Abstract
The present study compared longitudinal treatment outcomes for depressed substance-dependent veterans (N=206) assigned to Integrated Cognitive Behavioral Therapy plus standard pharmacotherapy (ICBT+P) or Twelve Step Facilitation Therapy plus standard pharmacotherapy (TSF+P). Drug and alcohol involvement and depressive symptomology were measured at intake and at three-month intervals during treatment and up to one year post-treatment. Participants in both treatment conditions showed decreased depression and substance use from intake. ICBT+P participants maintained improvements in substance involvement over time whereas TSF+P participants had more rapid increases in use in the months following treatment. Decreases in depressive symptoms were more pronounced for TSF+P than ICBT+P in the six months post-treatment. Within both treatment groups, higher attendance was associated with improved substance use and depression outcomes over time. Initial levels of depressive symptomology had a complex predictive relationship with long-term depression outcomes. Early treatment response predicted long-term substance use outcomes for a portion of the sample. Although both treatments were associated with improvements in substance use and depression, ICBT+P may lead to more stable substance use reductions compared to TSF+P.
Keywords: Depression, Substance use disorders, Cognitive behavioral therapy
Introduction
Substance use disorders (SUDs) and depressive disorders are prevalent and costly to society. The lifetime prevalence of major depressive disorder (16.6%) and alcohol abuse (13.2%) are higher than any other DSM-IV Axis I disorder (Kessler, Berglund, Demler, Jin, & Walters, 2005). SUDs and psychiatric illness frequently co-occur, and such comorbidity is associated with more severe psychopathology (Kessler, Chiu, Demler, & Walters, 2005), increased risk for negative outcomes (Hesselbrock, Meyer, & Hesselbrock, 1992; Brown & D’Amico, 2001), and substantially higher health service costs (Hoff & Rosenheck, 1999).
Depression is the most frequently co-occurring Axis I mental health disorder for most SUDs (Grant, 1995; Grant & Harford, 1995; Regier et al., 1990). Dually diagnosed patients demonstrate poorer treatment outcomes regardless of whether the intervention addresses the substance use or the depression (Greenfield et al., 1998; McKay et al., 2002; Dodge, Sindelar, & Sinha, 2005; Kodl et al., 2008; Hasin et al., 2002). Commonly used self-help approaches, such as involvement in traditional 12-Step groups, may be less accessible and beneficial for dually diagnosed individuals (Kelly, McKellar, & Moos, 2003).
Given the aforementioned issues, the establishment of effective interventions for this high-risk population is especially important. Historically, treatment was provided separately for each disorder, either sequentially (e.g., treatment for depression following completion of treatment for SUD) or in a parallel manner during the same timeframe (with separate appointments, in different settings, by different providers). For example, Brown and colleagues (1997) added a separate cognitive behavioral therapy for depression to the usual addiction treatment in a parallel fashion, which resulted in better alcohol and depression outcomes relative to the addition of a relaxation training control condition. A recent meta-analysis (Nunes & Levin, 2004) concluded that antidepressants were effective in reducing depression symptoms for dually diagnosed patients, but “concurrent therapy directly targeting the addiction is also indicated” (p. 1887).
Difficulties with sequential and parallel interventions became evident, including potential for conflicting treatment philosophies and recovery models, multiple locations, and scheduling problems associated with separate appointments (Drake et al., 1996). Integration of treatments was advocated to address these problems, placing the burden for resolving potential conflicts on treatment providers rather than patients. Thus, integrated interventions provide coordinated treatment that is compatible in approach and philosophy, is more efficient in terms of appointments and settings, and is delivered by providers trained in interventions for both disorders. The limited existing research suggests that interventions addressing both the SUD and comorbid psychiatric disorder may be more effective than interventions targeting either disorder alone (Hellerstein, Rosenthal & Miner, 1995; Kushner et al., 2006; Morrissey et al., 2005).
Since dually diagnosed individuals exhibit a more challenging clinical course, examination of longitudinal patterns of outcomes is important to understand timing and processes of change for both depression and SUDs. Such research is particularly important given that treatment effects may not become evident until the post-intervention follow-up phase (e.g., Rawson et al., 2002) or may wane in the months post-intervention (Smith & Glass, 1977).
The present study compares treatment outcomes of veterans with current alcohol/drug dependence and depression receiving one of two interventions: a newly developed integrated cognitive behavioral therapy (ICBT) or an evidence-based model of care for alcohol and drug treatment (Twelve Step Facilitation group therapy). Participants in both groups also received standard VA pharmacotherapy (P). A preliminary report of this study’s results (N=66) examined outcomes at intake, during treatment, and up to six months post treatment (Brown et al., 2006). Findings suggested that although both interventions were associated with reductions in depression and substance use during active treatment, gains made by ICBT+P participants were more stable up to six months post-treatment compared to gains made by TSF+P participants. The present study longitudinally extends the investigation to 18 months after treatment entry for an expanded sample of male and female veterans. The present paper addressed three questions: 1) In which treatment group do participants experience superior improvements in substance use and depression during and after treatment, 2) Do outcomes differ as a function of severity of substance use or depression evident in the early stages of treatment, and 3) Does amount of treatment add to the predictability of clinical outcomes? It is hypothesized that both interventions will be associated with improvements in depression and substance use during active treatment. Second, it is expected that ICBT+P assignment will be associated with more stable improvements in substance involvement and depression than TSF+P, and that these differences will persist when initial depression and substance use are included in the model. Further, it is hypothesized that increased treatment exposure will be associated with better outcomes for both intervention groups.
Method
Participants
This study was approved by VA and University of California, San Diego Institutional Review Boards. The current sample included 206 veterans recruited from referrals to the VA Substance Abuse Mental Illness (SAMI) program, a dual diagnosis outpatient clinic for veterans with SUD and concomitant Axis I disorders. Inclusion criteria for the study were: (1) presence of a current (past year) DSM-IV diagnosis of alcohol, cannabinol, and/or stimulant dependence, (2) DSM-IV diagnosis of lifetime major depressive disorder with at least one episode independent of alcohol/drug use, and (3) recent substance use (past 90 days) and elevated depressive symptoms (Hamilton Depression Rating Scale total score>20). Study exclusion criteria were: (1) lifetime DSM-IV diagnosis of bipolar disorder, psychotic disorder, or opiate dependence through intravenous administration (non-intravenous opiate use was acceptable), (2) residence too far from the study site or homeless, and (3) memory deficits sufficient to impair accurate recall of events.
The sample was characterized by extensive histories of dependence on multiple substances (average, 24 years) and depression episodes (average, 25 years), as well as multiple prior treatments (e.g., 3.8 alcohol/drug, 3.3 psychiatric; Table 1 details). Three-fourths of the sample met criteria for dysthymia as well as lifetime MDD (“double depression”), and participants averaged 20 lifetime major depressive episodes. As previously mentioned, all participants had experienced clinically significant depression symptoms in the week prior to intake. At intake, 88% of participants met DSM criteria for lifetime alcohol dependence, 39% for cocaine dependence, 32% for amphetamine dependence, 30% for cannabis dependence, 13% for opioid dependence, 10% for sedative dependence, 6% for hallucinogen dependence, 2% for PCP dependence, and 1% for inhalant dependence.
Table 1.
Characteristics of Substance Dependent Depressed Participants at Intake: TSF+P vs. ICBT+P Conditions
| Characteristic | Overall (N=206) |
TSF+P (n=99) |
ICBT+P (n=107) |
|---|---|---|---|
| Gender (% male) | 92 | 91 | 93 |
| Age, mean yrs (SD) | 48.2 (7.7) | 48.4 (8.1) | 48.0 (7.5) |
| Marital Status (%) | |||
| Married | 14.7 | 19.4 | 10.4 |
| Divorced/Widowed/Separated | 61.8 | 61.2 | 62.3 |
| Never married | 23.5 | 19.4 | 27.4 |
| Education, mean yrs (SD) | 13.5 (2.0) | 13.4 (2.0) | 13.5 (1.9) |
| Ethnicity (% Caucasian) | 71 | 69 | 73 |
| Monthly Income, mean dollars (SD) | 1227 (1543) | 1318 (1516) | 1146 (1570) |
| Treatment History | |||
| Lifetime substance treatments, mean (SD) | 3.8 (5.5) | 4.1 (5.7) | 3.5 (5.4) |
| Lifetime psychiatric treatments, mean (SD) | 3.3 (7.3) | 3.5 (7.8) | 3.1 (6.8) |
| Psychiatric inpatient pre-intake (%) | 30.6 | 30.3 | 30.8 |
| Substance inpatient pre-intake (%) | 40.8 | 35.4 | 45.8 |
| Yrs since first SUD onset, mean (SD) | 23.9 (11.0) | 24.6 (11.1) | 23.2 (10.8) |
| Yrs since MDD onset, mean (SD) | 24.55 (12.4) | 27.0 (11.7) | 22.1 (13.1) |
| Lifetime SUD Combinations (%) | |||
| Alcohol only | 21 | 23 | 19 |
| Drug(s) only | 1 | 0 | 1 |
| Alcohol and Drug(s) | 79 | 76 | 80 |
| Depression | |||
| HDRS Total Score, mean (SD) | 28.0 (11.1) | 27.3 (11.7) | 28.6 (10.6) |
| Substance Use | |||
| TLFB PDA, mean (SD) | .73 (.24) | .70 (.25) | .75 (.24) |
| Days since last use at intake (SD) | 37.2 (25.5) | 35.5 (25.2) | 38.8 (25.8) |
Note: HDRS = Hamilton Depression Rating Scale; TLFB = Timeline Follow Back; CIDI = Composite International Diagnostic Interview; PDA= Percent Days Abstinent.
Psychiatric inpatient pre-intake and substance inpatient pre-intake refer to percentage of sample hospitalized in the 90 days prior to intake. Substance inpatient includes 28-day inpatient rehabilitation and inpatient detoxification. Psychiatric inpatient includes psychiatric and dual-diagnosis hospitalization as well as placement in a crisis house. There were no significant differences observed between groups on any measures.
All participants met criteria for dependence on at least one substance in the past year. Specific current diagnostic rates for the sample are as follows: 66% alcohol dependent, 32% stimulant dependent (19% cocaine, 16% amphetamine), 12% marijuana dependent, 2% opioid dependent, 2% sedative dependent, and 1% hallucinogen dependent. No participants were currently dependent on PCP or inhalants at intake. Because a number of participants were hospitalized at treatment entry (and thus had no access to substances), average percent days using substances was relatively low. On average, participants used substances 27% of the 90 days prior to intake. Eighty-three percent of the sample had drunk alcohol, consuming an average of 13.8 drinks per drinking day.
Measures
Diagnostic Information
The computerized interview and scoring system of the Composite International Diagnostic Interview (CIDI; Robins et al., 1998) was used to assess Axis I diagnoses. DSM-IV diagnoses of lifetime and current substance use disorders (alcohol and 10 categories of drugs) were assessed using the CIDI. Additionally, the CIDI was used to establish independent and substance-induced lifetime and current psychiatric disorders.
Substance Use
The Time Line Follow Back (TLFB; Sobell & Sobell, 1992) is a calendar assisted structured interview with demonstrated reliability and validity in addiction treatment research (Fals-Stewart, O’Farrell, Freitas, McFarlin, & Rutigliano, 2000; Maisto, Sobell, & Sobell, 1979). In the present study, the TLFB was adapted to type and frequency of drug use as well as frequency and quantity of alcohol consumption (Brown et al., 2006; Ehrman & Robbins, 1994; Fals-Stewart et al., 2000).
Depression Symptoms
The Hamilton Depression Rating Scale (HDRS; Hamilton, 1960), a 21-item structured clinical interview, was used to assess depressive symptoms in the prior week. The HDRS has been proven to have good sensitivity and specificity among alcohol/substance dependent populations (Willenbring, 1986).
Addiction Severity
The Addiction Severity Index (ASI; McLellan et al. 1990) is a structured interview that was used to assess dysfunction in four domains (medical, legal, psychological, employment). The strong psychometric properties of the ASI have been well-established (Alterman et al., 1994).
Community 12-Step Affiliation
The AA Affiliation Scale (Humphreys, Kaskutas, & Weisner, 1998) is a 9-item self-report measure with good reliability. Items assess past month meeting attendance, presence of a current AA sponsor, reading AA literature, and calling another AA member for help.
Procedure
Recruitment of participants took place from March 2000 to August 2006. All referrals to the SAMI program completed a comprehensive clinical interview, including an assessment of lifetime psychiatric symptoms during periods of extended (> one month) abstinence. Individuals with independent depression diagnoses were screened for study participation. As part of this process, research staff assessed for an independent lifetime MDD diagnosis using the CIDI. A supervising licensed clinical psychologist reviewed all previously mentioned diagnostic information to determine DSM-IV diagnoses. For study purposes, depression independent of substance use was defined as depression symptoms meeting DSM-IV diagnostic criteria following a minimum of 30 days of abstinence from alcohol and illicit drug use.
Eligible and interested individuals gave informed consent and agreed to: (a) randomization to 24 weeks of ICBT+P or TSF+P group treatment, (b) assessment interviews at intake and quarterly thereafter with thirty dollar compensation for post-treatment interviews, (c) random toxicology screens, (d) no other formal treatment for depression or substance dependence during the 24 week intervention period other than psychiatrist appointments and 12-step meetings in the community.
Consecutive admissions to the study were sequentially allocated by cohorts into ICBT+P or TSF+P. Participants were sequentially assigned to the group with the next entry date. Groups had entry times every 4 weeks at the beginning of a new module, and the ICBT and TSF entry times were alternated and staggered such that participants entered with a maximum of a two-week waiting period. The feasibility of this design has been previously reported (Drapkin, Tate, McQuaid, & Brown, 2008). Given the nature of this study in a clinical context, participants, administrators, and interviewers were not blinded to the patient’s treatment assignment.
Both treatment conditions had two consecutive 12-week phases of treatment. In each condition, Phase I involved biweekly hour-long group sessions plus monthly individual medication management visits, and Phase II consisted of weekly hour-long group sessions plus monthly medication management. Each therapy session was videotaped, and a random sample (25%) of sessions was reviewed by a condition-specific clinical supervisor to ensure fidelity to the manual and avoid contamination of outside content/techniques.
Assessments were conducted at intake, end of Phase I (12 weeks), end of Phase II (24 weeks), and then quarterly at 3, 6, 9, and 12 months post-treatment. At each of the seven quarterly time points, HDRS assessed depressive symptoms in the past week and TLFB assessed alcohol and drug use in the preceding three months. During TLFB administration, participants were reminded of toxicology results (monthly drug screens are a SAMI program requirement) as an additional aid in recollection of dates of use. In the case of discrepancies between self-report and toxicology results, substance use was added to the TLFB. Addition Severity Index (ASI) scores obtained during Phases I and II of treatment were averaged into single treatment ASI subscores. Similarly, ASI scores from the quarterly follow-up periods were averaged to create single post-treatment subscores. The AA Affiliation Scale was administered during Phases I and II of treatment as well as 6 and 12 months post-treatment. In addition to the initial diagnostic interview, the CIDI was also administered 6 and 12 months following treatment completion.
Interventions
Integrated Cognitive Behavioral Therapy (ICBT)
Phase I of ICBT incorporated three modules (managing cognitions, increasing healthy activities, and building social network) spanning eight twice-weekly hour-long sessions each. Phase II included 12 structured once-weekly hour-long sessions during which the core skills acquired in Phase I were reviewed and reinforced.
ICBT was based on Cognitive-Behavioral Depression Treatment (Munoz, Ying, Perez-Stable, & Miranda, 1993) with Cognitive-Behavioral Coping Skills Training of Addiction (Kadden et al., 1994) integrated into the content and process of each session. The thoughts module focused on identifying maladaptive cognitions, generating alternative cognitions, and rehearsing thought challenging techniques in situations that could potentially lead to substance use relapse or increased depressive symptoms. The activities module involved identifying, scheduling, and assessing effectiveness of specific activities for increasing positive affect and managing pressure to relapse. The social module consisted of assertiveness and communication training with the goal of increasing positive social interactions and patient efficacy in resisting social pressure to use.
Twelve-Step Facilitation (TSF) Therapy
TSF employed herein is a therapist-guided group intervention based on the Project Match TSF treatment (Nowinski, Baker, & Carroll, 1994). TSF was chosen as the control condition because twelve-step principles are among the most common intervention elements across addiction treatment settings, and TSF has been associated with successful outcomes (Miller & Longabaugh, 2003). The TSF control intervention contains some “integrated” components (dually diagnosed veterans being treated together) but not all elements of fully integrated intervention (i.e., dual diagnosis disorder specific integrated content). For this study, the intervention was modified from the Project Match manual in the following ways: 1) hour-long group therapy sessions versus individual sessions, and entry points at the beginning of any module. The TSF+P treatment dosage was identical to that for ICBT+P (Phase I for 12 weeks with twice-weekly sessions and Phase II for 12 weeks with once-weekly sessions) and was accompanied by comparable monthly medication management sessions. One module addressed AA/NA Steps 1–3, another module focused on topics common in AA/NA meetings and Twelve Step literature, and a third module covered AA/NA Steps 4 and 5. Across both Phase I and II, each session involved review of relevant readings (e.g., AA Big Book), new didactic content, and discussion of recovery tasks (e.g., going to 12-step meetings, obtaining a sponsor). Depression issues were addressed following TSF themes, and therapists encouraged patients to discuss mood symptoms with the psychiatrist during medication management appointments. As with ICBT+P, Phase II included once-weekly group sessions, reviewing topics from Phase I.
Data Analytic Plan
Primary outcome variables selected a priori included Percent Days Abstinent (PDA) as measured on the TLFB and Hamilton Depression Rating Scale Total score (HDRS Total). PDA was chosen as the primary substance use variable because it reflects frequency of substance use, regardless of substance of choice. Additionally, given that both treatments involve an abstinence-based model, PDA (versus quantity variables) may be a better indicator of treatment results for any given individual.
Baseline Analyses
One-way ANOVAs and Chi Square tests were performed on baseline characteristics (demographics, intake substance use and depression, additional Axis I disorders, treatment history) to assess comparability of the two treatment groups.
Trajectory Analyses
Substance use and depression trajectories were ascertained using linear mixed effects (LME) models (Frees, 2004; West, Welch & Galecki, 2007). Both HDRS and PDA trajectories were modeled from intake to one year post-treatment. PDA was assessed for the entire 90 days prior to each interview and consequently includes one less time point than HDRS. PDA in the period prior to treatment had been recorded, but trajectories across the pretreatment period were not included in our models because: this paper’s focus is on the pattern of substance use and depression during and after treatment, all participants have a common starting-point at time zero because they were required to be abstinent for at least one week prior to entry, and the variation in the clinical routes that participants had experienced in the weeks prior to enrollment resulted in great variation in the recorded pre-treatment use patterns. These patterns were often not reflective of the severity of participants’ substance use. Because the temporal dynamics were expected to be complex, time was included in the models as a polynomial term, providing for curvilinear trajectories. The time parameters were orthogonalized to reduce the effects of collinearity (Hedeker & Gibbons, 2006). Number of therapy sessions attended, early abstinence (Phase I PDA), and intake HDRS were included as independent variables in extended models to determine the predictive role of treatment exposure and initial substance use and depression. Interactions between these covariates and time were also included in the model. Intercepts were treated as a random variable and all of the slopes as fixed. Incomplete cases were used in the analyses as linear mixed effects models can accommodate incomplete cases under the missing at random assumption (Singer & Willett, 2003). Models were estimated with Stata version 9.2 (Stata Corp., 2005). Comparisons of outcome summary data were made with adjusted means, which compensates for variation in covariate values and missing observations (Sall, Lehman & Crieghton, 2001).
Results
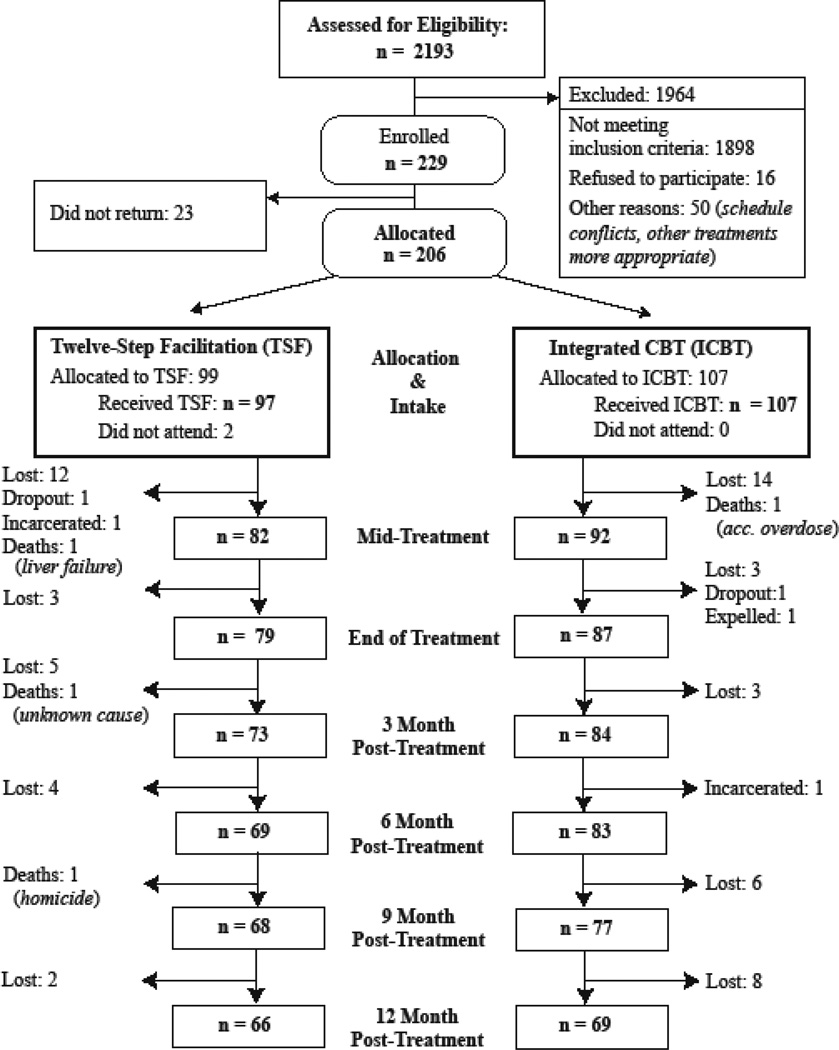
A total of 229 veterans met criteria for study inclusion and provided informed consent. Of these, 206 returned for initial assessment and were allocated to one of the two treatment conditions (Figure 1). The mean number of sessions attended was 20.2 (SD=9.5) for ICBT+P participants and 19.4 (SD=10.5) sessions for TSF+P participants. Participants completed an average of 5.3 PDA assessments and 5.2 HDRS assessments. Across the two groups, there were no adverse events that occurred as a result of treatment. For both TSF+P and ICBT+P conditions, there were no significant differences between participants included in analyses and early dropouts in terms of gender, ethnicity, years of education, intake percent days abstinent, or intake Hamilton depression scores (p>.16). However dropouts were 6 years younger on average (95%CI=2.9–8.5) although the ages were not significantly different between treatments among the dropouts (p=.11).
Figure 1.
Participant Flow Chart.
Group Equivalence and Sample Characterization
ICBT+P and TSF+P participants did not differ significantly (p>.05 on χ2 and ANOVA) on any of the demographic (age, gender, ethnic background, education), prior treatment (lifetime, past 90 days), intake substance use (ASI, drug use diagnoses, PDA, current abstinence status), or intake depression (HDRS) measures indicating that the two treatment groups were equivalent (Table 1).
Both groups had extensive history of depressive episodes (mean lifetime episodes = 22.1 for TSF and 17.4 for ICBT, FE, p=.34) and the majority of both treatment groups met criteria for a recent depressive episode (TSF = 71 %, ICBT = 88 %, FE, p = .01) approximately half of which were independent of recent substance use (40% and 42%, respectively). Further, rates of comorbid dysthymia were 75% in both treatment groups (FE, p=.53). Groups were also similar on proportion of participants with co-occurring Axis I disorders: social phobia (ICBT = 65%, TSF = 64%), posttraumatic stress disorder (ICBT = 43%, TSF = 36%), and obsessive compulsive disorder (ICBT = 22%, TSF = 21%).
Pharmacotherapy
The number of medication management appointments attended in each of the study periods was similar in the two treatments and not significantly different during any period. The average number of appointments during treatment was 4.60 (SD=3.17) for TSF+P and 4.55 (SD=3.34) for ICBT+P. The number of appointments consistently decreased through time concluding at 0.9 (95% CI= .6–1.3) for TSF+P and 1.3 (95% CI= .9–1.7) for ICBT+P, during the final follow-up period.
Most participants were prescribed an antidepressant during each phase of treatment and follow-up. The rate ranged from 92% to 98% across all waves. The rate was significantly lower for ICBT+P in two periods, Phase II of treatment (FE, p=.045), and the three months post-treatment (FE, p=.032). The rate for TSF+P during Phase II was 99% (95% CI= .92–.99) and 91% (95% CI= .82–.96) for ICBT+P. During the three months post-treatment the rate for TSF+P was 98% (95% CI= .90–.99) and 88% (95% CI= .78–.94) for ICBT+P.
A limited number of participants received pharmacotherapy for substance abuse. Only five participants received these types of medications during the entire study period. There were no periods where the rate was significantly different between TSF+P and ICBT+P (FE, p>0.14). The marginal rate of substance abuse medications across study periods was 2.7% (95% CI= .018–.041).
Substance Use Outcomes
Summary Statistics
The mean PDA in our TSF+P sample in Phase I of treatment was 92 (95% CI= 88–96), which decreased to 89 (95% CI= 85–94) at the end of treatment and later decreased to 74 (95% CI= 66–83) one year post-treatment. The mean PDA in the ICBT+P sample followed a similar pattern starting at 90 (95% CI=87–94), then dropping to 84 (95% CI=79–90) at the end of treatment and ending at 84 (95% CI = 77–92) one year post-treatment.
Clinical Trajectories of Abstinence (PDA)
ICBT+P was found to provide superior 18-month substance use outcomes than TSF+P. In the simple model (model 1), both TSF+P and +P produce significantly decreased PDA after Phase I of treatment (Table 2). TSF+P declined throughout the study whereas ICBT+P trajectories were significantly less negative (Table 2), such that TSF+P trajectories dropped 2 percentage points between Phase I and the end of Phase II, and then PDA decline accelerated. After treatment ended, TSF+P’s PDA trajectories deteriorated another 15 percentage points by one year post-treatment. ICBT+P trajectories decreased a similar amount (2% points) during treatment but only dropped 3% in the year after treatment. Of note, in the last nine months the ICBT+P trajectories were stable with only a one point drop. These trajectories result in the average ICBT+P trajectory ending at 84% days of abstinence at 18 months following intake compared to 75% days abstinent for TSF+P.
Table 2.
Mixed Effects Models for Percent Days Abstinent and Depression, Coefficient (SE).
| Model and terms | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Substance Use (PDA) | χ2(5)=41.80** | χ2 (7)=64.59** | χ2 (10)=95.88** |
| Time | −.055(.01)** | .065(.03)* | .048(.03) |
| Time (quadratic) | −.011(.01) | −.082(.03)* | −.076(.03)* |
| Treatment | .010(.03) | .014(.03) | .016(.03) |
| Treatment × Time | .041(.01)* | .041(.01)** | .041(.01)** |
| Treatment × Time (quadratic) | .015(.01) | .014(.01) | .015(.01) |
| Phase I PDA × Time | --- | −.132(.03)** | −.153(.04)** |
| Phase I PDA × Time (quadratic) | --- | .078(.03)* | .085(.04)* |
| Session Quantity | --- | --- | .008(<.01)** |
| Session Quantity × Time | --- | --- | .002(<.01)* |
| Session Quantity × Time (quadratic) | --- | --- | −.0006(<.01) |
| Depression (HDRS Total) | χ2(5)=76.43** | χ2 (7)=198.38** | χ2 (10)=210.37** |
| Time | .−1.96(.41)** | −2.23(.39)** | −1.667(.83)* |
| Time (quadratic) | 2.035(.42)** | 1.94(.39)** | .181(.85) |
| Treatment | 3.091(1.34)* | 3.104(1.31)* | 3.102(1.29)* |
| Treatment × Time | .054(.58) | .435(.55) | .434(.55)* |
| Treatment × Time (quadratic) | −1.207(.58)* | −1.182(.55)* | −1.206(.55)* |
| Intake HDRS Total × Time | --- | −.243(.02)** | −.245(.02)** |
| Intake HDRS Total × Time (quadratic) | --- | .087(.02)** | .095(.02)** |
| Session Quantity | --- | --- | −.186(.07)* |
| Session Quantity × Time | --- | --- | −.024(.03) |
| Session Quantity × Time (quadratic) | --- | --- | .076(.03)* |
Note: HDRS = Hamilton Depression Rating Scale; PDA= Percent Days Abstinent. Initial PDA, Hamilton, and session quantity was centered prior to analysis.
Significant at p<. 05
Significant at p<. 01
To determine whether initial abstinence during treatment was related to outcomes, initial PDA levels were added to the model and were found to be predictive of individual substance use trajectories (Likelihood Ratio χ2 (2)=21.1, p<.001, Table 2). In this model (model 2), the trajectories for participants with the mean initial level of PDA were similar to the model 1 findings, where ICBT+P was significantly superior (Table 2). Additionally, model 2 captures the substantial differences in clinical trajectories of individual participants who had either high or low initial PDA.
As expected, trajectories for participants with high levels of abstinence during Phase I had trajectories that decreased over time and to a significantly greater extent (Table 2). Trajectories for TSF+P participants starting at 100% PDA dropped 4 points more over the course of the study than those starting at 91% PDA, which was the mean. For ICBT+P, trajectories for those initially at 100% PDA only dropped 2 points more over the same period. Participants who started treatment with low initial PDA have qualitatively different trajectories. Their clinical trajectories initially rise quickly (more abstinence during treatment) and then turn negative or stabilize depending on treatment group. In TSF+P, those with low initial abstinence rise 10 percentage points to peak at 3 months post-treatment and then turn negative and drop 11 points by the end of the study. By contrast, those with low initial abstinence in ICBT+P rises 14 points to a peak at 6 months post-treatment and then drop only 3 percentage points by the end of the study.
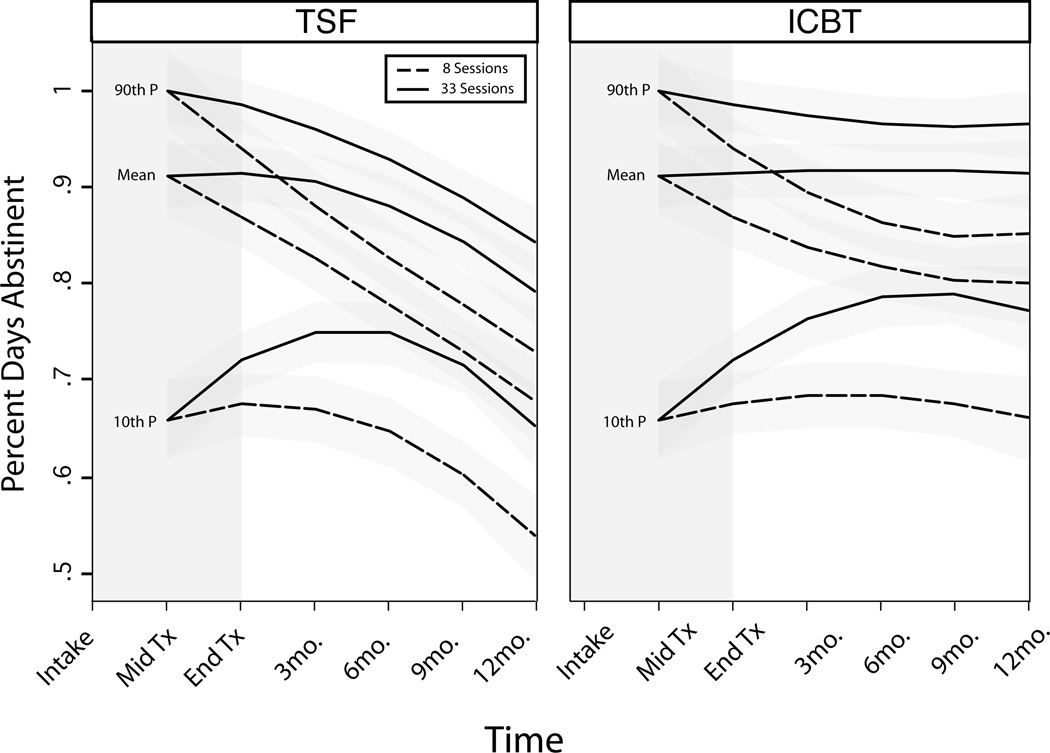
In the final analysis (model 3), we considered how PDA trajectories were related to the treatment exposure (number of sessions attended) and early abstinence level. Similar to models 1 and 2, PDA was found to deteriorate across time in both treatments with steeper declines in TSF+P for average participants (Table 2). As in model 2 the long-term patterns of substance use were found to be complex functions of early abstinence levels when also considering number of sessions attended. Trajectories for participants with poor levels of early abstinence differed qualitatively from other groups, such that they had trajectories with increasing PDA during the second half of the treatment (Phase II) and the early post-treatment period (Figure 2). This was followed by a pronounced decrease in PDA for TSF+P participants but a leveling of PDA in ICBT+P participants (Figure 2).
Figure 2.
Modeled Substance Use Trajectories Based on Group Attendance and Early Treatment Response (The three sets of lines represent predicted PDA when Phase I PDA is at the 90th, 50th, and 10th percentile, respectively).
There was a strong treatment exposure by time interaction, such that participants attending more sessions had better substance use trajectories (χ2 (3) =29.34, p<.0001). To exemplify this, we present two levels of session attendance in Figure 2. Trajectories for those attending 33 sessions (90th percentile) ended significantly higher than those attending only 8 sessions (10th percentile); however the direction of the trajectory was similar for all levels of participation by the end of the study (Figure 2).
In summary, differences in abstinence between treatments were most pronounced when adjusting for treatment exposure and initial PDA. Consequently, ICBT+P trajectories predict a persistence of higher abstinence than in TSF+P (Figure 2). In particular, after treatment the abstinence trajectories were higher for ICBT+P, when controlling for both number of sessions and Phase I PDA (Figure 2). As shown in Table 3, mean PDA for ICBT+P is significantly higher than TSF+P at 6, 9, and 12 months post-treatment, when controlling for number of sessions attended or initial PDA. Mean PDA for ICBT+P was 10 points higher than TSF+P by 12 months post-treatment.
Table 3.
Substance Use and Depression Adjusted Means (SE) for Substance Dependent Depressed Veterans, estimates based on median attendance (23), mean PDA (91%), and HDRS (28)
| Condition | Intake | Phase I | Phase II | 3m | 6m | 9m | 12m |
|---|---|---|---|---|---|---|---|
| Substance Use (PDA) | |||||||
| Attendance=23 | |||||||
| TSF+P | . | .92(.03) | .90(.02) | .88(.02) | .85(.02) | .80(.02) | .75(.03) |
| ICBT+P | . | .89(.02) | .88(.02) | .87(.02) | .86(.02) | .86(.02) | .85(.03) |
| PDA Phase I = 91% | |||||||
| TSF+P | . | .91(.03) | .90(.02) | .88(.02) | .84(.02) | .80(.02) | .75(.03) |
| ICBT+P | . | .89(.02) | .87(.02) | .86(.02) | .86(.02) | .85(.02) | .85(.03) |
| Depression (HDRS) | |||||||
| Attendance =23 | |||||||
| TSF+P | 27(1.2) | 23(1.0) | 21(1.0) | 19(1.1) | 19(1.1) | 20(1.1) | 21(1.3) |
| ICBT+P | 29(1.1) | 27(.95) | 25(1.0) | 24(1.0) | 23(1.0) | 23(1.0) | 23(1.2) |
| Hamilton Intake=28 | |||||||
| TSF+P | 27(1.2) | 24(1.0) | 21(1.0) | 19(1.1) | 19(1.1) | 20(1.1) | 21(1.3) |
| ICBT+P | 29(1.1) | 27(.94) | 25(.98) | 24(1.0) | 23(1.0) | 23(1.0) | 23(1.2) |
Note: HDRS = Hamilton Depression Rating Scale; PDA = Proportion Days Abstinent. Standard errors in parentheses. Both PDA and HDRS were recorded at the end of each period; PDA refers to abstinence across the entire 90 days and HDRS refers to depression in past 7 days.
Findings by Substance Type
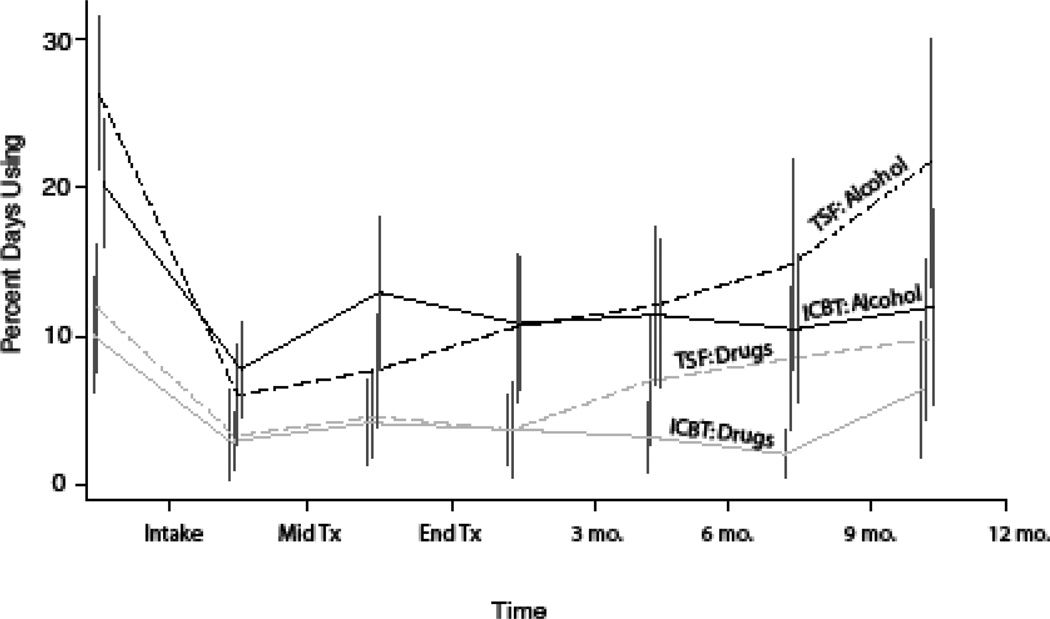
The differences in the patterns for percent days using drugs and percent days using alcohol between the two treatments were not strong enough to reach statistical significance (LME Models: Percent Days Drinking, p>.10; Percent Days Using, p>.61). Although not significant, the sample statistics for the two components are consistent with the findings for percent days abstinent, where TSF+P use increases more than ICBT+P in the later post-treatment time points (Figure 4).
Figure 4.
Percent Days Using Alcohol and Drugs for TSF+P and ICBT+P Participants.
To further exame potential substance related differences in treatment response, participants were classified into three groups depending on the types of substances used in the 90 days prior to treatment. Thirty-six percent were classified as stimulant users if they used stimulants regardless of other substances used. Twelve percent used marijuana and alcohol, and 52% used alcohol alone or alcohol and other drugs. Substance use classification was not significantly related to PDA trajectories (LME Model, LR χ2 (8) =8.3, p= .40) or HDRS trajectories (LME Model, LR χ2 (8) =13.2, p=.11).
Depression Outcomes
Summary Statistics
The mean HDRS in our TSF+P sample at intake was 27.5 (95% CI= 25.0–30.0), which decreased to 18.8 (95% CI=16.3–21.3) at the end of treatment and later increased to 21.1 (95% CI=18.0–24.0) one year post-treatment. The mean HDRS in the ICBT+P sample followed a similar pattern starting at 28.6 (95% CI=26.6–30.7), dropping to 24.5 (95% CI=21.4–27.5) at the end of treatment and ending at 22.5 (95% CI =19.5–25.5) one year post-treatment.
Clinical Trajectories of Depression
Depression trajectories for both treatment groups dropped dramatically during treatment. TSF+P was found to provide a superior early impact on HDRS scores compared to ICBT+P, however the greater improvement was lost by the end of the study. In the simple model (model 1), both TSF+P and ICBT+P experienced significant improvements in HDRS during the study (Table 2). In TSF+P, depression reached its lowest at 6 months post-treatment with mean HDRS of 19.1(95% CI= 16.9–21.2). ICBT+P trajectories were more linear dropping to a minimum of 23.0 (95% CI= 20.7–25.8) at 9 months post-treatment. For those receiving TSF+P, depression rose to 22.0 (95% CI= 19.4–24.6) by the end of the follow up whereas ICBT+P ended at a comparable level of 23.2 (95% CI= 20.7–25.8).
Next, HDRS levels at the beginning of treatment were added to the model (model 2) and found to be highly predictive of individual depression trajectories (LR χ2 (2) =105.5, p<.001, Table 2). In this conditional model, the depression trajectories for participants with initial HDRS scores at or near average scores (M=28) were similar to the model 1 findings (Table 2). However, participants with high initial HDRS scores (≥ 42) showed significantly greater drops over time, and to a significantly greater extent than for participants with low initial HDRS (Table 2). Trajectories for TSF+P and ICBT+P participants starting at 42 HDRS dropped 9 points more over the course of the study than those starting at 28 HDRS. Trajectories for ICBT+P and TSF+P participants who started with low initial HDRS have a qualitatively different course with a modest rise in both by the final assessment 18 months later.
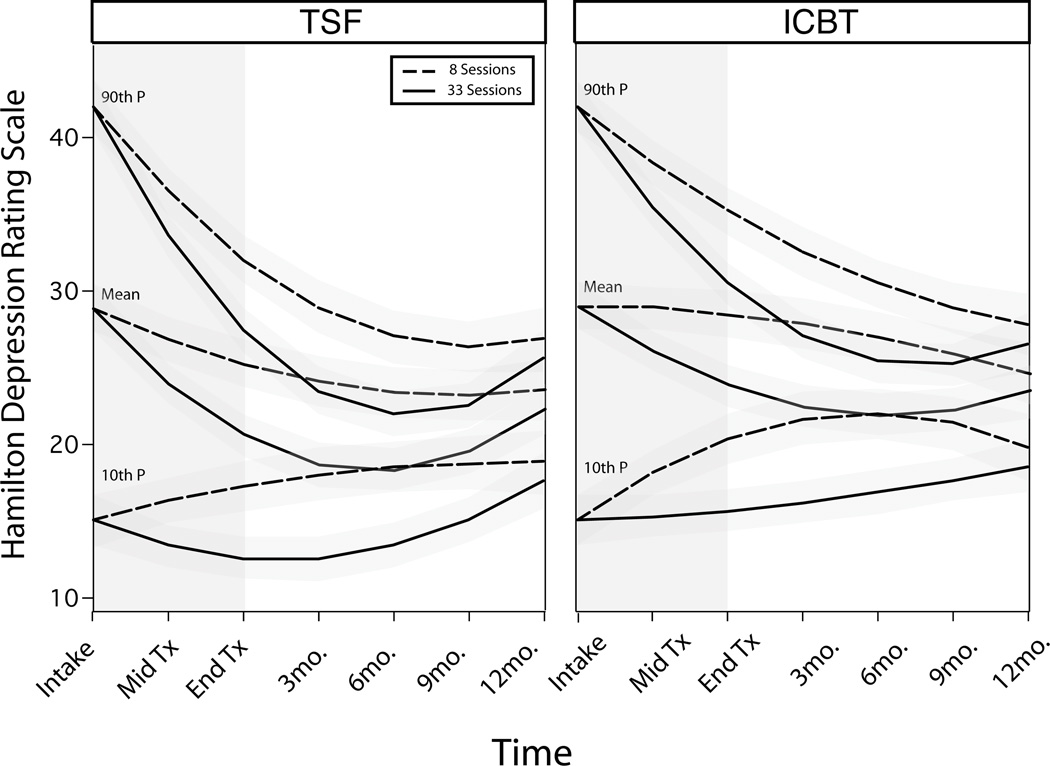
Finally, when attendance is added to the conditional model of clinical course, HDRS trajectories were found to be related to treatment condition, initial HDRS, and attendance (Table 2). The estimated unconditional effects of initial levels of depression and treatment type were similar in all three models (Table 2). However, model 3 demonstrates that number of sessions attended is a significant predictor of depression (LR χ2 (3) =10.3, p=.016).
When level of attendance is considered, treatment groups evidence different HDRS trajectories (Table 2). The differences were mild and reflect a faster drop for TSF+P during treatment than ICBT+P (Figure 3). HDRS trajectories curved differently for the two treatment groups: TSF+P produced a greater drop in depression scores in the six months post-treatment but increased during the final six months of study. By contrast, most of the ICBT+P sample had depression trajectories that were leveling off or still decreasing one year post-treatment.
Figure 3.
Modeled Depression Trajectories Based on Group Attendance and Intake Levels of Depression (The three sets of lines represent predicted HDRS when intake HDRS is at the 90th, 50th, and 10th percentile, respectively).
Note, HDRS adjusted means from model 3 for ICBT+P are higher than TSF+P at all time points, when modeled controlling for either number of sessions attended or HDRS at intake (Figure 3, Table 3). The greatest difference between treatment conditions was 5 points at 3 months post-treatment but shrank to 2 points by 18 months after entry into treatment.
As with PDA, the number of sessions attended was highly related to depression trajectories (Table 2). Those with high attendance had trajectories that displayed greater reductions in HDRS scores than low attendance trajectories in the months following treatment completion. However, the greater level of improvement is lost and approaches scores of low-attendance trajectories by 12 months post-treatment (Figure 3).
Secondary Analyses
Community 12-Step Involvement
Consistent with the focus of treatment and previous reports from this trial (Brown et al., 2006), participants in TSF+P were more likely to report 12-step attendance in the community than ICBT+P participants at mid-treatment (TSF+P=91.8%, ICBT+P=65.6%, respectively; FE, p<.001) and end of treatment (TSF+P=87.0%, ICBT+P=55.0%; FE, p<.001). In the year following treatment, the percentage of participants attending at least one 12-Step meeting in the community did not differ statistically across the two treatment groups (TSF+P =68%, ICBT+P= 60% at 6 months; FE, p=.42, and TSF+P= 57%, ICBT+P= 55% at 12 months; FE, p=1.0). Similarly, higher proportions of TSF+P versus ICBT+P participants volunteered at meetings by the end of treatment (TSF+P=56%, ICBT+P=32%; FE, p=.01) and had read AA/NA literature (TSF+P=78%, ICBT+P=60%; FE, p=.05). However, at six and 12 months post-treatment, the groups did not statistically differ on these 12-Step participation variables (p>.11).
Substance Use Disorders
At intake, the entire sample met criteria for dependence on at least one substance. At six months post-treatment, 73% of TSF+P and 73% of ICBT+P no longer met criteria for current alcohol or drug dependence (FE, p=.55), whereas by one year post-treatment, TSF+P rates dropped to 65% with no diagnosis but improved to 78% of ICBT+P without dependence (FE, p=.056).
Addiction Severity
ASI medical, legal, and psychological scales did not differ significantly by treatment type during treatment or follow-up, after controlling for intake severity (ANOVAs, p’s>0.06). The employment scale was significantly lower in ICBT+P during both periods (p=.0006), where adjusted means in ICBT+P were 0.10 (95% CI=.06–.14) points lower than TSF+P.
The higher of the ASI drug or alcohol severity pretreatment scores were entered into the PDA and HDRS trajectories models to assess the impact of initial severity on outcomes. Severity did not significantly relate to PDA trajectories (LR χ2 (6) =5.26, p=.51) or HDRS trajectories (LR χ2 (6) =4.06, p=.67).
Depression
At six months post-treatment, 52% of both TSF+P and ICBT+P participants had HDRS scores in the moderate to severe range (HDRS≥20; FE, p=1.0), and at one year post-treatment, 54% of TSF+P and 57% of ICBT+P had HDRS scores in this range (FE, p=.86). While four out of five participants met DSM diagnostic criteria for current depressive disorder at intake (Table 4), both groups had substantially reduced rates at follow up (TSF+P = 42% and ICBT+P = 36% at one year post-treatment).
Table 4.
Current diagnostic rates at intake, 6 months post-treatment, and 12 months post-treatment.
|
Intake |
6 mo. Post-tx | 12 mo. Post-tx | ||||
|---|---|---|---|---|---|---|
| TSF+P | ICBT+P | TSF+P | ICBT+P | TSF+P | ICBT+P | |
| Alcohol Dependence | 56% (46,67) | 74% (65,82) | 23% (14,35) | 27% (18,37) | 25% (16,37) | 20% (12,30) |
| Marijuana Dependence | 14% (8,23) | 10% (5,17) | 0% (0,6) | 1.5% (0,8) | 3.2% (0,11) | 1.7% (0,9) |
| Stimulant Dependence | 33% (24,44) | 32% (23,42) | 16% (8,27) | 9% (3,19) | 26%* (16,38) | 8%* (3,19) |
| Depression | 71%* (62,80) | 88%* (82,94) | 49% (38,61) | 46% (36,57) | 42% (31,54) | 36% (25,46) |
| Depression Exclusive of Substance Use | 40% (30,49) | 42% (33,52) | 8% (3,16) | 10% (5,18) | 4% (1,10) | 4% (1,10) |
Note: 95% confidence intervals in parentheses.
Significant at p<.02
For all other comparisons, the relationships between treatment type and diagnosis are non-significant at each of the time points (FE, p-values > .06).
Discussion
In the present study, we compared longitudinal patterns of clinical outcomes of two forms of psychotherapy for adults with comorbid depression and substance use disorders: Integrated Cognitive Behavioral Therapy (ICBT+P) and Twelve Step Facilitation (TSF+P). ICBT+P was associated with superior substance use outcomes compared to TSF+P at 18 months after treatment entry. Participants in the ICBT+P condition had greater reductions in the frequency of drug and alcohol use over time compared to TSF+P participants. Of note, reductions in drinking and drug use remained relatively stable in the year following ICBT+P treatment whereas the frequency of use increased following TSF+P treatment. These group differences in use patterns are consistent when drug and alcohol involvement is modeled separately. Additionally, clinical relevance of these findings is demonstrated by a lower proportion of ICBT+P participants meeting DSM criteria for current alcohol or drug dependence at one year post-treatment. These superior substance use outcomes for ICBT+P are consistent with shorter term outcomes from our previous paper (Brown et al., 2006). Our findings are also consistent with a prior study that provided a cognitive-behavioral treatment for depression (CBT-D) as an adjunct to partial hospital alcohol treatment for individuals with elevated depression symptoms (Brown, Evans, Miller, Burgess, & Mueller, 1997). CBT-D was compared to a relaxation training control condition, and similar to our findings, more stability in percentage days abstinent was observed in the CBT-D condition in the six months following treatment, whereas percentage days abstinent decreased significantly in the relaxation condition. These findings suggest that skills acquired in ICBT+P may promote continued utilization of coping skills for managing potential substance use situations. Importantly, the group difference in alcohol/use trajectories became increasingly pronounced in the year following treatment completion. Without the professional support provided during active treatment, TSF+P participants may not fare as well as their ICBT+P counterparts who were taught skills to manage high risk situations related to negative affective states common in such comorbid samples.
Early treatment response in our study also significantly predicted long-term substance use outcomes. Poor initial addiction treatment response was associated with improvement during and immediately after treatment, but was followed by increases in substance use following treatment. However, trajectories of poor initial responders never achieved abstinence levels as high as those of typical and good early responders. These results suggest the need for additional therapeutic efforts for the dually diagnosed with poor initial treatment response. For example, the addition of a motivational enhancement component to therapy for these individuals may increase readiness to change substance behaviors. Alternatively, a focus on the environmental and social context of individuals with early poor response may be productive (Tate et al., 2008).
Individuals with good initial treatment response demonstrated markedly different substance use outcomes over time as a function of the type of treatment received. ICBT+P good early responders showed decreases in frequency of substance use that remained more stable over time, whereas good early responders in TSF+P increased use in the months following treatment completion. Similarly, initial levels of depressive symptomology had a complex, curvilinear predictive relationship with long-term treatment outcomes for depression, and these trajectories were moderated by both initial symptom level and treatment attendance. Across treatment groups, participants with higher levels of depression at intake had pronounced drops in depressive symptoms over time. For participants with elevated early depression and high treatment attendance, depression scores drop initially then start to increase in the 6–12 months after treatment.
Consistent with hypotheses, greater treatment exposure was associated with decreases in frequency of substance use and reduced depressive symptoms over time for both treatments. These findings are consistent with substantial research documenting an association between treatment attendance and better post-treatment outcomes (e.g., Stark, 1992; Simpson, Joe, Rowan-Szal, 1997). Thus, efforts to improve treatment retention for depressed and substance dependent patients deserve attention. Such efforts may be particularly important in comorbid samples, as higher levels of depressive symptoms have predicted shorter addiction treatment stays (Brown et el., 1998). Findings of one prior study suggest a potential avenue for increasing treatment retention: the addition of supplemental case management was shown to reduce treatment dropout rates for cognitive behavioral therapy for depression (Miranda et al., 2003).
Depressive symptoms and diagnoses improved for dually diagnosed participants receiving both forms of behavioral intervention accompanied by pharmacotherapy. Inconsistent with our hypotheses, ICBT+P was not associated with superior improvements in depression compared to TSF+P. Although participants in both treatment conditions experienced decreases in depressive symptomology over time, improvements for ICBT+P were slightly more modest than those for TSF+P. Of note, ICBT+P participants had significantly lower rates of pharmacotherapy in the second phase of treatment and in the three months post-treatment. Group trajectory differences were not associated with clinically significant differences in depressive symptoms. Specifically, TSF+P and ICBT+P were similar in proportion of participants exceeding clinical cutoffs for moderate to severe depression symptoms and proportion meeting criteria for depressive episode at one year post-treatment. These findings are not wholly consistent with those of our preliminary study (Brown et al., 2006). Although both studies found a pattern of a symptom drop followed by increases in depression outcomes for TSF+P, with a larger sample over longer periods of time we found depression symptoms to be higher for ICBT+P than TSF+P. Our findings are also not consistent with the Brown and colleagues (1997) study mentioned previously that compared CBT-D to a relaxation training control as adjuncts to alcohol treatment. In that study, CBT-D participants had greater decreases in depression symptoms assessed at the end of a partial day treatment program compared to those in the relaxation condition. In addition to the differences in assessment timing, the study sample also differed from our sample in that alcohol dependent individuals who had “elevated depression symptoms” (BDI≥ 10) were included without the requirement of meeting diagnostic criteria for major depressive disorder. In fact, “only 1 participant met criterion for major depressive episode when the organic rule-out was applied (i.e., during a period while not drinking)” (Brown et al., 1997, p. 718). Thus, sample diagnostic criteria and assessment timing were substantially different from our study. The absence of superior depression outcomes for ICBT+P in the current study may reflect the fact that both treatment groups were comprised of chronic and severe dually diagnosed individuals. In applied settings, TSF+P groups also include patients with only a SUD. Perhaps the presence of other dually diagnosed participants contributed to improved depression outcomes for TSF+P participants, despite the manualized treatment’s avoidance of detailed depression-related discussions. Shared structural characteristics (e.g., group context, support, and discussions) and pharmacotherapy may have contributed to improvements for all participants. Additionally, the behavioral activation and social interactions inherent in the 12-step model (attending meetings, having a sponsor, 12 step social activities) may have contributed to TSF+P’s reduction in depressive symptoms. Community 12-Step involvement was higher for TSF+P participants than ICBT+P participants during treatment, but the two groups had similar rates of involvement in the year following treatment completion. However, as noted by Kelly et al. (2003), 12-Step involvement is not sufficient to address the depression symptoms of dually diagnosed patients. Future research should investigate mechanisms of change that contribute to long-term decreases in depression within ICBT+P and TSF+P.
Additionally, future studies should explore the relationship between substance use treatment outcomes and depressive symptoms. Numerous models have been proposed that link substance use with a variety of mental health symptoms (e.g., self-medication hypothesis whereby substance use is proposed to lessen symptoms: Khantzian, 1985; rebound hypothesis whereby substance use is proposed to worsen symptoms: Blume et al., 2000; relapse prevention model whereby negative affective states represent high risk situations for substance relapse: Marlatt, 1985). While evaluation of these substance use-symptom linkages is beyond the scope of the current investigation, future studies exploring the bi-directional, dynamic links between depression and substance use are critical. Additionally, future research could benefit from considering length and phase of depressive episode at treatment entry.
As noted above, TSF+P groups were composed solely of individuals with concomitant depression and addictions, and thus differed from usual 12-Step community meetings and 12-Step approaches often administered in treatment settings. In evaluating our findings, it is also important to consider other differences between our TSF+P intervention and those typically seen in community settings. Our TSF+P intervention provides a standardized presentation of specific elements of 12-Step principles with the goal of facilitating connection to widely available community resources supportive of abstinence. Our intervention was professionally led, manualized, and delivered in a VA hospital setting whereas 12-Step meetings are peer led, vary significantly in format and content, and are delivered in a variety of settings. Thus, caution is warranted in generalizing our findings to other 12-Step interventions in clinical and community settings.
Interpretation of the study’s results may be affected by several methodological limitations. The present sample was primarily male, comprised exclusively of veterans, and requires replication with women and non-veteran populations. Additionally, a slightly larger proportion of ICBT+P participants had completed an inpatient substance use program prior to treatment entry. The present study focused on the effectiveness of psychotherapy interventions, so while all participants received standard psychiatric medications and care, we did not monitor medication compliance or other factors related to psychopharmacology efficacy. Additionally, the study’s notable attrition rates, while consistent with multiply diagnosed addiction treatment in general, limit the interpretation of study results. Finally, cohort designs present unique analytic challenges (Morgan-Lopez and Fals-Stewart, 2006), and lack of direct quantitative assessment of cohort effects is an additional study limitation.
In summary, findings from the present clinical trial suggest that substance dependent individuals with comorbid depressive disorder receiving either Integrated Cognitive Behavioral Therapy (ICBT+P) or Twelve Step Facilitation therapy (TSF+P) show improvements in substance involvement and depressive symptomology. Substance-dependent depressed veterans who received ICBT+P demonstrated sustained decreases in substance involvement up to one year post-treatment. Additionally, the current study highlights the relationship between treatment attendance and early response to treatment for dual diagnosis populations.
Acknowledgements
This research was supported by a VA Medical Research Merit Review Grant awarded to Dr. Sandra A. Brown and VA Merit Review Entry Program Grant awarded to Dr. Susan Tate.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/adb
References
- Alterman AI, Brown LS, Zaballero A, McKay JR. Interviewer severity ratings and composite scores of the ASI– a further look. Drug and Alcohol Dependence. 1994;34:201–209. doi: 10.1016/0376-8716(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Blume AW, Schmaling KB, Marlatt GA. Revisiting the self-medication hypothesis from a behavioral perspective. Cognitive and Behavioral Practice. 2000;7:379–384. [Google Scholar]
- Brown RA, Evans M, Miller IW, Burgess ES, Mueller TI. Cognitive-behavioral treatment for depression in alcoholism. Journal of Consulting and Clinical Psychology. 1997;65:715–726. doi: 10.1037//0022-006x.65.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, D’Amico EJ. Outcomes for alcohol treatment for adolescents. In: Galanter M, editor. Recent developments on alcoholism: Vol. XVI. Selected treatment topics. New York: Plenum; 2001. pp. 307–327. [DOI] [PubMed] [Google Scholar]
- Brown SA, Glasner-Edwards SV, Tate SR, McQuaid JR, Chalekian MS, Granholm E. Integrated cognitive behavioral therapy versus twelve-step facilitation therapy for substance dependent adults with depressive disorders. Journal of Psychoactive Drugs. 2006;38:449–460. doi: 10.1080/02791072.2006.10400584. [DOI] [PubMed] [Google Scholar]
- Brown RA, Monti PM, Myers MG, Martin RA, Rivinus T, Dubreuil ME, Rohsenow DJ. Depression among cocaine abusers in treatment: Relation to cocaine and alcohol use and treatment outcome. American Journal of Psychiatry. 1998;155(2):220–225. doi: 10.1176/ajp.155.2.220. [DOI] [PubMed] [Google Scholar]
- Burnham K, Anderson D. Model selection and multimodel inference: A practical information-theoretic approach. second edition. New York: Springer Science and Business. Media, Inc; 2002. [Google Scholar]
- Dodge R, Sindelar J, Sinha R. The role of depression symptoms in predicting drug abstinence in outpatient substance abuse treatment. Journal of Substance Abuse Treatment. 2005;28:189–196. doi: 10.1016/j.jsat.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Drake RE, Mueser KT, Clark RE, Wallach MA. The course, treatment, and outcome of substance disorders in persons with severe mental illness. American Journal of Orthopsychiatry. 1996;66:42–51. doi: 10.1037/h0080153. [DOI] [PubMed] [Google Scholar]
- Drapkin ML, Tate SR, McQuaid JR, Brown SA. Does Initial Treatment Focus Influence Outcomes for Depressed Substance Abusers? Journal of Substance Abuse Treatment. 2008 doi: 10.1016/j.jsat.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ. Reliability and validity of 6-month timeline reports of cocaine and heroin use in a methadone population. Journal of Consulting & Clinical Psychology. 1994;62:843–850. doi: 10.1037//0022-006x.62.4.843. [DOI] [PubMed] [Google Scholar]
- Fals-Stewart W, O’Farrell T, Freitas T, McFarlin S, Rutigliano P. The Timeline Followback reports of psychoactive substance use by drug-abusing patients: Psychometric properties. Journal of Consulting & Clinical Psychology. 2000;68:134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- Frees EW. Longitudinal and Panel Data: Analysis and Applications in the Social Sciences. New York: Cambridge University Press; 2004. [Google Scholar]
- Grant BF. Comorbidity between DSM-IV drug use disorders and major depression: Results of a national survey of adults. Journal of Substance Abuse. 1995;7:481–497. doi: 10.1016/0899-3289(95)90017-9. [DOI] [PubMed] [Google Scholar]
- Grant BF, Harford TC. Comorbidity between DSM-IV alcohol use disorders and major depression: Results of a national survey. Drug & Alcohol Dependence. 1995;39:197–206. doi: 10.1016/0376-8716(95)01160-4. [DOI] [PubMed] [Google Scholar]
- Greenfield S, Weiss R, Muenz L, Vagge L, Kelly J, Bello L, Michael J. The effect of depression on return to drinking: A prospective study. Archives of General Psychiatry. 1998;55:259–265. doi: 10.1001/archpsyc.55.3.259. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, & Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin D, Liu X, Nunes E, McCloud S, Samet S, Endicott J. Effects of major depression on remission and relapse of substance dependence. Arch Gen Psychiatry. 2002;59:375–380. doi: 10.1001/archpsyc.59.4.375. [DOI] [PubMed] [Google Scholar]
- Hedeker D, Gibbons RD. Longitudinal Data Analysis. Hoboken, New Jersey: Wiley & Sons, Inc.; 2006. [Google Scholar]
- Hellerstein DJ, Rosenthal RN, Miner CR. A prospective study of integrated outpatient treatment for substance-abusing schizophrenic patients. The American Journal on Addictions. 1995;4:33–42. [Google Scholar]
- Hesselbrock V, Meyer R, Hesselbrock M. Psychopathology and addictive disorders: The specific case of antisocial personality disorder. In: O’Brien CP, Jaffe JH, editors. Addictive States. New York: Raven Press; 1992. pp. 179–191. [PubMed] [Google Scholar]
- Hoff RA, Rosenheck RA. The cost of treating substance abuse patients with and without comorbid psychiatric disorders. Psychiatric Services. 1999;50:1309–1315. doi: 10.1176/ps.50.10.1309. [DOI] [PubMed] [Google Scholar]
- Humphreys K, Kaskutas LA, Weisner C. The Alcoholics Anonymous Affiliation Scale: Development, reliability, and norms for diverse treated and untreated populations. Alcoholism: Clinical and Experimental Research. 1998;22:974–978. doi: 10.1111/j.1530-0277.1998.tb03691.x. [DOI] [PubMed] [Google Scholar]
- Kadden K, Carrol K, Donovan D, Cooney N, Monti P, Abrams D, Litt M, Hester R. Cognitive-Behavioral Coping Skills Therapy Manual. 1994 Project Match, NIAAA, NIH, 94–3724. [Google Scholar]
- Kelly JF, McKellar JD, Moos R. Major depression in patients with substance use disorders: Relationship to 12-Step self-help involvement and substance use outcomes. Addiction. 2003;98:499–508. doi: 10.1046/j.1360-0443.2003.t01-1-00294.x. [DOI] [PubMed] [Google Scholar]
- Kessler R, Berglund P, Demler O, Jin R, Walters E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler R, Chiu W, Demler O, Walters E. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey replication. Archives of General Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. American Journal of Psychiatry. 1985;142(11):1259–1264. doi: 10.1176/ajp.142.11.1259. [DOI] [PubMed] [Google Scholar]
- Kodl M, Fu S, Willenbring M, Gravely A, Nelson D, Joseph A. The impact of depressive symptoms on alcohol and cigarette consumption following treatment for alcohol and nicotine dependence. Alcoholism: Clinical and Experimental Research. 2008;32(1):92–99. doi: 10.1111/j.1530-0277.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- Kuha J. AIC and BIC: Comparisons of assumptions and performance. Sociological Methods & Research. 2004;33:188–229. [Google Scholar]
- Kushner M, Donahue C, Sletten S, Thuras P, Abrams K, Peterson J, Frye B. Cognitive behavioral treatment of comorbid anxiety disorder in alcoholism treatment patients: Presentation of a prototype program and future directions. Journal of Mental Health. 2006;15:697–707. [Google Scholar]
- Maisto SA, Sobell LC, Sobell MB. Comparison of alcoholics’ self-reports of drinking behavior with reports of collateral informants. Journal of Consulting & Clinical Psychology. 1979;47:106–122. [PubMed] [Google Scholar]
- Marlatt GA. Relapse prevention: Theoretical rationale and overview of the model. In: Marlatt GA, Gordon JR, editors. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors. New York: Guilford Press; 1985. [Google Scholar]
- McKay JR, Pettinati HM, Morrison R, Feeley M, Mulvaney FD, Gallop R. Relation of depression diagnoses to 2-year outcomes in cocaine-dependent patients in a randomized continuing care study. Psychology of Addictive Behaviors. 2002;16:225–235. [PubMed] [Google Scholar]
- McLellan AT, Parikh G, Braff A. Addiction Severity Index Administration Manual. 5th edition. Pennsylvania Veterans: Administration Center for Studies of Addiction; 1990. [Google Scholar]
- Miller WR, Longabaugh R. Summary and conclusions. In: Babor TF, Del Boca FK, editors. Treatment matching in alcoholism. New York: Cambridge University Press; [Google Scholar]
- Miranda J, Azocar F, Organista KC, Dwyer E, Areane P. Treatment of depression among impoverished primary care patients from ethnic minority groups. Psychiatric Services. 2003;54:219–225. doi: 10.1176/appi.ps.54.2.219. [DOI] [PubMed] [Google Scholar]
- Morgan-Lopez AA, Fals-Stewart W. Analytic complexities associated with group therapy in substance abuse treatment research: Problems, recommendations, and future directions. Experimental and Clinical Psychopharmacology. 2006;14:265–273. doi: 10.1037/1064-1297.14.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey JP, Jackson EW, Ellis AR, Amaro H, Brown VB, Najavits LM. Twelve-month outcomes of trauma-informed interventions for women with co-occurring disorders. Psychiatric Services. 2005;56:1213–1222. doi: 10.1176/appi.ps.56.10.1213. [DOI] [PubMed] [Google Scholar]
- Munoz RF, Ying Y, Perez-Stable EJ, Miranda J. The prevention of depression: Research and practice. Baltimore, MD: John Hopkins University Press; 1993. [Google Scholar]
- Nowinski J, Baker S, Carroll K. Washington, DC: Supt. of Docs., U.S. Govt. Print. Off.; 1994. Twelve-Step Facilitation Therapy Manual: A Clinical Research Guide for Therapists Treating Individuals With Alcohol Abuse and Dependence. National Institute on Alcohol Abuse and Alcoholism, Project MATCH Monograph series, Vol. 1. NIH Publication No. 94 – 3722. [Google Scholar]
- Nunes E, Levin F. Treatment of depression in patients with alcohol or other drug dependence. JAMA. 2004;291(15):1887–1896. doi: 10.1001/jama.291.15.1887. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Huber A, McCann M, Shoptaw S, Farabee D, Reiber C, Ling W. A comparison of contingency management and cognitive-behavioral approaches during methadone maintenance treatment for cocaine dependence. Archives of General Psychiatry. 2002;59:817–824. doi: 10.1001/archpsyc.59.9.817. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Journal of the American Medical Association. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, Farmer A, Jablenski A, Pickens R, Regier DA, Sartorius N, Towle L, et al. The Composite International Diagnostic Interview. An epidemiologic instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Archives of General Psychiatry. 1988;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- Sall J, Lehman A, Creighton L. JMP Start Statistics: A Guide to Statistics and Data Analysis. Pacific Grove, CA: Duxbury; 2001. [Google Scholar]
- Simpson DD, Joe GW, Rowan-Szal GA. Drug abuse treatment retention and process effects on follow-up outcomes. Drug and Alcohol Dependence. 1997;47:227–235. doi: 10.1016/s0376-8716(97)00099-9. [DOI] [PubMed] [Google Scholar]
- Singer J, Willett J. Applied longitudinal data analysis: Modeling change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- Smith ML, Glass GV. Meta-analysis of psychotherapy outcome studies. American Psychologist. 1977;32:752–760. doi: 10.1037//0003-066x.32.9.752. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-Back: A technique for assessing self-reported alcohol consumption. In: Litten RL, Allen JP, editors. Measuring alcohol consumption, psychosocial and biochemical methods. Totowa, New Jersey: Humana Press; 1992. [Google Scholar]
- Stark MJ. Dropping out of substance abuse treatment: A clinically oriented review. Clinical Psychology Review. 1992;12:93–116. [Google Scholar]
- Stata Corp. Stata Statistical Software: Release 9.2. College Station, TX: Stata Corporation; 2005. [Google Scholar]
- Tate SR, Wu J, McQuaid JR, Cummins K, Shriver C, Krenek M, Brown SA. Substance dependence and depression comorbidity: Role of self-efficacy and life stress in sustaining abstinence. Psychology of Addictive Behaviors. 2008;22:47–57. doi: 10.1037/0893-164X.22.1.47. [DOI] [PubMed] [Google Scholar]
- West BT, Welch KB, Galecki AT. Linear mixed models: A practical guide to using statistical software. Boca Raton, FL: Chapman & Hall; 2007. [Google Scholar]
- Willenbring ML. Measurement of depression in alcoholics. Journal of Studies on Alcohol. 1986;47:367–372. doi: 10.15288/jsa.1986.47.367. [DOI] [PubMed] [Google Scholar]