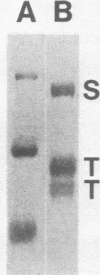
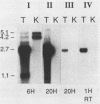
Abstract
Angiotensin-converting enzyme (ACE) is a zinc-containing dipeptidyl carboxypeptidase that catalyzes the conversion of angiotensin I to the potent vasoconstrictor angiotensin II. By analyzing cDNA and genomic DNA, we have constructed a consensus sequence encoding the testis isozyme of mouse ACE. Testis ACE cDNA contains 2,435 base pairs and encodes a protein of 732 amino acids. The N-terminal 66 amino acids are unique to the testis isozyme, while the remaining 666 are identical to the carboxyl half of mouse somatic ACE. The overall conservation of amino acid sequence between the testis isozymes of the mouse, rabbit, and human is 78 to 84%. The conservation of amino acids for the N-terminal domain uniquely expressed within the testis is 63 to 67% between these species. Primer extension and RNase protection experiments show that RNA transcription of the testis ACE isozyme begins 16 or 17 bases upstream from the translation start site. A sequence element resembling a TATA box is found 25 bases 5' of the transcription start site. To create its unique isozyme of ACE, the testis begins mRNA transcription in the middle of the exonic-intronic structure of somatic ACE, within a sequence treated as an intron by somatic tissues. Testis ACE is not the result of alternative RNA splicing but seems due to the start of transcription at a unique site within the ACE gene.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg T., Sulner J., Lai C. Y., Soffer R. L. Immunohistochemical localization of two angiotensin I-converting isoenzymes in the reproductive tract of the male rabbit. J Histochem Cytochem. 1986 Jun;34(6):753–760. doi: 10.1177/34.6.3009604. [DOI] [PubMed] [Google Scholar]
- Bernstein K. E., Martin B. M., Bernstein E. A., Linton J., Striker L., Striker G. The isolation of angiotensin-converting enzyme cDNA. J Biol Chem. 1988 Aug 15;263(23):11021–11024. [PubMed] [Google Scholar]
- Bernstein K. E., Martin B. M., Edwards A. S., Bernstein E. A. Mouse angiotensin-converting enzyme is a protein composed of two homologous domains. J Biol Chem. 1989 Jul 15;264(20):11945–11951. [PubMed] [Google Scholar]
- Bernstein K. E., Martin B. M., Striker L., Striker G. Partial protein sequence of mouse and bovine kidney angiotensin converting enzyme. Kidney Int. 1988 Mar;33(3):652–655. doi: 10.1038/ki.1988.48. [DOI] [PubMed] [Google Scholar]
- Cushman D. W., Cheung H. S. Concentrations of angiotensin-converting enzyme in tissues of the rat. Biochim Biophys Acta. 1971 Oct;250(1):261–265. doi: 10.1016/0005-2744(71)90142-2. [DOI] [PubMed] [Google Scholar]
- Dobner P. R., Kislauskis E., Wentworth B. M., Villa-Komaroff L. Alternative 5' exons either provide or deny an initiator methionine codon to the same alpha-tubulin coding region. Nucleic Acids Res. 1987 Jan 12;15(1):199–218. doi: 10.1093/nar/15.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll D. M., Wynne J. K., Wallis S. C., Scott J. An in vitro system for the editing of apolipoprotein B mRNA. Cell. 1989 Aug 11;58(3):519–525. doi: 10.1016/0092-8674(89)90432-7. [DOI] [PubMed] [Google Scholar]
- Ehlers M. R., Fox E. A., Strydom D. J., Riordan J. F. Molecular cloning of human testicular angiotensin-converting enzyme: the testis isozyme is identical to the C-terminal half of endothelial angiotensin-converting enzyme. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7741–7745. doi: 10.1073/pnas.86.20.7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers M. R., Riordan J. F. Angiotensin-converting enzyme: new concepts concerning its biological role. Biochemistry. 1989 Jun 27;28(13):5311–5318. doi: 10.1021/bi00439a001. [DOI] [PubMed] [Google Scholar]
- El-Dorry H. A., Bull H. G., Iwata K., Thornberry N. A., Cordes E. H., Soffer R. L. Molecular and catalytic properties of rabbit testicular dipeptidyl carboxypeptidase. J Biol Chem. 1982 Dec 10;257(23):14128–14133. [PubMed] [Google Scholar]
- El-Dorry H. A., Pickett C. B., MacGregor J. S., Soffer R. L. Tissue-specific expression of mRNAs for dipeptidyl carboxypeptidase isoenzymes. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4295–4297. doi: 10.1073/pnas.79.14.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdös E. G. Angiotensin I converting enzyme. Circ Res. 1975 Feb;36(2):247–255. doi: 10.1161/01.res.36.2.247. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gold B., Fujimoto H., Kramer J. M., Erickson R. P., Hecht N. B. Haploid accumulation and translational control of phosphoglycerate kinase-2 messenger RNA during mouse spermatogenesis. Dev Biol. 1983 Aug;98(2):392–399. doi: 10.1016/0012-1606(83)90368-8. [DOI] [PubMed] [Google Scholar]
- Heindell H. C., Liu A., Paddock G. V., Studnicka G. M., Salser W. A. The primary sequence of rabbit alpha-globin mRNA. Cell. 1978 Sep;15(1):43–54. doi: 10.1016/0092-8674(78)90081-8. [DOI] [PubMed] [Google Scholar]
- Iatrou K., Dixon G. H. Protamine messenger RNA: its life history during spermatogenesis in rainbow trout. Fed Proc. 1978 Sep;37(11):2526–2533. [PubMed] [Google Scholar]
- Iwata K., Blacher R., Soffer R. L., Lai C. Y. Rabbit pulmonary angiotensin-converting enzyme: the NH2-terminal fragment with enzymatic activity and its formation from the native enzyme by NH4OH treatment. Arch Biochem Biophys. 1983 Nov;227(1):188–201. doi: 10.1016/0003-9861(83)90362-4. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug M. S., Berger S. L. First-strand cDNA synthesis primed with oligo(dT). Methods Enzymol. 1987;152:316–325. doi: 10.1016/0076-6879(87)52036-5. [DOI] [PubMed] [Google Scholar]
- Kumar R. S., Kusari J., Roy S. N., Soffer R. L., Sen G. C. Structure of testicular angiotensin-converting enzyme. A segmental mosaic isozyme. J Biol Chem. 1989 Oct 5;264(28):16754–16758. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lattion A. L., Soubrier F., Allegrini J., Hubert C., Corvol P., Alhenc-Gelas F. The testicular transcript of the angiotensin I-converting enzyme encodes for the ancestral, non-duplicated form of the enzyme. FEBS Lett. 1989 Jul 31;252(1-2):99–104. doi: 10.1016/0014-5793(89)80897-x. [DOI] [PubMed] [Google Scholar]
- Patchett A. A., Cordes E. H. The design and properties of N-carboxyalkyldipeptide inhibitors of angiotensin-converting enzyme. Adv Enzymol Relat Areas Mol Biol. 1985;57:1–84. doi: 10.1002/9780470123034.ch1. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shai S. Y., Langford K. G., Martin B. M., Bernstein K. E. Genomic DNA 5' to the mouse and human angiotensin-converting enzyme genes contains two distinct regions of conserved sequence. Biochem Biophys Res Commun. 1990 Mar 30;167(3):1128–1133. doi: 10.1016/0006-291x(90)90640-9. [DOI] [PubMed] [Google Scholar]
- Soubrier F., Alhenc-Gelas F., Hubert C., Allegrini J., John M., Tregear G., Corvol P. Two putative active centers in human angiotensin I-converting enzyme revealed by molecular cloning. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9386–9390. doi: 10.1073/pnas.85.24.9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter S. M., Snyder S. H. Angiotensin-converting enzyme in the male rat reproductive system: autoradiographic visualization with [3H]captopril. Endocrinology. 1984 Dec;115(6):2332–2341. doi: 10.1210/endo-115-6-2332. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. Y., Erdös E. G., Levin Y. A dipeptidyl carboxypeptidase that converts angiotensin I and inactivates bradykinin. Biochim Biophys Acta. 1970 Aug 21;214(2):374–376. doi: 10.1016/0005-2795(70)90017-6. [DOI] [PubMed] [Google Scholar]