Abstract
There are several reports showing that hyperleptinemia positively correlates with atherogenic process including promotion of platelet aggregation, thrombosis, and production of inflammatory cytokines. Thereby endothelial dysfunction takes place and underlies metabolic and vascular alterations that contribute to the development of both cardiovascular disease and type 2 diabetes. It has been also proposed that endothelial dysfunction may antecede the development of insulin resistance and diabetes. Endothelial cells participate in vasoregulation by the modulation of nitric oxide production and prostaglandin; in addition these cells are major vector in angiogenesis and recruitment of leukocytes and adhesion molecules. Nevertheless mechanisms underlying vascular dysfunction remain to be fully elucidated. This experiment in vitro revealed that the addition of leptin to cultivated endothelial cells elicited a significant molecular expression of both COX 2 and ICAM-1: in addition, the response showed a positive relationship with leptin concentration and the time of incubation. Thus, it may be suggested that leptin acts directly on the endothelium by activating its specific receptor which in turn initiates the molecular response related with the production of factors involved in the inflammatory response. Alterations on prostaglandins and recruiting molecules of adhesion are relevant stages of the endothelial damage.
Keywords: Endothelium, leptin, receptor Ob, ICAM-1, COX-2
Introduction
Leptin is a hormone expressed in a variety of tissues, mainly adipocytes, and it is a key molecule in the regulation of food intake and energy expenditure in the hypothalamus [1], however it is also involved in the regulation of inflammatory response and endothelial function [2-4]. Leptin itself is a member of the cytokine superfamily and resembles IL-6 modulates T cell in immune system. In addition leptin promotes angiogenesis by increasing vascular endothelial growth factor (VEGF) [5,6].
Endothelial dysfunction is thought to be a principal event in the development of atherosclerosis and predates vascular pathology by many years [7-9]. This is because endothelial dysfunction is associated with diminished anticoagulant properties as well as increased adhesion molecule expression and cytokine release and oxidative stress, all of which play roles in the development of atherosclerosis. Therefore, study of endothelial function is considered important for the detection of cardiovascular disease [10,11]. The aim of this study was to investigate the effect of leptin on the expression of inflammatory markers which are associated with endothelial dysfunction.
Material and methods
Animals
The present study utilized male Wistar rats of 3-month-old. Animals were maintained under controlled conditions (22°C, 12:12-h light-dark cycle) with water and food provided ad libitum. Body weights of male were of 250±20g.
Cell culture
After an acclimatization period, male rats following euthanasia, the thoracic aorta was excised from each animal were isolated endothelium from aorta of rat. The endothelial cells were maintained at 37°C in a humid 95% air/5% CO2 atmosphere, cells were cultured in Dulbecco’s modified eagle’s medium (DMEM-high glucose, phenol red-free), supplemented with penicillin (100u/ml), streptomycin (100μg/ml), glutamine (2mM), and 5% fetal bovine “calf” serum. After the formation of a confluent monolayer, the cells were incubated for 24 to 48h with different concentrations of leptin (10-10 or 10-8M).
The study was approved by the Ethics Committee of the IMSS. The present experimental protocol was approved and is in accordance with the U.S. National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Total RNA isolation for analysis of ICAM-1, COX-2 and leptin receptor expression
Total cellular RNA was isolated from rat endothelial cells using 1ml of TRIzol reagent in 1 x 106 cells (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Briefly, RNA was precipitated from the TRIzol solution after the addition of chloroform, followed by isopropyl alcohol, and then washed in 75% ethanol in diethyl pyrocarbonate-treated (DEPC) water. Ethanol was then removed, and RNA pellets were briefly air-dried before the addition of 20 μl of RNase-free water. Contaminant genomic DNA was removed by DNase treatment (DNA-free; Ambion, Austin, TX). Total RNA was eluted in DEPC-treated water (pH 7.5). Total RNA concentration and quality was determined spectrophotometrically at 260nm/280nm and isolated RNA was stored at -70°C.
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Purified total RNA (1μg) was used as a template to generate cDNA (Kit First Strand cDNA Synthesis, Fermentas Life Sciences), RT-PCR were performed using a kit from Invitrogen Life Technologies which was amplified with a specific primer for ICAM-1, COX-2, Ob-Rb receptor and actin. PCR mixture contained 0.5U per sample of Taq DNA polymerase and buffers (PCR amplification buffer 30mM MgCl), 10mM dNTP; 15mM each 5’ and 3’ primers were added to cDNA samples from ICAM-1. Forward primers used were 5’-AGGTATCCATCCATCCCACA-3’ and reverse primer 5’-CTTCAGAGGCAGGAAACAGG-3’ (product size 600 bp); COX-2 forward primers used were 5’-AACTCAAGTTCGACCCAG-3’ and reverse primer 5’-TTTCAACTCTGCAGCCAT-3’ (product size 404bp). From Ob-Rb receptor forward primers used were 5’-CCAGGTGAGGAGCAAGAGAC-3’ and reverse primer 5’-CTGCACAGTGCTTCCCACTA-3’ (product size 470bp). From b-actin, forward primer 5’-CCTGGGTATGGAATCCTGTG-3’ and reverse primer 5’-TTGTAAAGAAAGGGTGTAAA-3’ (product size 374bp) were used. Initial denaturation at 95°C for 5min was followed by 35 cycles of denaturation for 1min at 95°C, annealing for 1 min at 55°C and extension for 1min at 72°C, and the PCR was terminated by a final extension at 72°C for 5min using a thermocycler MasterCycler epGradient S (Eppendorf North America, Westbury, NY). Subsequent assay results were analyzed relative to a housekeeping gene (b-actin) within the same sample to normalize for possible variations in RNA quality and quantity and RT-PCR efficiency actin levels were analyzed independently and did not vary in any of the experimental groups (Table 1).
Table 1.
Endothelial genes determined using RT-PCR
| Gen | Sense primer | Antisense primer | RT-PCR products Amplified (bp) |
|---|---|---|---|
| Ob-Rb | CCAGGTGAGGAGCAAGAGAC | CTGCACAGTGCT TCCCACTA | 472 |
| ICAM-1 | AGGTATCCATCCATCCCACA | CTTCAGAGGCAGGAAACAGG | 600 |
| COX-2 | AACTCAAGTTCGACCCAG | TTTCAACTCTGCAGCCAT | 404 |
| b-actin | CCTGGGTATGGAATCCTGTG | TTGTAAAGAAAGGGTGTAAA | 374 |
Electrophoresis
The total RNA and expected PCR products were analyzed on 2% agarose gels containing ethidium bromide. Finally, the optical density (OD) of bands was measured using a Kodak Transilluminator Gel Logic 200 (Estman-Kodak, Rochester, USA). Data are show as a ratio of ICAM-1, COX-2 expression to b-actin.
Statistical analysis
Experimental results are reported as mean ± SD. were compared by an unpaired two-tailed Student t test analysis and two-way analysis of variance (ANOVA). Statistical analyses were performed using Prism Software (Graph Pad Prism 5 for Windows, San Diego, CA); p<0.05 was considered significant.
Results
Leptin stimulates expression Ob-Rb, ICAM-1 and COX-2
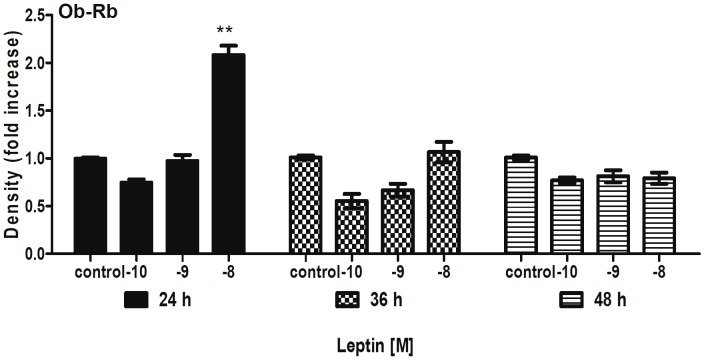
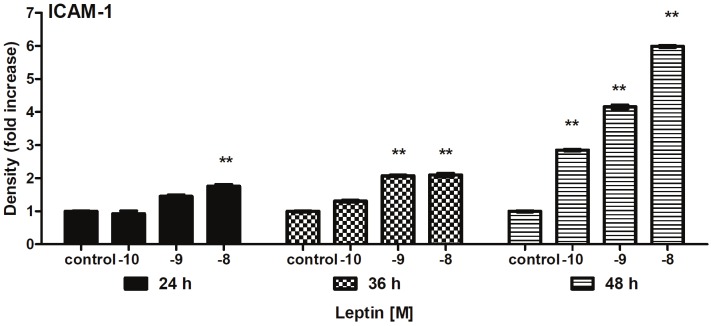
After the addition of leptin (10-10 or 10-8M) to endothelial cells; showed an increased expression of the receptor Ob-Rb was observed for 1-fold with 24h incubation compared with the control (C) group; p<0.001) (Figure 1). Likewise, ICAM-1 expression reached a maximal 5-fold increase by 48h incubation (p<0.001) (Figure 2).
Figure 1.
Changes in expression of Ob-Rb in cultured endothelium cells (ECs) were incubated in the absence and presence of leptin (10-8 a 10-10 M) for 24 to 48h. Values represent means ± SD for 4 experiments. **P<0.01 difference between C and leptin group.
Figure 2.
Changes in expression of ICAM-1 in cultured endothelium cells were incubated in the absence and presence of leptin (10-8 a 10-10M) for 36 to 48h. Values represent means ± SD for 4 experiments. **P<0.01 difference between C and leptin group.
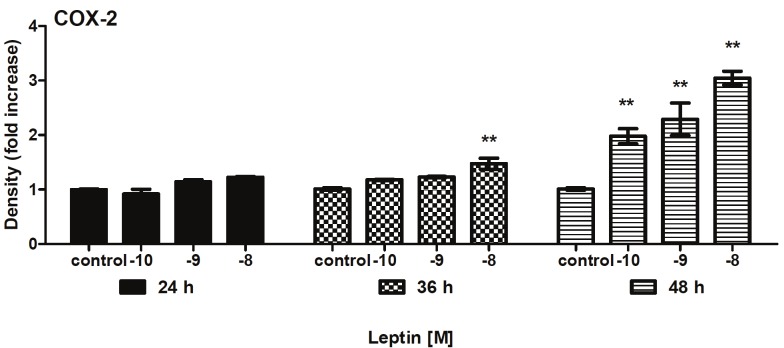
Leptin also stimulated COX-2 expression was relatively higher in the 48h with a significant increase of 3-fold compared to the C group (p<0.01) (Figure 3).
Figure 3.
Changes in expression of COX-2 in cultured endothelium cells were incubated in the absence and presence of leptin (10-8 a 10-10M) for 36 to 48h. Values represent means ± SD for 4 experiments. **P<0.01 difference between C and leptin group.
The dose response for leptin-stimulated ICAM-1 and COX-2 expression showed that a maximal effect was observed at 10-8M concentration and 48hr incubation in aort from rat. In summary these demonstrated that leptin exerts a direct effect on endothelium inducing ICAM-1 and COX-2 expression. The result confirm the relevance of leptin-stimulates ICAM-1 and COX-2 activity for leptin angiogenesis.
Discussion
It is well known that endothelial cells exert critical functions in the maintenance of vascular homeostasis; therefore any perturbation of these cells may be initiating the expression of proinflammatory adhesion molecules that may be associated with atherosclerosis and cardiovascular disease. It has been demonstrated that adipose tissue secretes bioactive substances that positively or negatively modulate endothelial function; thus leptin may induce monocyte adhesions and expression of vascular cell adhesion molecule which causes significant endothelial dysfunction [12-14].
There are several reports showing that hyperleptinemia positively correlates with atherogenic process including promotion of platelet aggregation, thrombosis, and production of inflammatory cytokines, e.g., TNF alpha, IL 6 [15,16]. Thereby endothelial dysfunction takes place and underlies metabolic and vascular alterations that contribute to the development of both cardiovascular disease and type 2 diabetes [17,18]. It has been also proposed that endothelial dysfunction may antecede the development of insulin resistance and diabetes. Endothelial cells participate in vasoregulation by the modulation of nitric oxide production and prostaglandin; in addition these cells are major vector in angiogenesis and recruitment of leukocytes and adhesion molecules. Nevertheless mechanisms underlying vascular dysfunction remain to be fully elucidated.
This experiment in vitro revealed that the addition of leptin to cultivated endothelial cells elicited a significant molecular expression of both COX 2 and ICAM-1: in addition, the response showed a positive relationship with leptin concentration and the time of incubation. Thus, it may be suggested that leptin acts directly on the endothelium by activating its specific receptor which in turn initiates the molecular response related with the production of factors involved in the inflammatory response. Alterations on prostaglandins and recruiting molecules of adhesion are relevant stages of the endothelial damage.
Acknowledgments
We wish to acknowledge financial support of FIS/IMSS 991.
Disclosure statement
The authors have nothing to declare.
References
- 1.Zhang Y, Proenca R, Maffei M, Barone L, Leopold L, Friedman JM. Positional cloning of the mouse ob gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Boulomie A, Drexler HC, Lafontan M, Busse R. Leptin, the product of Ob gene promotes angiogenesis. Circ Res. 1998;83:1059–1066. doi: 10.1161/01.res.83.10.1059. [DOI] [PubMed] [Google Scholar]
- 3.Park HY, Kwon HM, Lim HJ, Hong BK, Lee JY, Park BE, Jang Y, Cho SY, Kim HS. Potential role of leptin in angiogenesis: leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro. Exp Mol Med. 2001;33:95–102. doi: 10.1038/emm.2001.17. [DOI] [PubMed] [Google Scholar]
- 4.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 5.Oda A, Taniguchi T, Yokoyama M. Leptin stimulates rat aortic smooth muscle cell proliferation and migration. Kobe J Med Sci. 2001;47:141–150. [PubMed] [Google Scholar]
- 6.Park HY, Kwon HM, Lim HJ, Hong BK, Lee JY, Park BE, Jang Y, Cho SY, Kim HS. Potential role of leptin in angiogenesis: leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro. Exp Mol Med. 2001;33:95–102. doi: 10.1038/emm.2001.17. [DOI] [PubMed] [Google Scholar]
- 7.Libby P, Ricker PM, Masei A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 8.Fernández-Riejos P, Najib S, Santos-Álvarez J, Marín-Romero C, González-Yanes C, Sanchez-Margalet V. Role of leptin in the activation of immune cells. Mediators Inflamm. 2010;2010:568343. doi: 10.1155/2010/568343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Osika W, Dangardt F, Gan LM, Strandvik B, Friberg P. High levels of soluble intercellular adhesion molecule-1, insulin resistance and saturated fatty acids are associated with endothelial dysfunction in healthy adolescents. Atherosclerosis. 2010;211:638–42. doi: 10.1016/j.atherosclerosis.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein BJ, Scalia R. Adiponectin: A Novel Adipokine Linking Adipocytes and Vascular Function. J Clin Endocrinol Metab. 2004;89:2563–2568. doi: 10.1210/jc.2004-0518. [DOI] [PubMed] [Google Scholar]
- 11.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction: implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dick G, Tune J. Leptin receptors are expressed in coronary arteries and hyperleptinemia causes significant coronary endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2005;289:H48–H56. doi: 10.1152/ajpheart.01159.2004. [DOI] [PubMed] [Google Scholar]
- 13.Santos-Alvarez J, Goberna R, Sánchez-Margalet V. Human leptin stimulates proliferation and activation of human circulating monocytes. Cellular Immunology. 1999;194:6–11. doi: 10.1006/cimm.1999.1490. [DOI] [PubMed] [Google Scholar]
- 14.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction: implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zerani M, Boiti C, Dall’Aglio C, Pascucci L, Maranesi M, Brecchia G, Mariottini C, Guelfi G, Zampini D, Gobbetti A. Leptin receptor expression and in vitro leptin actions on prostaglandin release and nitric oxide synthase activity in the rabbit oviduct. J Endocrinol. 2005;185:319–25. doi: 10.1677/joe.1.05983. [DOI] [PubMed] [Google Scholar]
- 16.Knudson JD, Dincer UD, Zhang C, Swafford AN, Ryoji Koshida J, Picchi A, Focardi M, Dick G, Tune J. Leptin receptors are expressed in coronary arteries and hyperleptinemia causes significant coronary endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2005;289:H48–H56. doi: 10.1152/ajpheart.01159.2004. [DOI] [PubMed] [Google Scholar]
- 17.Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, Ferraro A, Chello M, Mastroroberto P, Verdecchia P, Schillaci G. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001;104:191–196. doi: 10.1161/01.cir.104.2.191. [DOI] [PubMed] [Google Scholar]
- 18.Zheng J, Fang J, Yin Y, Wang J. Leptin protects cardiomyocytes from serum-deprivation-induced apoptosis by increasing anti-oxidant defence. Clin Exp Pharmacol Physiol. 2010;37:955–962. doi: 10.1111/j.1440-1681.2010.05415.x. [DOI] [PubMed] [Google Scholar]