Abstract
Background
Heart failure (HF) is increasing in prevalence and associated with prolonged morbidity, repeat hospitalizations, and high costs. Drug therapies and lifestyle changes can reduce hospitalizations, but non-adherence is high, ranging from 30–80%. There is an urgent need to identify cost-effective ways to improve adherence and reduce hospitalizations.
Trial Design
HART evaluated the benefit of patient self-management (SM) skills training in combination with HF education, over HF education alone, on the composite endpoints of death/HF hospitalizations and death/all-cause hospitalizations in patients with mild to moderate systolic or diastolic dysfunction. Secondary endpoints included progression of HF, quality of life, adherence to drug and lifestyle regimens, and psychosocial function. The HART cohort was comprised of 902 patients including 47% women, 40% minorities, and 23% with diastolic dysfunction. After a baseline exam, patients were randomized to SM or education control, received 18 treatment contacts over one year, annual follow-ups, and 3-month phone calls to assess primary endpoints. SM treatment was conducted in small groups and aimed to activate the patient to implement HF education through training in problem-solving and 5 SM skills. The education control received HF education in the mail followed by a phone call to check comprehension.
Conclusions
The significance of HART lies in its ability to determine the clinical value of activating the patient to collaborate in his/her care. Support for the trial hypotheses would encourage interdisciplinary HF treatment, drawing on an evidence base not only from medicine but also from the behavioral sciences.
The epidemic of acute decompensated heart failure (HF) is becoming a major public health problem.1,2 Although the incidence of new cases has been stable over the past 2 decades,3 survival has improved, resulting in increased prevalence, particularly among women and the elderly.2 Increased prevalence translates into increased hospitalizations. There has been a doubling of HF hospitalizations over the past 10 years.4 Fifty percent of those discharged will be rehospitalized within 6 months5. Currently, there are over 1 million HF hospitalizations annually6, accounting for the largest single Medicare expenditure.7 In 2007, the estimated direct and indirect cost of HF in the United States was $33.2 billion, equivalent to $5,912 per hospital discharge.6
The combined burdens of increasing prevalence, prolonged morbidity, repeat hospitalizations, and high costs make HF a prime target for determining cost-effective ways to manage this disease. Although a number of pharmacologic agents have been identified that can improve outcomes,8 pill counts and electronic pill caps suggest that about 30% of HF patients in general, and 60% of disadvantaged subgroups, do not take these medications as prescribed.9 Non-adherence to lifestyle recommendations, such as salt restriction, physical activity, and daily weighing, tends to be higher, ranging from 50% to more than 80%, with the higher rates again occurring in the more disadvantaged subgroups.9
In light of the importance of the patient side of effective HF management, there has been a proliferation of disease management10–13 and patient self-management (SM)14 programs. In general, these programs have been successful in achieving approximately a 25% reduction in HF hospitalizations, but their benefits on mortality have been more inconsistent. Disease management programs aim to create a close connection between the patient and needed medical care. However, they often tend to keep patients in a passive role and, as such, raise questions about optimum duration and sustainability of effects. SM programs involve patients in their disease by teaching them to manage their symptoms, their treatments, and the physical and psychosocial consequences and needed lifestyle changes.15 As such, they may offer more promise for sustainability of effects. Sustainability is key to the cost-effectiveness of any approach.
Pilot data suggested that a patient SM intervention could improve patient self-efficacy at being able to manage their HF.16 These promising data encouraged further testing of the value of this SM intervention on important clinical endpoints. The Heart Failure Adherence and Retention Trial (HART) was a behavioral randomized clinical trial that evaluated the benefits of a patient SM intervention on the clinical endpoints of death or repeat hospitalizations. The purpose of this paper is to describe the rationale for, and design of, the trial.
METHODS
Aims and Hypotheses
HART was designed to determine whether a patient SM intervention significantly improved clinically important outcomes, relative to an education control, in patients with mild to moderate systolic and/or diastolic HF. Two clinically important composite outcomes served as the primary endpoints: (1) death or hospitalizations for HF; and (2) death or all-cause hospitalizations.
Figure 1 portrays the pathway by which the SM intervention was hypothesized to translate into benefit on the primary endpoints. The intervention provided training in SM skills and was, as such, hypothesized to improve self-efficacy at SM. Since belief about one’s self-efficacy at SM is a prerequisite for performance of SM behaviors,17 improvement in self-efficacy was hypothesized to result in improved adherence to recommended drug therapy and lifestyle changes. Since negative mood undermines adherence18 and may also have a direct effect on clinical endpoints,19 several SM skills were directed toward the management of psychosocial stress, resulting in the expectation that the intervention would improve psychosocial function. Improvement in adherence and psychosocial function were hypothesized to translate into improvement on the primary endpoints and on the secondary endpoints of progression of HF and quality of life.
Figure 1.
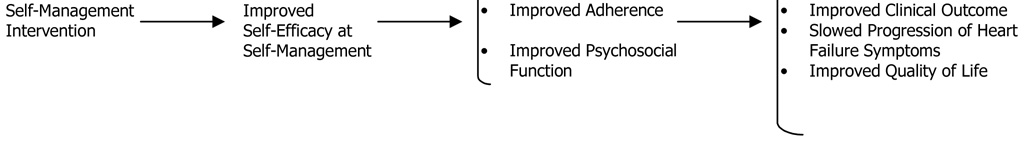
Hypothesized Pathway
Design
HART was a single-site, multi-hospital behavioral randomized efficacy trial based at Rush University Medical Center in Chicago and including 9 other collaborating hospitals from the Chicago metropolitan area. The aim was to randomize approximately 900 patients to one of two treatment arms between October, 2001 and October, 2004. Patients underwent their respective HART treatment for one year and were then followed for an additional 1 to 2 years, depending upon the timing of recruitment, resulting in a total on-trial time of 2–3 years. All data collection ended in October, 2006.
Sample size was based upon the assumption that the SM intervention would produce a 25% reduction in the primary event rate, relative to the education control group, from results of prior SM trials.20,21 The base rate for the primary endpoint of death or hospitalization for HF in the education control group was assumed to be 15% per year, from rates observed in the treatment arms of drug trials with similar patients22 since these drugs would likely be the standard of care when HART ended. Dropouts and losses were estimated to be 15% over the duration of the trial and sample size was adjusted by approximately 3% to allow for interim analyses. To account for both of these factors, an inflation factor of 20% was incorporated into the sample size calculations. Assuming a 2-sided alpha of 0.05 and 80% power, this led to a sample size of 900 participants, evenly distributed between the two treatment arms.
Because HART was a behavioral trial, double-blinding is impossible. However, HART was a partially blinded trial. All staff, except for the senior investigators, and all participants were blinded to trial hypotheses by providing neutral names for the randomized treatment arms. The SM treatment was called “Skills Training” and the attention control was called “Enhanced Education.” All investigators and staff, except for 2 biostatisticians and the recruiting nurses, were blinded to the randomization status of the participant. Treatment teams within each trial arm had no contact with patients in the other arm.
Eligibility
Eligibility for HART was kept broad to maximize the generalizability of results. Because the HART intervention focused on the development of skills, not simply the correction of deficits, eligibility was open to all patients, regardless of their current success with adherence. Eligible patients had HF for not less than the prior 3 months defined as either: (1) left ventricular ejection fraction ≤40% by echocardiography, radiographic ventriculography, or radionuclide ventriculography; or (2) diuretic therapy for at least 3 months and 1 previous hospitalization for HF.
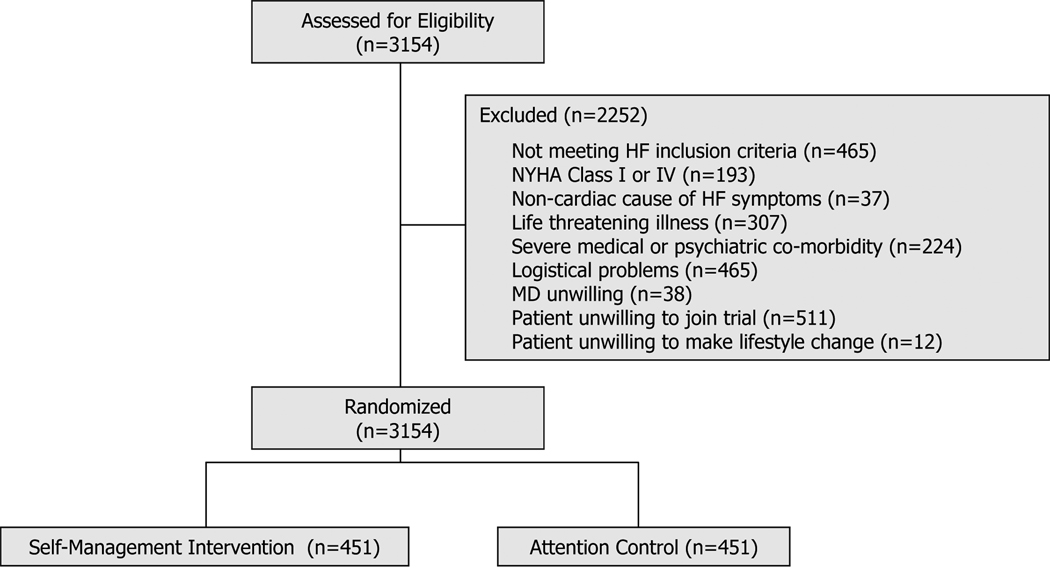
Exclusions were factors that would jeopardize the conduct or rigor of the trial. These included: (1) patients for whom the 12-month prognosis was uncertain (i.e., NYHA Class IV, likelihood of cardiac transplant over the next year, symptomatic or sustained ventricular tachycardia not controlled by therapy within the last 3 months, or other illnesses or disorders that limit 12-month survival); (2) patients classified as NYHA Class I who were unlikely to have a primary endpoint over the course of the trial; (3) patients who were unlikely to undergo or benefit from the behavioral treatment (i.e., presence of cognitive dysfunction or psychological co-morbidity such as substance abuse, psychotic disorder, or active suicidal ideation); (4) patients whose symptoms may be eliminated by surgery (e.g., severe aortic stenosis); (5) logistical issues (e.g., patients were already enrolled in a conflicting protocol, patients were non-English speaking); (6) patients whose physicians refused access; (7) patients who indicated that they were unwilling to make lifestyle changes now or in the near future; and (8) patients who had unstable angina, MI, CABG, or PTCA within the last month (this was a temporary exclusion).
Figure 2 shows the number of screened patients who were excluded for each of these reasons. Of all patients screened, 988 (31%) excluded themselves due to logistical problems, being unwilling to join the trial, or being unwilling to make lifestyle changes.
Figure 2.
Patient flow from screening to enrollment
Recruitment
Nine recruiting hospitals were chosen because they had a substantial number of patients, had potential to promote diversity in gender and ethnicity, and were located in one of 4 geographic clusters, thus making patient travel to a group treatment feasible. Each collaborating hospital had a local cardiologist in place who served as the local principal investigator of the trial. In general, 3 recruiting strategies were used: inpatient screening, outpatient screening at ongoing clinics, and referrals from local cardiologists and internists. Each local principal investigator could tailor the recruitment approach to the features of his/her setting. In the early phase of recruitment, direct marketing to community volunteers was conducted using mass media but this strategy was abandoned due to unfavorable cost-effectiveness.
From pilot data, the screening to enrollment ratio was expected to be 4 patients screened for every patient enrolled, necessitating a recruitment period of between 2–3 years to obtain the target of 900 patients. Figure 2 shows that Rush and the 9 recruiting hospitals screened 3,154 patients over 3 years to produce the 902 patients enrolled in HART. Thus, 3.5 patients were screened for every patient enrolled.
Table 1 presents the final cohort of 902 patients by geographic area, age, gender, ethnicity, and HF type. The average age of the HART participant was 63.6 years. Approximately half were women, 40% were minority and 23% had diastolic dysfunction, reflecting the diversity in HF seen in clinical practice. The achievement of this diversity was due to the choice of recruiting hospitals. For example, patients from the 3 central Chicago sites were younger (average age = 58.0 years) and more often ethnic minorities (80.2%) than patients from the northern suburbs (average age = 69.2 years; 11.0% minority).
Table I.
Recruitment by geographic area, age, gender, ethnicity, and heart failure type
| Age (yrs.) | Female | Minority | Diastolic HF | ||
|---|---|---|---|---|---|
| Geographic Area* | N (%) | X (sd) | N (%) | N (%) | N (%) |
| Central Chicago | 349 (38.7) | 58.0 (13.8) | 183 (52.4) | 280 (80.2) | 79 (22.6) |
| Northern Suburbs | 310 (34.4) | 69.2 (11.5) | 134 (43.2) | 34 (11.0) | 85 (27.4) |
| South Chicago and Suburbs | 124 (13.7) | 63.3 (11.8) | 63 (50.8) | 36 (29.0) | 19 (15.3) |
| Western Suburbs | 82 (9.1) | 66.1 (12.6) | 35 (42.7) | 5 (6.1) | 14 (17.0) |
| Community Volunteers | 37 (4.1) | 65.1 (11.6) | 12 (32.4) | 7 (18.9) | 11 (29.8) |
| TOTAL | 902 (100.0) | 63.6 (13.5) | 427 (47.3) | 362 (40.1) | 208 (23.1) |
Central Chicago: Rush University Medical Center; Stroger Hospital of Cook County; University of Illinois at Chicago.
Northern Suburbs: Advocate Lutheran General Hospital; Evanston Hospital; Rush North Shore Medical Center; Glenbrook Hospital.
South Chicago and Suburbs: Advocate Christ Medical Center/Advocate Clinics; South Suburban Hospital.
Western Suburbs: Midwest Heart.
Community Volunteers: Recruited through mass media.
Protocol
Once a potential participant was identified, a HART nurse coordinator obtained permission from the physician to examine medical records and screen for the presence of exclusion criteria. If the patient was eligible, he/she was contacted and the trial was described. If the patient was interested in participating, a baseline exam was scheduled at the local recruiting hospital. Upon arrival at the baseline exam, the trial was described again, eligibility was confirmed, informed consent was obtained, and the baseline exam was conducted.
At the conclusion of the baseline exam, the patient was provided with instructions for receiving the results of the randomization, beginning the treatment, and upcoming exams and phone calls. The nurse coordinator called the automated randomization service (Moffitt Cancer Center, University of South Florida) to obtain treatment assignment and then mailed a letter of notification to the patient. Follow-up by staff leading the relevant trial arm commenced approximately 2–5 days after receipt of the letter.
Baseline and all annual exams consisted of: (1) a clinical exam assessing height, weight, pulse, respiratory rate, blood pressure, 6-Minute Walk, and a blood draw; (2) a medical history including current medical conditions, current medications, sociodemographic status, and risk factors such as smoking, alcohol consumption, and physical activity; (3) an interview for the assessment of HF symptoms, health-related quality of life, problems with HF management, and depression; and (4) a questionnaire assessing satisfaction with quality of life, self-efficacy, social support, hopelessness, irritability, purpose in life, and salt intake. At the conclusion of each exam, the patient was asked to put a month’s supply of an ace inhibitor (or beta blocker if the patient was not taking an ace inhibitor) into a MEM’s pill cap container (MEMS V Trackcap, AARDEX, Zug, Switzerland) and was taught how to use it for the ensuing month.
The trial protocol was approved by the institutional committees on human research at the central coordinating center and at each of the 9 collaborating hospitals.
Randomization
The randomization protocol was developed to insure that 10 patients from the same geographic location would be randomized as quickly as possible to a SM group. A stratified block design was used with strata defined as 4 geographic locations in metropolitan Chicago. Within each stratum, a block size of 20 was used to insure that after 20 randomizations, 10 patients were assigned to each treatment arm.
Self-Management Treatment
SM treatment featured group-based counseling to help patients develop mastery in problem-solving and in 5 SM skills deemed to be helpful in adhering to medical advice and alleviating any negative affect that would undermine adherence. Group, rather than individual, treatment was chosen because modeling by coping peers, and the resulting vicarious learning, has been shown to be a powerful agent of change.23
Eighteen 2-hour group meetings of 5–10 patients were spread out over the course of one year. At each group meeting, patients received HF education in the form of 18 one-page Heart Failure Tip Sheets from the American Heart Association which summarized basic elements of HF management including medication adherence, sudden weight gain, salt restriction, moderate physical activity, and stress management.
Implementation of these health education tips was accomplished through the mastery of 5 SM skills, chosen because of their relevance for the lifestyle changes required. These skills were: (1) self-monitoring of such targets as weight and daily sodium intake; (2) environmental restructuring of the home and workplace by, for example, placing pill boxes in visible places to remind patients to take them; (3) social support where patients were encouraged to discuss efforts at making lifestyle changes with family and friends as a way to elicit their support; (4) cognitive restructuring where patients were taught to replace stress inducing thoughts with stress reducing ones; and (5) deep breathing as an immediate response to physical or emotional stress. To foster proactivity in invoking these skills when needed, a problem-solving format was used in which patients identified barriers to implementing the HF tips and were encouraged to use the SM skills to overcome them.
All groups were led by health professionals with advanced degrees, experience in conducting groups, and a willingness to follow a protocol. All prospective group leaders underwent standardized training over the course of 2 days. Only those certified at the end of this training were invited to lead. All treatment sessions were taped and randomly selected tapes were reviewed by the supervisor (KJF) to check adherence to the protocol and provide feedback to the leader, as needed. Data reports focusing both on group leaders and individual patients were reviewed by a SM Subcommittee to identify problems and strategies for their resolution. To prevent drift, group leaders met monthly to share successes and strategies for overcoming challenges. These meetings were conducted using the same format as that used in the SM groups for the purpose of modeling desired behaviors.
Education Control
The use of an attention control, rather than a usual care control, minimizes potential problems that could occur in a behavioral trial with an unblinded usual care control. Among these problems are: (1) the inability to determine whether it is any attention, rather than the specific treatment, that promotes benefit; (2) the inability to mask patients to trial hypotheses; (3) the potential to produce an “underdog” effect that would promote differential dropout in the controls; (4) the potential to produce a “John Henry” effect in which the controls seek treatment on their own and thus essentially cross-over into treatment; and (5) the minimization of any generalized placebo effect where patients in the treated group get better simply because they perceive they are receiving treatment.
Patients randomized to the education control received a phone-based educational intervention which was believed to be the standard of care that would be in place when HART was concluded. Patients received the same 18 American Heart Association Heart Failure Tip Sheets, on the same schedule as the group meetings in the SM group. However, they were mailed to the home and, to insure receipt and check comprehension, a study coordinator placed a phone call within 2–3 days of receipt. If at the time the first call was placed the patient had not read the Tip Sheet, another call was scheduled. Questions about the Tip Sheets were answered but if questions were asked about SM or non-Tip Sheet concerns, the patient was referred back to his/her provider. No new information beyond the Tip Sheet was provided.
Study coordinators conducting the phone calls were trained on purpose, structure, content, data reporting responsibilities, and quality control procedures. Training included role playing to simulate phone call interactions. Ongoing training, as needed, took place in response to special problems (e.g., difficulty in reaching patients, patients asking for SM support).
Endpoints
Table 2 summarizes the primary and secondary endpoints in HART. Patients or, in the case of death, their family members were queried every 3 months for the occurrence of death or hospitalizations. All secondary endpoints were assessed annually during the physical exam, interview, and self-reported questionnaire. Mortality was confirmed by medical record, death certificate, emergency medical services record, or queries from the national mortality registry. HF admissions were adjudicated by the presence of either shortness of breath, peripheral edema, or chest x-ray evidence of pulmonary edema without evidence of another disease process accounting for symptoms or signs. HF admissions were confirmed if the patient responded to anti-failure therapy or had a documented decrease in LV function.
Table II.
Description of primary and secondary endpoints
| Variable | Measure | Description |
|---|---|---|
| PRIMARY ENDPOINTS | ||
| Death or hospitalization | blind adjudication | Ascertanment of death from death certificate, emergency room notes, full hospitalization record, summary from emergency medical services records, autopsy. Ascertainment of hospitalization for HF from full medical record, dated chest x-ray, dated ECG, serum enzymes of CK, CK-MB, troponins, and all consultation and progress notes. |
| Death or all-cause hospitalization | blind adjudication | Ascertainment of death same as above. Ascertainment of hospitalization documented from medical record. |
| SECONDARY ENDPOINTS | ||
| Progression of HF | Repeat Hospitalizations for HF | Rate of repeated hospitalizations for HF over the duration of the follow-up. |
| NYHA Functional Class24 | Symptom-based rating of functional capacity. Only Class II (symptoms on ordinary exertion) and Class III (symptoms on less than ordinary exertion) patients eligible for the trial. | |
| 6-Minute Walk25 | Maximal functional capacity assessed as distance patient can walk in 6 minutes. Cutpoint of ≤ 300m (984 ft.) is associated with increased risk of death or hospitalization.26 | |
| Acute Physiology Score27 | Composite acuity score comprised of heart rate, respiratory rate, oxygenation, acid base, sodium, potassium, creatinine, Hct, white blood cell count, and Glasgow coma scale. | |
| Heart Failure Symptom Checklist28 | 4 selected self-reported scales assessing symptoms in last month: Cardiopulmonary (12 items); Gastrointestinal (11 items); Genitourinary (3 items); Sensory/Cognitive/Neurological/Muscular (14 items). Internal consistency reliability range (alpha=0.80–0.87), except for Genitourinary (alpha=0.46). | |
| Quality of Life | Satisfaction: Quality of Life Index Cardiac Version29 | 2 self-reported subscales that measure satisfaction in important areas of life: Health/Functioning (11 items) and Psychological/Spiritual (11 items). Internal consistency reliability ranges from 0.70–0.93. Test-retest reliability is over 0.70. |
| Health-Related: RAND SF-3630 | 2 selected self-reported subscales: Physical Role Function (10 items) assesses health limitations in basic activities such as walking, bathing, lifting groceries and Vitality (4 items) assesses general feelings of energy and tiredness. Internal consistency reliabilities, respectively (alpha=0.93; 0.86). | |
| Adherence | Adherence to Drug Therapy31 | MEMS electronic pill caps used to track a single medication, typically an ACE inhibitor, to serve as a marker of overall adherence. Monitoring continues for 1 month after baseline/annual exam. Produces % missed pills of those prescribed. |
| Salt Intake32 | Salt intake over the past week is assessed using the CALS Food Frequency Questionnaire, specifically developed to focus on dietary items that are main sources of sodium. It is computer-scored and results in an estimated total sodium intake (in milligrams) per day. | |
| Physical Activity33 | Two questions assessing, in a typical week, minutes spent walking and hours/day watching TV. | |
| Smoking | 5 self-reported questions assessing current cigarettes smoked/day and past smoking history. | |
| Alcohol Intake | 3 self-reported questions assessing average intake separately by beer, wine, and hard liquor. | |
| Problems with Heart Failure Management--Pt. B34 | 10-item self-report scale measuring adherence to medications, diet, fluid restrictions, exercise, smoking, taking vital signs, daily weighing, calling with problems, clinic attendance, and getting lab tests done. | |
| Self Efficacy | Self-Efficacy at Self-Management | 5 self-reported items, developed for this trial, to assess self-efficacy in the use of 5 self-management skills: environmental restructuring, self-monitoring, cognitive restructuring, relaxation, and problem-solving. |
| Psychosocial | Geriatric Depression Scale35 | 30 self-reported items answered in “yes/no” format, summed to yield score between 0–30 (alpha=0.94). A score over 10 is a sensitive and specific screen for major depressive symptoms. |
| Hopelessness36 | 2 self-reported items assessing degree to which “it is impossible to reach goals” and “the future is hopeless”. Score of ≥6 indicates high hopelessness. | |
| Irritability37 | 9 self-reported items focusing on easily provoked anger and irritability in daily situations, plus 3 additional items assessing ability to control reactions to interpersonal and situational provocations. | |
| MOS Social Support38 | 4 subscales assessing: emotional support (8 items), tangible support (4 items), affectionate support (3 items), and positive social interactions (4 items). All scales have internal consistency alphas of >0.90. An additional item was added to assess number of close friends/relatives. | |
| Purpose in Life Scale39 | 14 self-reported items that are a subscale of the Psychological Well-Being Scale assessing degree to which a person has goals in life, a sense of directedness, and feels there is meaning to present and past life. | |
Adverse Events
The safety of the patients was monitored continuously by an In-House Safety Committee. Potential adverse events that could be related to the nature or content of the interventions included suicidal attempts, suicidal ideation, a new or recurrent episode of depression or anxiety, substance abuse or recidivism, a report of unusually high levels of distress, depression, or anxiety not meeting diagnostic criteria, and physical injury. Potential serious adverse events that could be directly related to the nature or content of the interventions included death, life-threatening adverse drug/device/product events, inpatient hospitalization or prolonged hospitalization, and persistent or significant disability/incapacity.
Special Populations
The HART cohort was 40% African American and 36% had incomes <$20,000/year. Many of these patients had low literacy levels and functional limitations. To insure that this trial was culturally sensitive to the special barriers that occur in these subgroups, all aspects of the recruitment protocol and treatment arms were studied to determine patient perceptions of the entire research process.40 This resulted in several important protocol modifications. For example, for underserved African Americans, the development of trust came more slowly than in other subgroups, translating into missed SM group meetings early in treatment. Thus, the SM protocol was modified to permit make-up sessions later in the year after trust had developed. For low income patients, reimbursement for transportation was arranged. For low literacy patients, Literacy Chicago, a non-profit organization dedicated to improving literacy in the underserved, was employed to improve the readability and comprehensibility of all treatment and outcome measures. Offers were made to read consent forms and questionnaires. For patients with functional limitations, all examination and treatment sites were evaluated for their ability to accommodate wheelchairs, consistent with recommendations from the Americans with Disabilities Act.
Planned Analyses
Trial results will be analyzed according to the intention-to-treat principle. Data from all randomized patients will be analyzed according to the original treatment assignment, regardless of the actual dose of treatment received or possible cross-over between treatment arms. Baseline characteristics will be compared between the 2 treatment arms to assess covariate balance and any imbalances will be adjusted for in multivariate models. Event rates over time will be summarized using Kaplan-Meier survival curves and differences in these curves by treatment arm will be analyzed using the Mantel-Haenszel (logrank) test. The semi-parametric Cox model will be used for multivariate modeling. In the event that the proportionality assumption is not met, treatment differences will be analyzed using the Wilcoxon test and multivariate modeling will be analyzed using logistic regression.
Differential dependency in the HART data exists because the SM intervention was delivered in groups whereas the education control was delivered individually. To assess the significance of this dependency, we will use hierarchical linear models to analyze endpoint data in the SM participants. If group assignment and/or any relevant interactions are found to be significant covariates, they will be incorporated as covariates in subsequent analyses of the trial endpoints.
Treatment differences in secondary endpoints will be examined using t-tests as the primary analytic tool. Change in secondary outcomes will be analyzed using generalized linear models (GLM) which will take into account the full richness of the data obtained via annual assessments. Analyses of treatment efficacy are planned for prespecified subgroups which include age, gender, income, education, ethnicity, functional capacity, systolic/diastolic dysfunction, and adherence. For these analyses, interaction terms (subgroup × treatment) will be evaluated in multivariate modeling that includes the interaction, its main effects, and sociodemographic and medical covariates.
TRIAL ORGANIZATION
HART incorporates features of phase 3 therapeutic clinical trials, modified as needed to address the unique nature of the trial. The large size of the Chicago metropolitan area made it possible to conduct the trial entirely in the city and its suburbs, with Rush University Medical Center serving as the coordinating center. The trial was run by an Executive Committee (which also served as the Steering Committee), chaired by the Principal Investigator (LHP), and co-chaired by the two Co-Principal Investigators (JEC, Jr, CMdL). Seven organizational units directed the progress of the trial, each of which had their own director: (1) Medical Issues/Recruitment (JEC, Jr.); (2) Operations (CE); (3) Self-Management (KJF); (4) Education Control (KLG); (5) Special Populations (CSR-W); (6) Data Management (CMdL); and (7) Data Analysis (DR). In addition, an In-House Safety Committee reviewed potential adverse events and a blinded Adjudication Committee, comprised of cardiologists, adjudicated all deaths and hospitalizations.
The trial was overseen by an NHLBI-appointed Data and Safety Monitoring Board (DSMB) which met semiannually and was responsible for reviewing and monitoring the protocol and the feasibility of the operations, patient safety, and outcome events. Proposed changes to the protocol were brought to the DSMB by the investigators for approval. The unblinded senior and junior biostatisticians (DR, IJ) provided reports requested by the DSMB. Stopping rules for the primary endpoints were developed using the O’Brien-Fleming procedure with Lan-DeMets boundaries, the log-rank statistic, and an overall 2-sided significance level of 0.05. Using total patient-months on study as a gauge, interim analyses were conducted at yearly intervals after 8%, 33%, 67%, and 100% of the information had been accumulated. These data formed the basis of recommendations to the NHLBI to continue or discontinue the trial, the final decision of which rested with the NHLBI.
DISCUSSION
The significance of HART, one of the largest behavioral trials in HF conducted, lies in its ability to determine the clinical value of activating the patient to collaborate in his/her care. HART was designed on the assumption that a chronic illness like HF requires a shift from an acute care model of disease to one of collaborative management.41 This is essentially equivalent to shifting from a model where the provider prescribes and the patient passively receives, to one in which the provider recommends and the patient actively implements.
Activation of patients requires that they have the knowledge, skills, and motivation to implement medical advice. A basic design assumption of HART was that knowledge by itself was necessary but not sufficient to activate patients. As such, an education control group was used which essentially equalized the amount of HF knowledge received in the two treatment arms. HART was then in a position to determine the value of adding skills training and motivation enhancers above and beyond the benefits of HF knowledge. Among the skills taught in the SM arm were how to organize the home and work setting to encourage lifestyle change, monitor systematically for symptoms, and enlist the social environment to support SM efforts. Transfer of responsibility for making lifestyle changes from the provider to the patient was most directly targeted through the use of a problem-solving format for group treatment where patients, not providers, took initiative in identifying barriers to change and ways to overcome them. Since psychosocial distress undermines motivation and is in excess prevalence in HF populations,19 two stress-management skills were among the 5 SM skills taught. This was expected to minimize the chance that patients who were depressed, hopeless, or had lost their will to live would have insufficient motivation to implement medical advice.
The HART recruitment strategy was designed to result in a cohort that was representative of HF as it is seen during routine clinical practice. Past trials have focused primarily on white men with HF due to ischemic cardiomyopathy. Few women, ethnic minorities, and patients with preserved left ventricular function have been studied. Inattention to these important population subgroups may have resulted in some unfortunate trends. Women, relative to men, are at greater risk for a HF hospitalization and their survival after diagnosis is poorer.3,6 African Americans, relative to other groups, have four times the rate of HF emergency department visits and have twice the rate of non-adherence to recommended therapies and lifestyle changes.42 Patients with diastolic dysfunction comprise between 20–40% of the HF population but there are few effective treatments for them.4
While it is clear that the diversity of the HART cohort is a scientific strength, at the same time it poses clear challenges. The task of promoting lasting lifestyle change in underserved minorities or elderly patients on fixed incomes is daunting. For patients who cannot afford to buy their medications, SM training to adhere to these medications must be accompanied by additional efforts such as referring patients to indigent drug programs. For patients who distrust the value of these medications for the promotion of their health and longevity, SM training must be accompanied by trust-building. Mindful of the importance of these challenges, HART devoted significant attention to ways to tailor the treatment to overcome unique barriers in its diverse subgroups.
Several design decisions in HART were made because of the unique challenges posed by behavioral trials, relative to drug trials. In a drug trial, the mechanism of action is known, change in this mechanism is insured by titrating the dose of the drug to the individual patient, and the drug is taken continuously over the follow-up. In a behavioral trial, however, the mechanism of action is hypothesized, patients tend to receive the same dose of treatment regardless of need, and treatment is finite often ending before the follow-up ends. Mindful of these challenges, HART investigators sought to make the SM treatment as powerful as possible. Group treatment was chosen because of the large body of research that suggests that the most powerful agent of change is the coping peer,23 and a recent meta-analysis of HF interventions that supports the value of face-to-face contact.10 The potential power of group treatment was believed to outweigh the logistical risks associated with getting sick patients to attend. Moreover, the HART investigators developed a proposed pathway by which the HART treatment would translate into benefit on the primary endpoints to aid in the interpretation of the data. In the event of a null result, for example, it will be possible to determine whether this was a failure to support the hypothesis that SM treatment improves outcomes or a failure of SM treatment to affect the proposed mechanisms of action.
In a drug trial, the use of a placebo control blinds patients to their treatment status. In a behavioral trial, patients know exactly which treatment they are receiving and may respond in ways that could compromise the integrity of the trial. Indeed, one of the most difficult choices in designing a behavioral trial is a determination of the appropriate control group. The HART investigative team, in conjunction with the HART DSMB, considered a variety of control groups including usual care and didactic lectures. But ultimately the choice was to use a control that was expected to be the optimum standard of care in place when HART was completed. This choice had the effect of posing a rigorous question for the SM intervention: Would it be superior not just to usual care but to an optimum standard of care?
In summary, HF is one of the few cardiovascular conditions for which prevalence is increasing over time. Effective therapies exist for its optimal treatment, but patients are underutilizing them. HART focuses on the patient side of HF and aims to activate the patient to more fully implement medical recommendations. Support for the hypotheses posed by HART would encourage interdisciplinary treatment of HF which draws on the evidence base not only from medicine but also from the behavioral sciences.
Acknowledgments
Support for HART from HL065547. Clinical Trials Registration NCT00018005.
Appendix
Executive Committee
Co-authors listed on this paper.
Sponsor Contacts at NHLBI
Jared Jobe, PhD; Peter Kaufmann, PhD
Recruiting Hospital Principal Investigators and Collaborators
Cheryl Brody, DO: South Suburban Hospital/ Leslie Brookfield, MD, FACC: Advocate Lutheran General Hospital/ Stephanie Dunlap, MD: University of Illinois/ Philip Krause, MD, FACC: Rush North Shore Medical Center/Stuart Rosenbush, MD (PI), William Elliott, MD, PhD, Donald Tanis, MD: Rush University Medical Center/ Mitchell Saltzberg (PI), Maria Rosa Costanzo, MD: Midwest Heart Specialists/ Michael Shapiro (PI), Thomas Stamos, MD: John H. Stroger Hospital of Cook County/ Marc A. Silver, MD: Advocate Christ Medical Center/ Michael Waligora, MD (PI), Randall Williams, MD: Evanston Northwestern Healthcare and Glenbrook Hospital.
Self-Management Group Leaders
Kristin J. Flynn (Supervisor); Andrea Kozak (Assistant Supervisor); Carl Thoresen, Virginia Price, Susan Gilkey (Trainers); Maureen Gecht; Jim Jarzenbowski; Kevin McClone; Carmen Lynas; Sybil Madison-Boyd; Marilynn Rochon; Bruce Bybarczyk; Sarah Sellergren; Stephen Tate; Tamara Gathright; Rocio Munoz; Cheryl S. Rucker-Whitaker; Lynda H. Powell; Kim Lebowitz; Patty Roberts; Ayesha Shaikh; Marilyn Vander Werf.
Endpoints Adjudication Committee
James E. Calvin, Jr. (Chair); Stamatis Dimitropoulos, MD; William Elliott, MD, PhD; Philip Kraus, MD; Payman Sattar, MD; Sujata Shanbhag, MD; Thomas Stamos, MD; Patricia Vassallo, MD.
Data and Safety Monitoring Board
Lawrence S. Cohen, MD (Chair); Baruch A. Brody, PhD (starting 2002); Byron W. Brown (through 2004); Nancy R. Cook, ScD (starting 2005); Julie Buring, ScD; Robert M. Kaplan; Lynn Warner Stevenson, MD; David Thomasma, PhD (through 2002).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Braunwald E. Shattuck lecture: cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–1369. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 2.McCullough PA, Philbin EF, Spertun JA, et al. Confirmation of a heart failure epidemic: findings from the resource utilization among congestive heart failure (REACH) study. J Am Coll Cardiol. 2002;39:60–69. doi: 10.1016/s0735-1097(01)01700-4. [DOI] [PubMed] [Google Scholar]
- 3.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 4.Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2001;38:2101–2113. doi: 10.1016/s0735-1097(01)01683-7. [DOI] [PubMed] [Google Scholar]
- 5.Aghababian RV. Acutely decompensated heart failure: opportunities to improve care and outcomes in the emergency department. Rev Cardiovasc Med. 2002;3(suppl 4):S3–S9. [PubMed] [Google Scholar]
- 6.Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics—2007 Update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e3171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 7.Massie BM, Shah NB. Evolving trends in the epidemiologic factors of heart failure: rationale for preventive strategies and comprehensive disease management. Am Heart J. 1997;133:703–712. doi: 10.1016/s0002-8703(97)70173-x. [DOI] [PubMed] [Google Scholar]
- 8.Jessup M, Brozena S. Medical progress: heart failure. New Engl J Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 9.van der Wal MHL, Jaarsma T. Adherence in heart failure in the elderly: Problem and possible solutions. Intl J Cardiology. 2007 doi: 10.1016/j.ijcard.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Kozak AT, Rucker-Whitaker C, Basu S, et al. Elements of nonpharmacologic interventions that prevent progression of heart failure: a meta-analysis. Congestive Heart Failure. 2007;13:280–287. doi: 10.1111/j.1527-5299.2007.07236.x. [DOI] [PubMed] [Google Scholar]
- 11.McAlister FA, Stewart S, Ferrua S, et al. Multidisciplinary strategies for the management of heart failure patients at high risk for admission. A systematic review of randomized trials. J Am Coll Cardiol. 2004;44:810–819. doi: 10.1016/j.jacc.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 12.Gwadry-Sridhar F, Elintoft V, Lee DS, et al. A systematic review and meta-analysis of studies comparing readmission rates and mortality rates in patients with heart failure. Arch Intern Med. 2004;164:2315–2320. doi: 10.1001/archinte.164.21.2315. [DOI] [PubMed] [Google Scholar]
- 13.Phillips CO, Wright SM, Kern DE, et al. Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure. JAMA. 2004;291:1358–1367. doi: 10.1001/jama.291.11.1358. [DOI] [PubMed] [Google Scholar]
- 14.Jovicic A, Holroyd-Leduc JM, Straus SE. Effects of self-management intervention on health outcomes of patients with heart failure: a systematic review of randomized controlled trials. BMC Cardiov Disorders. 2006;6:43–50. doi: 10.1186/1471-2261-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barlow J, Wright C, Sheasby J, et al. Self-management approaches for people with chronic conditions: a review. Patient Educ Couns. 2002;48:177–187. doi: 10.1016/s0738-3991(02)00032-0. [DOI] [PubMed] [Google Scholar]
- 16.Flynn KJ, Powell LH, Mendes de Leon CG, et al. Increasing self-management skills in heart failure patients: A pilot study. Congestive Heart Failure. 2005;11:297–302. doi: 10.1111/j.1527-5299.2005.04361.x. [DOI] [PubMed] [Google Scholar]
- 17.Bandura A. Self-Efficacy. The Exercise of Control. New York: W. H. Freeman and Company; 1997. [Google Scholar]
- 18.Evangelista LS, Berg J, Dracup K. Relationship between psychosocial variables and compliance in patients with heart failure. Heart and Lung. 2001;30:294–301. doi: 10.1067/mhl.2001.116011. [DOI] [PubMed] [Google Scholar]
- 19.MacMahon KMA, Lip GYH. Psychological factors in heart failure. A review of the literature. Arch Intern Med. 2002;162:509–516. doi: 10.1001/archinte.162.5.509. [DOI] [PubMed] [Google Scholar]
- 20.Friedman M, Thoresen CE, Gill JJ, et al. Alteration of Type A behavior and reduction in cardiac recurrences in post-myocardial infarction subjects. Am Heart J. 1984;108:237–248. doi: 10.1016/0002-8703(84)90606-9. [DOI] [PubMed] [Google Scholar]
- 21.Lorig KR, Sobel DS, Stewart AL, et al. Evidence suggesting that a chronic disease self-management program cn improve health status while reducing hospitalization. A randomized trial. Medical Care. 1999;37:5–14. doi: 10.1097/00005650-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 22.The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. NEJM. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 23.Bandura A. Social Foundations of Thought & Action. A Social Cognitive Theory. Englewood Cliffs, New Jersey: Prentice-Hall, Inc.; 1986. [Google Scholar]
- 24.The Criteria Committee of the New York Heart Association. Diseases of the Heart and Blood Vessels: Nomenclature and Criteria for Diagnosis. 6th ed. Boston, MA: Little Brown; 1964. [Google Scholar]
- 25.Faggiano P, Aloia A, Gualeni A, et al. Assessment of oxygen uptake during the 6-minute walking test in patients with heart failure: Preliminary experience with a portable device. Am Heart J. 1997;134:203–206. doi: 10.1016/s0002-8703(97)70125-x. [DOI] [PubMed] [Google Scholar]
- 26.Bittner V, Weiner DH, Yusuf S, et al. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. JAMA. 1993;270:1707–1707. [PubMed] [Google Scholar]
- 27.Knaus WA, Zimmerman JE, Wagner DP, et al. APACHE: acute physiology and chronic health evaluation: a physiologically based classification system. Crit Care Med. 1981;9:591–597. doi: 10.1097/00003246-198108000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Grady KL, Jalowiec AJ, Grusk BB, et al. Symptom distress in cardiac transplant candidates. Heart and Lung. 1992;21:434–439. [PubMed] [Google Scholar]
- 29.Ferrans CE, Powers MJ. Quality of Life Index: Development and psychometric properties. Adv Nurs Sci. 1985;8:15–24. doi: 10.1097/00012272-198510000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Ware JE, Jr, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 31.Kruse W, Eggert-Druse W, Rampmaier J, et al. Compliance with short-term high-dose oestradiol in young patients with primary infertility. New insights from the use of electronic devices. Agents Actions. 1990;29:105–115. doi: 10.1007/978-3-0348-7292-8_12. [DOI] [PubMed] [Google Scholar]
- 32.DeBusk RF. MULTIFIT. A new approach to risk factor modification. Cardiol Clin. 1996;14:143–157. doi: 10.1016/s0733-8651(05)70267-8. [DOI] [PubMed] [Google Scholar]
- 33.National Center for Health Statistics. Series 10. Publication No. 160 PHHS (PHS) 86-1568. Hyattsville, MD: Public Health Service; 1985. [Google Scholar]
- 34.Grady KL, Jalowiec A, White-Williams C. Patient compliance at one year and two years after heart transplantation. J Heart Lung Transplant. 1998;17:383–394. [PubMed] [Google Scholar]
- 35.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiat Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 36.Everson SA, Goldberg DE, Kaplan GA, et al. Hopelessness and risk of mortality and incidence of myocardial infarction and cancer. Psychosom Med. 1996;58:112–121. doi: 10.1097/00006842-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Buss AH, Durkee A. An inventory for assessing different kinds of hostility. J Consult Psychol. 1957;42:155–162. doi: 10.1037/h0046900. [DOI] [PubMed] [Google Scholar]
- 38.Sherbourne CD, Stewrt AL. The MOS Social Support Survey. Soc Sci Med. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 39.Ryff CD, Keyes CL. The structure of psychological well-being revisited. J Pers Soc Psychol. 1995;69:719–727. doi: 10.1037//0022-3514.69.4.719. [DOI] [PubMed] [Google Scholar]
- 40.Rucker-Whitaker CS, Flynn KJ, Kravitz G, et al. Understanding African-American participation in a behavioral intervention: Results from focus groups. Contemp Clin Trials. 2006;27:274–286. doi: 10.1016/j.cct.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 41.Von Korff M, Gruman J, Schaefer J, et al. Collaborative management of chronic illness. Ann Intern Med. 1997;127:1097–1102. doi: 10.7326/0003-4819-127-12-199712150-00008. [DOI] [PubMed] [Google Scholar]
- 42.Hope CJ, Wu J, Tu W, et al. Association of medication adherence, knowledge, and skills with emergency department visits by adults 50 years or older with congestive heart failure. Am J Health-Syst Pharm. 2004;61:2043–2049. doi: 10.1093/ajhp/61.19.2043. [DOI] [PubMed] [Google Scholar]