with no detectable coronary calcium deposits (CT-no Ca). Individual values are shown and the group mean and SEM are indicated as line and error bars. * = p<0.05; *** = p<0.0005.
ROC values determined in the clinical cohorts
The levels of VCANM with the highest significant ROC values were found in the group of ACS patients (AUC: 0.775, p-value <0.0001). Furthermore the groups of patients with CT-plus Ca and stable IHD also displayed significant ROC values (Table 2).
Table 2.
ROC curves in high coronary calcium (CT-plus Ca), acute coronary syndrome (ACS) and ischemic heart disease (IHD) group compared to the control group with no detectable coronary calcium (CT-no Ca). AUC is given with standard error and p-value
| ROC curve; AUC [std error] p-value | |||
|---|---|---|---|
|
| |||
| Parameter | CT-plus Ca vs CT-no Ca | ACS vs CT-no Ca | IHD vs CT-no Ca |
| VCANM | 0.748 | 0.775 | 0.669 |
| [0.068] | [0.062] | [0.077] | |
| 0.0003 | <0.0001 | 0.03 | |
Discussion
In this study we identified a fragment of versican, VCANM, neo-epitope generated in vitro by MMP degradation, preferentially MMP-12. We presented the development of a novel, technically robust ELISA, detecting the VCANM neo-epitope in plasma. The assay was specific for the neo-epitope, as demonstrated by the ability of VCANM mAb to recognize only MMP-12 cleaved versican and not intact versican or elongated peptide variants.
The developed assay was used to preliminary assess the possibility of VCANM to separate patients with different manifestations of atherosclerotic heart disease from healthy subjects. The purpose of this analysis was to demonstrate VCANM potential as marker of extracellular matrix remodelling in lipid laden vulnerable plaques. The results of our measurements showed that the mean concentration of the VCANM neo-epitope was increased in three different patient groups with atherosclerotic heart diseases. We observed significantly higher levels of the VCANM neo-epitope in patients with stable IHD bound for the bypass surgery and in a group of patients suffering from ACS compared to an asymptomatic group with no signs of coronary calcifications.
Interestingly, VCANM was also able to separate with statistical significance two gender-, age-, cholesterol-, and blood pressure matched asymptomatic groups with or without the presence of coronary calcium deposits (AUC CT-plus Ca vs CT-no Ca: 0.748, p-value 0.0003). Both groups were recruited from a population-based screening of individuals without cardiovascular symptoms, which have been separated only on the basis of the amount of coronary calcium, detected by a coronary-CT. The degree of detected calcium (CAC score) reflects the amount of coronary atherosclerosis [14,15] and therefore also to a large degree the total amount of lesions throughout the complete arterial tree, since the occurrence of atherosclerosis is associated at different locations [16]. However, absence of coronary calcification does not rule out calcification in other arteries, which in part may explain the variation in the control group. The ROC value of VCANM for CT-plus Ca vs CT-no Ca is at the same level of the ROC value for the prediction of coronary heart disease events using the traditional risk factors [17]. Since the CAC score improves the risk prediction when added to the traditional risk factors, a marker useful to detect subclinical atherosclerosis (as VCANM in subjects belonging to the CT-plus Ca cohort) can be a breakthrough in term of preventive cardiology.
The mean value of VCANM was not higher in the two groups with manifest atherosclerotic disease, compared to asymptomatic individuals with CT signs of coronary atherosclerosis. Although it may be assumed that patients with ACS or IHD have larger plaque burden than CT-plus Ca individuals, it is important to point out that in the screening program we chose patients with the highest calcium scores and that we do not have measures for the combined plaque burden, which may also be of considerably size in some individuals in the screen-negative asymptomatic group.
Our hypothesis is that the amount of VCANM may not simply reflect the amount of plaque material, but could relate to the activity in atherosclerotic areas. VCANM is in fact a fragment of versican, a prominent component of restenotic and atherosclerotic lesions, and its elevated presence in circulation can indicate the presence of intense remodelling at the plaque level. The tissue specificity is increased by the combination of versican with MMP-12, which is a crucial protease for the initiation of the atherosclerotic lesion [18,19]. The MMP-12 generated versican fragment VCANM found in circulation is likely to be generated in the arteries subjected to high ECM remodelling that can lead to pathological outcomes. For this reason we believe VCANM can have a potential as prognostic marker reflecting the levels of extracellular matrix remodelling in atherosclerotic plaques, but this hypothesis needs to be verified in larger longitudinal studies. A limitation of this work is the possibility that VCANM can be generated in locations different than arteries, being versican and MMP-12 not exclusively expressed in blood vessels. To demonstrate that VCANM is generated in arteries we performed an immunohistochemical analysis of VCANM in human coronary artery sections. Our results showed that the VCANM neo-epitope was present at the edges of the necrotic core of the plaque in individuals with different degrees of atherosclerosis. These findings are consistent with previous findings in literature, where versican has been identified in both early and advanced atherosclerotic lesions, as well as in plaque thrombus interface [20-23]. In early developing lesions involving intimal thickening, versican has been primarily associated with arterial SMCs, especially in arteries more susceptible for atherosclerosis, such as coronary arteries [24]. In advanced stages of lesion development versican was most prominent at the edges of the necrotic core, in close proximity to deposited lipoproteins [21,25]. The immunohistological evidences suggest that VCANM was generated in vivo in the atherosclerotic plaque, supporting the hypothesis that the neo-epitope found in plasma of atherosclerotic heart disease affected individual could be originated in the atherosclerotic plaque and then released into circulation.
The generation of neo-epitopes by protease cleavage is a much more complex process in vivo than in vitro, involving different proteases in different moments of the pathological events, therefore it is not possible to exclude the possibility that in vivo proteolytical events can generate different pathological relevant versican fragments than those generated in vitro. Even if neo-epitope peptides have proven to be a central part of the disease pathogenesis in other diseases [26], further investigations are needed to understand how VCANM is generated in vivo and on how different proteases contribute to the generation of this neo-epitope and its subsequent release into the circulation.
Conclusions
In summary, we developed an assay measuring a MMP generated neo-epitope of versican, which was able to separate individuals with different degree of atherosclerosis from subjects with no coronary calcium deposit. These results were supported by the immunohistological evaluation of human coronary arteries, where VCANM was present in the atherosclerotic plaque. Our data suggest that the level of VCANM may reflect the activity of matrix remodelling in atherosclerotic plaques. Larger longitudinal clinical studies are needed to confirm the diagnostic and prognostic features of this novel biomarker.
Acknowledgments
This research was funded by the Danish “Ministry of Science, Technology and Science” and the Danish science foundation (“Den Danske Forskningsfond”).
The study from which human arterial specimens were retrieved was supported in part by grant HL14164 (SCOR in Arteriosclerosis) contract NOI HV53029 from the National Heart, Lung, and Blood Institute, and by contract NOI HD82827 from the National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Md, and from a grant from the Translational Science Institute of Wake Forest School of Medicine.
References
- 1.Lendon C, Born GV, Davies MJ, Richardson PD. Plaque fissure: the link between atherosclerosis and thrombosis. Nouv Rev Fr Hematol. 1992;34:27–29. [PubMed] [Google Scholar]
- 2.Katsuda S, Kaji T. Atherosclerosis and extracellular matrix. J Atheroscler Thromb. 2003;10:267–274. doi: 10.5551/jat.10.267. [DOI] [PubMed] [Google Scholar]
- 3.Moreno PR, Falk E, Palacios IF, Newell JB, Fuster V, Fallon JT. Macrophage infiltration in acute coronary syndromes. Implications for plaque rupture. Circulation. 1994;90:775–778. doi: 10.1161/01.cir.90.2.775. [DOI] [PubMed] [Google Scholar]
- 4.Arroyo LH, Lee RT. Mechanisms of plaque rupture: mechanical and biologic interactions. Cardiovasc Res. 1999;41:369–375. doi: 10.1016/s0008-6363(98)00308-3. [DOI] [PubMed] [Google Scholar]
- 5.Koenig W, Khuseyinova N. Biomarkers of atherosclerotic plaque instability and rupture. Arterioscler Thromb Vasc Biol. 2007;27:15–26. doi: 10.1161/01.ATV.0000251503.35795.4f. [DOI] [PubMed] [Google Scholar]
- 6.Johnson JL. Matrix metalloproteinases: influence on smooth muscle cells and atherosclerotic plaque stability. Expert Rev Cardiovasc Ther. 2007;5:265–282. doi: 10.1586/14779072.5.2.265. [DOI] [PubMed] [Google Scholar]
- 7.Asplund A, Friden V, Stillemark-Billton P, Camejo G, Bondjers G. Macrophages exposed to hypoxia secrete proteoglycans for which LDL has higher affinity. Atherosclerosis. 2011;215:77–81. doi: 10.1016/j.atherosclerosis.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 8.Wight TN, Merrilees MJ. Proteoglycans in atherosclerosis and restenosis: key roles for versican. Circ Res. 2004;94:1158–1167. doi: 10.1161/01.RES.0000126921.29919.51. [DOI] [PubMed] [Google Scholar]
- 9.Llorente-Cortes V, Otero-Vinas M, Hurt-Camejo E, Martinez-Gonzalez J, Badimon L. Human coronary smooth muscle cells internalize versican-modified LDL through LDL receptor-related protein and LDL receptors. Arterioscler Thromb Vasc Biol. 2002;22:387–393. doi: 10.1161/hq0302.105367. [DOI] [PubMed] [Google Scholar]
- 10.Karsdal MA, Henriksen K, Leeming DJ, Mitchell P, Duffin K, Barascuk N, Klickstein L, Aggarwal P, Nemirovskiy O, Byrjalsen I, Qvist P, Bay-Jensen AC, Dam EB, Madsen SH, Christiansen C. Biochemical markers and the FDA Critical Path: how biomarkers may contribute to the understanding of pathophysiology and provide unique and necessary tools for drug development. Biomarkers. 2009;14:181–202. doi: 10.1080/13547500902777608. [DOI] [PubMed] [Google Scholar]
- 11.Veidal SS, Karsdal MA, Nawrocki A, Larsen MR, Dai Y, Zheng Q, Hagglund P, Vainer B, Skjot-Arkil H, Leeming DJ. Assessment of proteolytic degradation of the basement membrane: a fragment of type IV collagen as a biochemical marker for liver fibrosis. Fibrogenesis Tissue Repair. 2011;4:22. doi: 10.1186/1755-1536-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Combet C, Blanchet C, Geourjon C, Deleage G. NPS@: network protein sequence analysis. Trends Biochem Sci. 2000;25:147–150. doi: 10.1016/s0968-0004(99)01540-6. [DOI] [PubMed] [Google Scholar]
- 13.Clarkson TB, Prichard RW, Morgan TM, Petrick GS, Klein KP. Remodeling of coronary arteries in human and nonhuman primates. JAMA. 1994;271:289–294. [PubMed] [Google Scholar]
- 14.Diederichsen AC, Sand NP, Norgaard B, Lambrechtsen J, Jensen JM, Munkholm H, Aziz A, Gerke O, Egstrup K, Larsen ML, Petersen H, Hoilund-Carlsen PF, Mickley H. Discrepancy between coronary artery calcium score and HeartScore in middle-aged Danes: the Dan-Risk study. Eur J Prev Cardiol. 2012;19:558–564. doi: 10.1177/1741826711409172. [DOI] [PubMed] [Google Scholar]
- 15.Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46:158–165. doi: 10.1016/j.jacc.2005.02.088. [DOI] [PubMed] [Google Scholar]
- 16.Dalager S, Paaske WP, Kristensen IB, Laurberg JM, Falk E. Artery-related differences in atherosclerosis expression: implications for atherogenesis and dynamics in intima-media thickness. Stroke. 2007;38:2698–2705. doi: 10.1161/STROKEAHA.107.486480. [DOI] [PubMed] [Google Scholar]
- 17.Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, Greenland P. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303:1610–1616. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamada S, Wang KY, Tanimoto A, Fan J, Shimajiri S, Kitajima S, Morimoto M, Tsutsui M, Watanabe T, Yasumoto K, Sasaguri Y. Matrix metalloproteinase 12 accelerates the initiation of atherosclerosis and stimulates the progression of fatty streaks to fibrous plaques in transgenic rabbits. Am J Pathol. 2008;172:1419–1429. doi: 10.2353/ajpath.2008.070604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson JL, Devel L, Czarny B, George SJ, Jackson CL, Rogakos V, Beau F, Yiotakis A, Newby AC, Dive V. A selective matrix metalloproteinase-12 inhibitor retards atherosclerotic plaque development in apolipoprotein E-knockout mice. Arterioscler Thromb Vasc Biol. 2011;31:528–535. doi: 10.1161/ATVBAHA.110.219147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin H, Wilson JE, Roberts CR, Horley KJ, Winters GL, Costanzo MR, McManus BM. Biglycan, decorin, and versican protein expression patterns in coronary arteriopathy of human cardiac allograft: distinctness as compared to native atherosclerosis. J Heart Lung Transplant. 1996;15:1233–1247. [PubMed] [Google Scholar]
- 21.Evanko SP, Raines EW, Ross R, Gold LI, Wight TN. Proteoglycan distribution in lesions of atherosclerosis depends on lesion severity, structural characteristics, and the proximity of platelet-derived growth factor and transforming growth factor-beta. Am J Pathol. 1998;152:533–546. [PMC free article] [PubMed] [Google Scholar]
- 22.Kolodgie FD, Burke AP, Farb A, Weber DK, Kutys R, Wight TN, Virmani R. Differential accumulation of proteoglycans and hyaluronan in culprit lesions: insights into plaque erosion. Arterioscler Thromb Vasc Biol. 2002;22:1642–1648. doi: 10.1161/01.atv.0000034021.92658.4c. [DOI] [PubMed] [Google Scholar]
- 23.Luttun A, Lupu F, Storkebaum E, Hoylaerts MF, Moons L, Crawley J, Bono F, Poole AR, Tipping P, Herbert JM, Collen D, Carmeliet P. Lack of plasminogen activator inhibitor-1 promotes growth and abnormal matrix remodeling of advanced atherosclerotic plaques in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2002;22:499–505. doi: 10.1161/hq0302.104529. [DOI] [PubMed] [Google Scholar]
- 24.Yao LY, Moody C, Schonherr E, Wight TN, Sandell LJ. Identification of the proteoglycan versican in aorta and smooth muscle cells by DNA sequence analysis, in situ hybridization and immunohistochemistry. Matrix Biol. 1994;14:213–225. doi: 10.1016/0945-053x(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 25.Halpert I, Sires UI, Roby JD, Potter-Perigo S, Wight TN, Shapiro SD, Welgus HG, Wickline SA, Parks WC. Matrilysin is expressed by lipid-laden macrophages at sites of potential rupture in atherosclerotic lesions and localizes to areas of versican deposition, a proteoglycan substrate for the enzyme. Proc Natl Acad Sci U S A. 1996;93:9748–9753. doi: 10.1073/pnas.93.18.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karsdal MA, Henriksen K, Leeming DJ, Woodworth T, Vassiliadis E, Bay-Jensen AC. Novel combinations of Post-Translational Modification (PTM) neo-epitopes provide tissue-specific biochemical markers--are they the cause or the consequence of the disease? Clin Biochem. 2010;43:793–804. doi: 10.1016/j.clinbiochem.2010.03.015. [DOI] [PubMed] [Google Scholar]


