Abstract
Lymphoepithelial carcinoma (LEC), also called lymphoepithelioma-like carcinoma, is defined as an undifferentiated carcinoma or poorly differentiated squamous cell carcinoma, accompanied by a prominent reactive lymphoplasmacytic infiltrate. LEC can occur in many organs, but is most common in head and neck regions including pharynx. LEC may be associated with Epstein-Barr virus (EBV) infection. LEC of the esophagus is extremely rare; only nine cases have been reported. A 79-year-old man presented epigastralgia and dysphagia. A blood laboratory test showed no significant findings. He was a hepatitis C virus healthy carrier. Tumor markers of CEA and SCC were normal. Upper gastrointestinal endoscopy showed a tumor in the lower esophagus. Biopsies were taken, and they identified malignant epithelioid cells and heavy infiltration of mature lymphocytes. The epithelioid cells showed large size, nuclear atypia, mitotic figure, hyperchromasia, and increased nucleo-cytoplasmic ratio. The lymphocytes were free from atypia. Immunohistochemically, the epithelioid cells were positive for cytokeratin (CK) AE1/3, CK CAM5.2, CK WSS, CK MNF16, CK KL1, CK5/6, CK7, CK8, CK14, CK18, CK19, p53, and Ki-67 (labeling=27%). They were negative for CK34BE12, CK20, p63, CEA, CA19-9, NSE, synaptophysin, CD56, chromogranin, KIT (CD117), desmin, vimentin, MUC apomucins, and several leukocytic markers. The epithelioid cells were positive for EBV associated molecules including EBV-encoded nuclear antigen2 (EBNA2), EBV latent membrane protein-1 (LMP-1), and EBV early RNAs (EBER). The lymphocytes were positive for CD45 and vimentin, and were composed of B-cells positive for CD20, CD79α, bcl-2, and CD10, T-cells positive for CD3 and CD45RO, NK-cells positive for CD56, and plasma cells positive for CD38, CD138, CD79α, κ-chain, and λ-chain. No light chain restriction was seen. Most of the lymphocytes were B and T-cells, and NK-cells and plasma cells were very scant. The lymphoplasma cells were reactive cells, because of no atypia and also because no p53 and very low Ki-67 labeling (3%). The lymphocytes were negative for CD21 and other antigens such as CKs and EMA. The pathological diagnosis was primary LEC of the esophagus. Imaging techniques revealed lymph nodes metastasis of the perigastric and periaortic regions, but identified no other tumors in the body. The patient was inoperative, and was treated by chemoradiation. The esophageal LEC and lymph nodes metastases were markedly reduced in size.
Keywords: Esophagus, lymphoepithelial carcinoma, immunohistochemistry, Epstein-Barr virus
Introduction
Lymphoepithelial carcinoma (LEC), previously called lymphoepithelioma-like carcinoma, is defined as an undifferentiated carcinoma or poorly differentiated squamous cell carcinoma, accompanied by a prominent non-neoplastic reactive lymphoplasmacytic infiltrate [1-4] The morphological features are indistinguishable from those examples of nasopharyngeal non-keratinizing carcinoma with a rich lymphoplasmacytic infiltrates [1-5]. Nasopharyngeal carcinoma is defined as a carcinoma arising in the nasopharyngeal mucosa that shows light microscopic or ultrastructural evidence of squamous differentiation [5]. It encompasses squamous cell carcinoma non-keratinizing carcinoma (differentiated or undifferentiated) and basaloid squamous cell carcinoma. Adenocarcinoma and salivary gland-type carcinoma are excluded [5]. However, most of the nasopharyngeal carcinoma shows morphological features of LEC [5], and it seems that LEC occurring in the nasopharyngeal regions is called nasopharyngeal carcinoma.
LEC and nasopharyngeal carcinoma most commonly develop in the head and neck regions including nasopharynx, paranasal sinus, oral cavity, larynx, and salivary glands. LEC can also occur in many organs such as lung [6]. Stomach [7], skin [8], breast [9,10], bile ducts [11], esophagus [12-20], and other many organs. Primary LEC of the esophagus is extremely rare; only nine cases have been reported in the world literature [12-20].
LEC and nasopharyngeal carcinoma may be associated with Epstein-Barr virus (EBV), although there are many controversies [21-25]. The association between LEC and EBV seems to be different in geographic areas, races, affected organs [1-25]. In general, association of LEC with EBV is strong in head and neck LEC, and relatively weak in LEC of other sites. Further, the association is strong in east Asia, and relatively weak in western countries.
Comprehensive immunohistochemical studies of LEC have rarely been performed. Immunoprofile of esophageal LEC have not been investigated [12-20].
The author herein reports a case of primary LEC of the esophagus with extensive immunohistochemical study and investigation of EBV.
Case report
A 79-year-old man presented epigastralgia and dysphagia. A blood laboratory test showed mild anemia (426 x 104 /μl, normal 450-550), elevated blood urea nitrogen (BUN) (23 mg/dl, normal 8-20), and elevated creatinine (1.31 mg/dl, normal 0.4-1.2). He was a hepatitis C virus healthy carrier. The serum liver enzymes such as ALT and AST and ductal enzymes such as alkaline phosphatase and LDH were within normal limits. The tumor markers were within normal ranges (CEA, 2.0 ng/ml normal 0-5.0: SCC, 0.6 ng/ml normal 0-1.5). Upper gastrointestinal endoscopy was performed because the symptoms are related to gastrointestinal tract. The endoscopy showed a projected type 1 tumor measuring 3 x 3 x 2 cm in the lower esophagus (Figure 1). The endoscopic features were somewhat similar to those of the submucosal tumor (Figure 1).
Figure 1.

Endoscopy of the esophagus. A type I elevated tumor measuring 3 x 3 x 2 cm is seen in the lower esophagus.
Biopsies were taken, and they identified malignant epithelioid cells and heavy infiltration of mature lymphocytes (Figure 2A-D). The atypical epithelioid cells and lymphocytes were mainly located in the subepithelial areas (Figure 1A). The epithelioid cells showed large size, nuclear atypia, mitotic figure, hyperchromasia, and increased nucleo-cytoplasmic ratio (Figure 2B-D). Some epithelioid cells had clear cytoplasm (Figure 2C and 2D). The lymphocytes were completely free from atypia (Figure 2B-D).
Figure 2.

Histological features of the tumor. A: Low power view. The tumor is largely located under the squamous epithelium of the esophagus. The presence of lymphoid tissue and scattered atypical cells are vaguely seen. HE; x40. B: Medium power view of the esophageal tumor. The tumor is composed of mature lymphocytes and atypical cells. In this area and magnification, the characteristics of atypical cells are unclear. Some atypical cells have clear cytoplasm. The atypical cells are epithelioid and also lymphocytoid. Distinction between carcinoma and lymphoma is unclear. HE; x200. C, D: High power view. The atypical cells show epithelioid features. No differentiation is seen. They show hyperchromatic nuclei, increased nucleocytoplasmic ratio, distinct nucleoli, mitosis, and apoptosis. Some atypical cells have clear cytoplasm. In contrast, the lymphocytes are mature, and free from atypia. HE; x400.
Periodic acid Schiff (PAS), Alcian blue (AB) at pH2.5, mucicarmine, and combined periodic acid-Schiff (d-PAS) reaction with diastase digestion and AB at pH2.5 (d-PAS/AB) were performed to detect glycogen and mucins. An immunohistochemical study was performed with the use of Dako Envision method (Dako Corp, Glostrup, Denmark), as previously described [26-40].
The histochemical stains revealed a small amount of glycogen in the tumor epithelioid cells. PAS showed a small amount of glycogen in the cytoplasm of tumor epithelioid cells. Mucicarmine, d-PAS, AB at pH2.5, d-PAS/AB showed no mucins in the tumor cells.
Immunohistochemically, the malignant epithelioid cells were positive for cytokeratin (CK) AE1/3 (Figure 3A), CK CAM5.2, CK WSS, CK MNF16, CK KL1, CK5/6, CK7 (Figure 3B), CK8 (Figure 3C), CK14, CK18 (Figure 3D), CK19, p53 (Figure 3E), and Ki-67 (labeling=27%) (Figure 3F). They were negative for CK34BE12, CK20, p63, CEA, CA19-9, NSE, synaptophysin, CD56, chromogranin, KIT (CD117), desmin, vimentin, MUC1, MUC2, MUC5AC, MUC6, CD45, CD20, CD79α, CD3, CD45 RO, CD10, bcl-2, CD38, CD138, κ-chain, and λ-chain.
Figure 3.
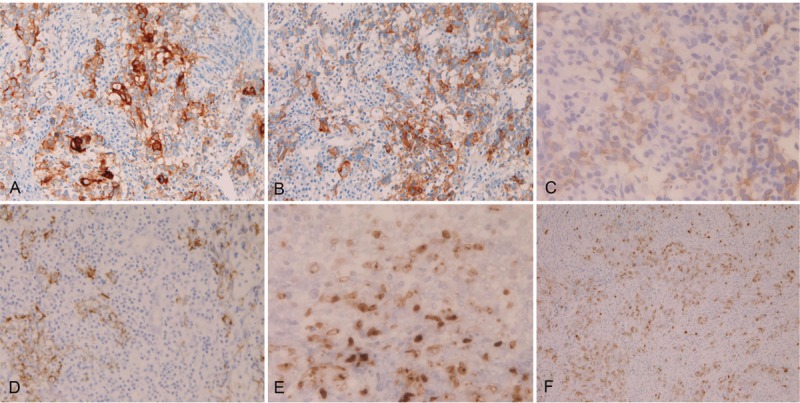
Immunohistochemical findings of the primary esophageal lymphoepithelial carcinoma. The epithelioid atypical cells are positive for CK AE1/3 (A), CK7 (B), CK8 (C), CK18 (D), p53 (E), and Ki-67 (labeling=27%) (F). Immunostaining. A-D: x200. E; x400. F; x100.
The malignant epithelioid cells were positive for EBV associated molecules including EBV-encoded nuclear antigen2 (EBNA2), EBV latent membrane protein-1 (LMP-1) (Figure 4A), and EBV early RNAs (EBER) (Figure 4B).
Figure 4.
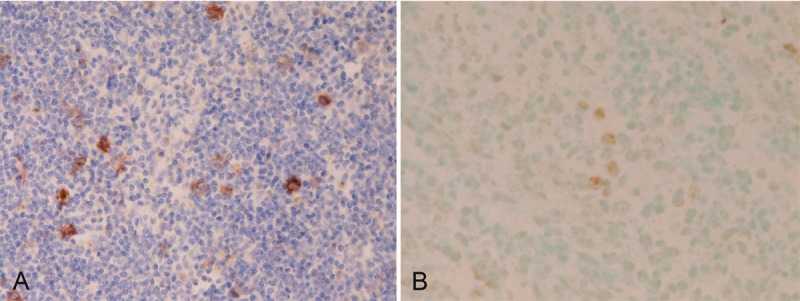
Expression of Epstein-Barr virus (EBV) associated molecules in esophageal lymphoepithelial carcinoma. The malignant epithelioid cells are positive for EBV latent membrane protein-1(LMP-1) (A) and EBV early RNAs (EBER) (B, center and right). Immunostaining and in situ hybridization; x200.
The lymphocytes were positive for CD45 (Figure 5A) and vimentin, and were composed of B-cells positive for CD20 (Figure 5B), CD79α, bcl-2, and CD10, T-cells positive for CD3 (Figure 5C) and CD45RO, NK-cells positive for CD56 (Figure 5D), and plasma cells positive for CD38, CD138 (Figure 5E), CD79α, κ-chain, and λ-chain. No light chain restriction was seen. Most of the lymphocytes were B and T-cells, and NK-cells and plasma cells were very scant. The lymphoplasma cells were reactive cells, because of no atypia and also because no p53 and very low Ki-67 labeling (3%) (Figure 3F). The lymphocytes were negative for CD21, and were also negative for other antigens (CK AE1/3, CK CAM5.2, CK WSS, CK MNF16, CK KL1, CK34BE12, CK5/6, CK7, CK8, CK14, CK18, CK19, CK20, EMA, p63, CEA, CA19-9, NSE, synaptophysin, CD56, chromogranin, KIT (CD117), desmin, p53, MUC1, MUC2, MUC5AC and MUC6).
Figure 5.

Immunohistochemical findings of mature lymphoplasmacytic cells in esophageal lymphoepithelioma. The mature lymphocytes are positive for CD45 (A), CD20 (B), CD3 (C), CD56 (D), and CD138 (E). Expression of CD45, CD20 and CD3 are broad, while expression of CD56 and CD138 is only focal. Immunostaining, x200.
The pathological diagnosis of primary LEC of the esophagus associated with EBV was made by the author. Imaging techniques revealed lymph nodes metastasis of the perigastric and periaortic regions, but identified no other tumors in the body. The patient was inoperative, and was treated by chemoradiation. The esophageal LEC and lymph nodes metastases were markedly reduced in size.
Discussion
In the present case, tumor formations were seen only in the esophagus and several lymph nodes. The largest is the esophageal tumor. In addition, the esophageal tumor took the shape of primary esophageal malignancy. Thus, the present case is a primary esophageal tumor.
Histologically, the tumor was composed of malignant epithelioid cells and mature lymphoid cells. The epithelioid cells showed cellular atypia including hyperchromatic nuclei, increased nucleus-cytoplasmic ratio, mitotic figures, and prominent nucleoli. These features are highly suggestive of malignant nature. In addition, the epithelioid cells showed prominent p53 expression and high Ki-67 labeling index (27%). Taken together, the epithelioid cells are definitely malignant cells. The malignant epithelioid showed immunoreactive epithelial markers such as CKs. From these overall findings, it is concluded that the epithelioid cells are carcinoma cells. The carcinoma cells did not show any differentiations histologically. Therefore, the carcinoma cells are undifferentiated carcinoma cells. No differentiations into squamous cell carcinoma or adenocarcinoma were seen. Taken together, it is concluded that the carcinoma cells are undifferentiated carcinoma cells. The lymphoid cells consisted of mature lymphoplasmacyte, and showed no atypia. Immunohistochemically, the lymphocytes were composed of various types of lymphocytes, and showed no p53 expression and low Ki-67 labeling index (3%). From these overall examination, it can be concluded that the lymphocytes are not tumor cells but are reactive non-neoplastic lymphocytes. LEC is defined as an undifferentiated carcinoma or poorly differentiated squamous cell carcinoma, accompanied by a prominent non-neoplastic reactive lymphoplasmacytic infiltrate [1-4]. The present case fulfills the criteria of LEC. Thus, the present primary esophageal tumor is primary LEC of the esophagus.
The cell origin of LEC is unclear [1-25]. In the present case, the endoscopic features resembled submucosal tumor. In addition, the biopsies showed that LEC cells were largely located in the subepithelial areas. The LEC in the present case may be derived from the surface squamous epithelia, but the current these finding may also suggest that the current LEC had arisen from the esophageal glands. More studies are required to elucidate histogenesis and pathogenesis of LEC of various organs.
In the present case, several mucin stains were performed, because the carcinoma cells occasionally had clear cytoplasm. It was found that there were no mucins in the tumor cells in histochemical staining of mucicarmine, AB at pH2.5, d-PAS, and d-PAS/AB. PAS showed glycogen in the cytoplasm of tumor epithelioid cells. Therefore, the clearness of the cytoplasm of the some tumor cells is thought to be due to glycogen. These findings also indicate that the tumor cells are not poorly cohesive signet-ring cell carcinoma-like cells seen in various organs [26-36].
There have been no comprehensive immunohistochemical studies of LEC of various locations, to the best of the author’s knowledge. The present case employed an immunohistochemical study using many antibodies. The cytokeratin profile of the epithelioid cells of the present case was CK AE1/3 +, CK CAM5.2 +, CK WSS +, CK MNF16 +, CK KL1 +, CK5/6 +, CK7 +, CK8 +, CK14 +, CK18 +, CK19 +, CK34BE12 -, and CK20 -. It was shown that the CK profiles of various carcinomas is not limited, but shows diverse different patterns of CK in a given tumor [26-36]. The CK7+/CK20- pattern in the current case is compatible with esophageal primary [41]. The positive CKs in the epithelioid cells of the current LEC also definitely conclude that the epithelioid cells of the current tumor are epithelial cells. In addition, p53 was positive and Ki-67 showed a high labeling index (27%) in the epithelioid cells, suggesting that the epithelioid cells are carcinoma cells. The negative immunoreactions of CEA, CA19-9, MUC1, MUC2, MUC5AC and MUC6, all of which are adenocarcinoma-related molecules, in the epithelioid cells suggest that the epithelioid cells shows no adenocarcinomatous differentiation. The negative reactions of high molecular weight CK34BE12 and p63, both of which are molecules of squamous cell epithelium, in the epithelioid cells of the present NEC suggest that the epithelioid carcinoma cells of the present LEC shows no squamous differentiation. The negative reactions of NSE, synaptophysin, CD56, chromogranin, KIT, desmin, vimentin in the epithelioid cells shows that the current tumor cells shows no endocrine differentiation. Thus, the present tumor is not neuroendocrine carcinoma. The negative desmin and vimentin in the epithelioid cells indicate that the carcinoma cells of the present LEC show no mesenchymal and muscular differentiation. The negative reactions of CD45, CD20, CD79α, CD3, CD45 RO, CD10, bcl-2, CD38, CD138, κ-chain and λ-chain definitely indicate that the tumor is not malignant lymphoma including anaplastic large cell lymphoma, which is histologically very similar to undifferentiated carcinoma.
It is thought that the lymphoplasmacytes in LEC and nasopharyngeal carcinoma are of reactive and non-neoplastic characteristics. However, there have been no comprehensive immunohistochemical studies have been performed in the lymphocytes element of LEC. In the current case, it was found that the lymphocytes of LEC were composed of various lymphocytes subpopulations; B-cells positive for CD20, CD79α, bcl-2, and CD10, T-cells positive for CD3 and CD45RO, NK-cells positive for CD56, and plasma cells positive for CD38, CD138, CD79α, κ-chain, and λ-chain. Most of the lymphocytes in the current LEC were B and T-cells, and NK-cells and plasma cells were very scant. The lymphoplasma cells were reactive cells, because of no atypia and also because no p53 and very low Ki-67 labeling (3%). The lymphocytes were negative for CD21, and were also negative for other various antigens examined. The heterogeneity of lymphocytes, absence of histological atypia, absent p53, very low Ki-67 labeling (3%), and no light chain restriction in the lymphoid cells element in the present LEC certainly demonstrate that the lymphoid element of LEC is a reactive and non-neoplastic lymphoid component. It is concluded that these lymphocytes in LEC are not tumor cells of LEC but may be lymphocytes accumulating around the carcinoma cells, in which the lymphocytes may functions as mediator cells of tumor immunology.
LEC of various organs may be associated with EBV [1-11,21-25]. However, there are many controversies about the association. Some authors showed positive association [1-5,7,20,22,24,25], while other authors negative association [8-11,21,23]. In the gastrointestinal tract, association between LEC and EBV have been reported to be positive in the stomach [7,20], esophagus [14,20], and intrahepatic bile ducts [11]. Recently association of LEC and human papilloma virus (HPV) has been described [10]. The association status between LEC and EBV is different among organs involved, geographical locations, and races [1-11,21-25]. This association is high in LEC of head and neck including nasopharyngeal carcinoma [1-5], but it is relatively weak in LEC of other organs [1-11,21-25]. The association of LEC and HBV is prevalent in East Asia [1-5]. The association is strong in Mongoloids but not strong in Caucasians [1-5]. Much more studies of this association remain to be elucidated.
The LEC of the esophagus is extremely rare; only nine cases have been reported in the world literature [12-20]. All are case reports [12-20]. The present case is the tenth case of primary LEC in the esophagus in the world. There have been no comprehensive immunohistochemical studies of primary esophageal LEC. The present study performed a relatively extensive immunohistochemical study using a large battery of antibodies. This is the first case of immunohistochemical findings in esophageal LEC. With regard to the association of esophageal LEC and EBV, there are two important case reports of esophageal LEC. The association was found in the reports of Chen et al [14] and Mori et al [20]. The present report is the third case with positive association between esophageal LEC and EBV. Of particular importance is that all the three cases that investigated EBV status in esophageal LEC detected the association of LEC and EBV. Thus the association is 100%, though the number is very small. It can be concluded that esophageal LEC is frequently related to EBV infection.
In summary, the author reported a case of EBV associated primary esophageal LEC. This case is very rare, and is the tenth report of primary esophageal LEC in the world literature. An extensive histochemical and immunohistochemical studies were performed.
Conflict of interest statement
The author has no conflict of interest.
References
- 1.Tsang WYW, Chan JKC. Lymphoepithelial carcinoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization Classification of Tumours. Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. p. 18. [Google Scholar]
- 2.Tsang WYW, Chan JKC. Lymphoepithelial carcinoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization Classification of Tumours. Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. p. 132. [Google Scholar]
- 3.Tsang WYW, Chan JKC, Westra W. Lymphoepithelial carcinoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization Classification of Tumours. Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. p. 176. [Google Scholar]
- 4.Tsang WYW, Kuo TT, Chan JKC. Lymphoepithelial carcinoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization Classification of Tumours. Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. pp. 251–252. [Google Scholar]
- 5.Chan JKC, Pilch BZ, Bray F, Wenig BM, McCarron P, Huang D, Foo W, Lo KW, Lee AWM, Zeng YX, Yip T, Jia WH, Kuo TT. Nasopharyngeal carcinoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization Classification of Tumours. Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. pp. 85–97. [Google Scholar]
- 6.Chang YL, Wu CT, Shih JY, Lee YC. New aspects in clinicopathologic and oncogene studies of 23 pulmonary lymphoepithelioma-like carcinomas. Am J Surg Pathol. 2002;26:715–723. doi: 10.1097/00000478-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Herrera-Goepfert R, Reyes E, Hernández-Avila M, Mohar A, Shinkura R, Fujiyama C, Akiba S, Eizuru Y, Harada Y, Tokunaga M. Epstein-Barr virus-associated gastric carcinoma in Mexico: analysis of 135 consecutive gastrectomies in two hospitals. Mod Pathol. 1999;12:873–878. [PubMed] [Google Scholar]
- 8.Ferlicot S, Plantier F, Rethers L, Bui AD, Wechsler J. Lymphoepithelioma-like carcinoma of the skin: a report of 3 Epstein-Barr virus (EBV)-negative additional cases. Immunohistochemical study of the stroma reaction. J Cutan Pathol. 2000;27:306–311. doi: 10.1034/j.1600-0560.2000.027006306.x. [DOI] [PubMed] [Google Scholar]
- 9.Dadmanesh F, Peterse JL, Sapino A, Fonelli A, Eusebi V. Lymphoepithelioma-like carcinoma of the breast: lack of evidence of Epstein-Barr virus infection. Histopathology. 2001;38:54–61. doi: 10.1046/j.1365-2559.2001.01055.x. [DOI] [PubMed] [Google Scholar]
- 10.Kulka J, Kovalszky I, Svastics E, Berta M, Fule T. Lymphoepithelioma-like carcinoma of the breast: not Epstein-Barr virus-, but human papilloma virus-positive. Hum Pathol. 2008;39:298–301. doi: 10.1016/j.humpath.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Szekely E. Lymphoepithelioma-like cholangiocarcinoma (LELC) not associated with Epstein-Barr virus. Am J Surg Pathol. 2001;25:1464–1466. doi: 10.1097/00000478-200111000-00023. [DOI] [PubMed] [Google Scholar]
- 12.Nakasono M, Hirokawa M, Suzuki M, Takizawa H, Okitsu H, Okamura S, Muguruma N, Ito S, Sano T. Lymphoepithelioma-like carcinoma of the esophagus: report of a case with non-progressive behavior. J Gastroenterol Hepatol. 2007;22:2344–2347. doi: 10.1111/j.1440-1746.2006.03445.x. [DOI] [PubMed] [Google Scholar]
- 13.Angulo-Pernett F, Smythe WR. Primary lymphoepithelioma of the esophagus. Ann Thorac Surg. 2003;76:603–605. doi: 10.1016/s0003-4975(03)00156-5. [DOI] [PubMed] [Google Scholar]
- 14.Chen PC, Pan CC, Hsu WH, Ka HJ, Yang AH. Epstein-Barr virus-associated lymphoepithelioma-like carcinoma of the esophagus. Hum Pathol. 2003;34:407–411. doi: 10.1053/hupa.2003.71. [DOI] [PubMed] [Google Scholar]
- 15.Takubo K, Lambie NK. Barrett’s adenocarcinoma of the esophagus with lymphoid stroma. J Clin Gastroenterol. 2001;33:141–144. doi: 10.1097/00004836-200108000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Squillaci S, Martignoni G, Chiodera PL, Vago L, Polonioli S, Capitanio A. Lymphoepitheliomalike carcinoma of the esophagus: description of a case. Pathologica. 2001;93:221–225. (In Italian) [PubMed] [Google Scholar]
- 17.Parra P, Aguilar J, López-Garrido J, Meléndez B, Merino E, Martinez , Gordillo E, Roldán JP. Primary esophageal lymphoepithelioma. Tumori. 1999;85:519–522. doi: 10.1177/030089169908500619. [DOI] [PubMed] [Google Scholar]
- 18.Yamada T, Tatsuzawa Y, Yagi S, Fujioka S, Kitagawa S, Nakagawa M, Minato H, Kurumaya H, Matsunou H. Lymphoepithelioma-like esophageal carcinoma: report of a case. Surg Today. 1999;29:542–544. doi: 10.1007/BF02482349. [DOI] [PubMed] [Google Scholar]
- 19.Sashiyama H, Nozawa A, Kimura M, Nomura E, Tamaru JI, Ninomiya E, Koide Y, Iino M, Ozawa K. Case report: A case of lymphoepithelioma-like carcinoma of the oesophagus and review of the literature. J Gastroenterol Hepatol. 1999;14:534–9. doi: 10.1046/j.1440-1746.1999.01911.x. [DOI] [PubMed] [Google Scholar]
- 20.Mori M, Watanabe M, Tanaka S, Mimori K, Kuwano H, Sugimachi K. Epstein-Barr virus-associated carcinomas of the esophagus and stomach. Arch Pathol Lab Med. 1994;118:998–1001. [PubMed] [Google Scholar]
- 21.Cerilli LA, Holst VA, Brandwein MS, Stoler MH, Mills SE. Sinonasal undifferentiated carcinoma: immunohistochemical profile and lack of EBV association. Am J Surg Pathol. 2001;25:156–163. doi: 10.1097/00000478-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Kuo T, Hsueh C. Lymphoepithelioma-like salivary gland carcinoma in Taiwan: a clinicopathological study of nine cases demonstrating a strong association with Epstein-Barr virus. Histopathology. 1997;31:75–82. doi: 10.1046/j.1365-2559.1997.5830814.x. [DOI] [PubMed] [Google Scholar]
- 23.Tardío JC, Cristóbal E, Burgos F, Menárguez J. Absence of EBV genome in lymphoepithelioma-like carcinomas of the larynx. Histopathology. 1997;30:126–128. doi: 10.1046/j.1365-2559.1997.d01-582.x. [DOI] [PubMed] [Google Scholar]
- 24.Tsai CC, Chen CL, Hsu HC. Expression of Epstein-Barr virus in carcinomas of major salivary glands: a strong association with lymphoepithelioma-like carcinoma. Hum Pathol. 1996;27:258–62. doi: 10.1016/s0046-8177(96)90066-0. [DOI] [PubMed] [Google Scholar]
- 25.Weiss LM, Movahed LA, Butler AE, Swanson SA, Frierson HF, Jr, Cooper PH, Colby TV, Mills SE. Analysis of lymphoepithelioma and lymphoepithelioma-like carcinomas for Epstein-Barr viral genomes by in situ hybridization. Am J Surg Pathol. 1989;13:625–631. doi: 10.1097/00000478-198908000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Terada T. Primary signet-ring cell carcinoma of the lung: a case report with an immunohistochemical study. Int J Clin Exp Pathol. 2012;5:171–174. [PMC free article] [PubMed] [Google Scholar]
- 27.Terada T. Primary signet-ring cell carcinoma of the ampulla of Vater: a case report with an immunohistochemical study. Appl Immunohistochem Mol Morphol. 2012;20:427–428. doi: 10.1097/PAI.0b013e31823b7052. [DOI] [PubMed] [Google Scholar]
- 28.Terada T. Primary signet-ring cell carcinoma of the pancreas diagnosed by endoscopic retrograde pancreatic duct biopsy: a case report. Endoscopy. 2012;44(Suppl 2):E141–142. doi: 10.1055/s-0030-1257045. [DOI] [PubMed] [Google Scholar]
- 29.Terada T. Primary pure signet ring cell adenocarcinoma of the non-Barrett’s esophagus: a case report with immunohistochemical study. Endoscopy. 2011;43(Suppl 2 UCTN):E397–8. doi: 10.1055/s-0030-1256944. [DOI] [PubMed] [Google Scholar]
- 30.Terada T. Primary pure signet-ring cell adenocarcinoma of the urinary bladder: a report of three cases with an immunohistochemical study Med Oncol. Med Oncol. 2012 Dec;29:2866–9. doi: 10.1007/s12032-011-0122-7. [DOI] [PubMed] [Google Scholar]
- 31.Terada T. Signet-ring cell carcinoma of the nonampullary duodenum and proximal jejunum: a case report with an immunohistochemical study. Endoscopy. doi: 10.1055/s-0031-1291528. (in press) [DOI] [PubMed] [Google Scholar]
- 32.Terada T. Primary pure signet-ring cell carcinoma of the anus: a case report with immunohistochemical study. Endoscopy. doi: 10.1055/s-0031-1291516. (in press) [DOI] [PubMed] [Google Scholar]
- 33.Terada T. An immunohistochemical study of a primary signet-ring cell carcinoma of the ampulla of Vater: A case report. Int J Gastrointest Cancer. 2012 Dec 19; doi: 10.1007/s12029-012-9469-z. [DOI] [PubMed] [Google Scholar]
- 34.Terada T. Signet-ring cell carcinoma of the esophagus in dermatomyositis: a case report with immunohistochemical study. J Gastrointest Cancer. 2013 Jan 6; doi: 10.1007/s12029-012-9473-3. [DOI] [PubMed] [Google Scholar]
- 35.Terada T. Ovarian malignant Mullerian mixed tumor (heterologous) whose epithelial component is composed predominantly of signet ring cell carcinoma. Arch Gynecol Obstet. 2011;83:1403–1406. doi: 10.1007/s00404-010-1591-1. [DOI] [PubMed] [Google Scholar]
- 36.Terada T. Small cell carcinoma of the ileum that developed 10 years after total gastrectomy for gastric signet-ring cell carcinoma. Appl Immunohistochem Mol Morphol. 2012;20:618–619. doi: 10.1097/PAI.0b013e31823eb34f. [DOI] [PubMed] [Google Scholar]
- 37.Terada T. Primary esophageal small cell carcinoma with brain metastasis and with CD56, KIT, and PDGFRA expressons. Pathol Oncol Res. 2012;18:1091–1093. doi: 10.1007/s12253-011-9374-y. [DOI] [PubMed] [Google Scholar]
- 38.Terada T. KIT and PDGFRA in esophageal pure small cell carcinoma. Int J Clin Exp Pathol. 2011;4:718–721. [PMC free article] [PubMed] [Google Scholar]
- 39.Terada T. KIT and PDGFRA in esophageal pure small cell carcinoma. Int J Clin Exp Pathol. 2011;4:718–721. [PMC free article] [PubMed] [Google Scholar]
- 40.Terada T. A clinical-histopathologic study of esophageal 860 benign and malignant lesions in 910 cases of consecutive esophageal biopsies. Int J Clin Exp Pathol. 2013;6:191–198. [PMC free article] [PubMed] [Google Scholar]
- 41.Chu P, Wu E, Weiss LM. Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod Pathol. 2000;13:962–972. doi: 10.1038/modpathol.3880175. [DOI] [PubMed] [Google Scholar]


