Abstract
Rationale
Screening and treating newly arriving immigrants for latent tuberculosis infection (LTBI) in low-incidence countries could be promising to reduce the tuberculosis incidence among this population. The effectiveness of screening with the tuberculin skin test (TST) is unknown.
Objectives
To estimate the risk of progression to tuberculosis within two years after entry, stratified by TST result at entry.
Methods
In a case-base design, we determined the prevalence of TST positives (10 mm and 15 mm) among a representative cohort of immunocompetent immigrants (n = 643) aged ≥18 years who arrived between April 2009 and March 2011 in the Netherlands (base cohort). Immigrants who progressed to tuberculosis within two years after arrival in 2005, 2006 or 2007 were extracted from the Netherlands Tuberculosis Register (case source cohort). The prevalence of TST positives from the base cohort was projected on the case source cohort to estimate the risk of progression to active tuberculosis by using Bayesian analyses to adjust for the sensitivity of the TST and Poisson regression analyses to take into account the random error of the number of extracted cases.
Results
The prevalence of TST positives was 42% and 23% for a cut-off value of 10 mm and 15 mm, respectively. The overall risk of progression to tuberculosis if TST positive was 238 per 100,000 population (95% CI 151–343) and 295 per 100,000 population (95% CI 161–473) for a cut-off value of ≥10 mm and ≥15 mm, respectively. The corresponding risk for TST negatives was 19 (95% CI 0–59) and 58 (95% CI 25–103).
Conclusion
The TST has the discriminatory ability to differentiate between individuals at low and high risk of disease.
Introduction
In countries with a low-incidence of tuberculosis (TB), TB is primarily prevalent among first generation immigrants. In the Netherlands, 73% of the new patients with TB in 2010 were first generation immigrants, corresponding to an incidence of 45.6 per 100,000 persons, whereas this incidence was 1.6/100,000 for the native population [1]. Immigrants from high incidence countries have a high risk of developing TB in the first five years after immigration [2]. From the Netherlands Tuberculosis Register (NTR) it appeared that in 2010, 12% of the first generation immigrants with TB were diagnosed at entry, while another 25% were diagnosed with TB within 2.5 years after entry [1]. Furthermore, the majority of immigrants with TB had a unique strain of Mycobacterium TB (MTB), and the ones who belonged to a cluster (patients with identical DNA-fingerprint isolates) were often not epidemiologically linked [1]. These data suggest that immigrants acquire TB infection in their country of origin and that incident TB is a consequence of reactivation of latent TB infection (LTBI).
All immigrants aged >12 years from high–incidence countries intending to stay more than three months in the Netherlands, currently only undergo a mandatory screening for TB by chest x-ray (CXR) [3]. Immigrants are not screened for LTBI because the specificity of the tuberculin skin test (TST) is considered limited, due to cross-reactivity with bacille Calmette-Guérin (BCG) vaccination and environmental mycobacteria [4].
Interferon gamma release assays (IGRAs) were developed to improve the diagnosis of LTBI. These are based on identifying cellular production of interferon gamma (IFN-γ) in response to MTB specific antigens and do therefore not react on BCG and most environmental mycobactaria. One of those IGRAs is the QuantiFERON®-TB Gold In-Tube (QFT-GIT). In a recent study we have shown that immigrants with a positive QFT-GIT at entry had a considerable higher risk of progression to active TB within two years compared to immigrants with a negative QFT-GIT at entry [5]. This implies that targeted testing for LTBI, at least with the QFT-GIT, and subsequently offering preventive therapy, might contribute to decrease the incidence of TB.
The effectiveness of using the TST in screening immigrants for LTBI is still unknown since it has never been used in the Dutch setting. Several studies found that, among immigrant contacts of TB patients, the TST and QFT-GIT were both indicative in predicting the risk of developing TB [6], [7]. Kik et al. found a similar positive predictive value (PPV) for QFT-GIT and TST, irrespective whether a cut-off value for the TST of 10 mm or 15 mm was used. Also the negative predictive values of both tests were comparable [6]. The TST therefore might also have potential in screening immigrants for LTBI at entry, especially since the TST is relatively cheap, easy to administer, and extensively used in routine practice for many years. Whether the TST would be useful depends on its discriminatory ability in comparison with the QFT-GIT. The objective of this study was to predict the risk of progression to TB within two years after entry given the TST result at entry.
Materials and Methods
Ethics Statement
Ethical approval was obtained from the Netherlands Central Committee on Research Involving Subjects.
Design
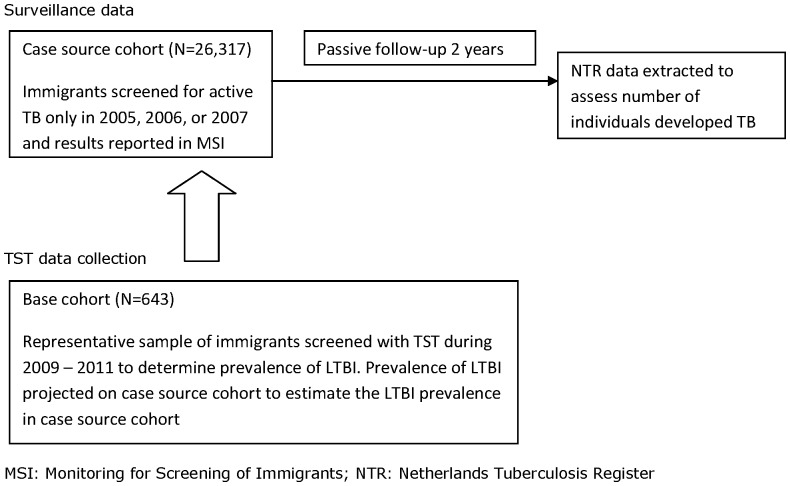
This study is a post-hoc analysis of data derived from the study assessing the relationship between QFT-GIT result and risk of active TB in immigrants within two years of time of entry screening [5]. A case-base design was used, also labelled as ‘case-cohort’ design [8]. A representative group of controls is selected from which the cases originated, regardless of future disease status. A case-base design was used, because in a prospective design we would have needed a very large sample to obtain accurate risk estimates given the relatively low risk of progression to active TB [9]. The prevalence of TST positives (≥10 mm and ≥15 mm) was assessed in a representative sample of newly arriving immigrants at seven Public Health Services (PHSs) between April 2009 and March 2011, denoted as ‘base cohort’ (Figure 1). We projected this prevalence on a cohort of immigrants who were screened for active TB at arrival in 2005, 2006, or 2007 at the same seven PHS and were registered in the Monitoring for Screening of Immigrants (MSI), denoted as ‘case source cohort’. From this cohort the immigrants who developed TB within two years after arrival were extracted, denoted as ‘cases’, by matching the MSI with the NTR by sex, country of birth and date of birth. The TST status of the case source cohort was therefore “assessed indirectly” by assuming a similar distribution of TST test results as directly assessed in the base cohort. As a matter of fact, since the immigrants from the case source cohort were not tested for LTBI at entry, none of them were offered a course of preventive therapy.
Figure 1. Schematic overview of study design.
All immigrants aged ≥18 years who visited the PHS for their entry screening, reported to be immunocompetent and provided written informed consent, were eligible for enrolment in the base cohort. We excluded immigrants who were diagnosed with active TB within six months after entry, because in the NTR these are reported as detected at entry screening and not during follow-up. Regarding the sample size, in our previous study 1570 immigrants consented. Out of these, 643 received a TST. The sample of 643 gave us enough power to find a prevalence of TST positives of 30% with a precision of 7%, and to do stratified analyses (sex, age, incidence in country of origin). The TST was performed according to the Mantoux method with 0.1 ml purified protein derivative RT23 (Statens Serum Institute, Copenhagen, Denmark). Experienced medical staff read the induration in millimetres after 48–120 hours (69% of the TST-results was read within 72 hours, 30% was read hereafter; the prevalence positive TST-results was similar for both reading times).
Statistical Analyses
To calculate the risk of progression to active TB per 100,000 population we used a Bayesian approach with non-informative priors. The numerator (number of cases identified from the case source cohort) was modelled using the Poisson distribution to allow for uncertainties in the number of identified patients. The denominator (number of immigrants with positive/negative TST) took into account differences in sensitivity of the TST reported in the published data [6], [10], [11], [12], [13], [14]. The Bayesian model provided a posterior distribution for the risk of progression to active TB from which 20,000 random samples were drawn to arrive at a point estimate and 95% credibility interval (CI). We estimated the risk stratified by sex, age and incidence in country of origin (see Text S1 for more details).
The risk estimates of progression to active TB enabled us to calculate the numbers needed to treat (NNT), and the numbers needed to screen (NNS) to prevent one TB patient within two years, for TST ≥10 mm and TST ≥15 mm. We assumed a 60% efficacy rate of the preventive treatment [15].
Eligible patients could have 1 of 3 reasons for not being included in the present study. First, they could not have consented to participate in the original study; second, they did participate but did not want to have a TST administered, or third, they had a TST administered but there was no available result (test failure or no return for test reading). Given the post-hoc analysis, there is a need to carefully check for selection bias at each of these instances. We therefore compared the baseline characteristics of the base cohort with the case source cohort, the non-consenters and the participants without a TST result by Pearson χ2. Baseline characteristics were sex, age (18–24 years, 25–34 years and ≥35 years), region of origin (Europe & the Americas, North Africa & Middle East, Asia, Sub-Saharan Africa, Unknown), incidence of TB in country of origin (<100 (low), 100–199 (intermediate) and ≥200 (high) per 100,000 population), BCG vaccination (yes if BCG-scar was present and/or participant indicated being vaccinated), previous diagnosis of TB (yes/no), smoking (yes if ever smoked/no) and time in the Netherlands before screening (yes if ≤3 months in the Netherlands before screening/no). Associations were considered statistically significant when p-values were ≤0.05. Statistical analyses were conducted using SPSS 17.0 (Chicago, IL, USA) and WinBUGS 1.4.3 (Imperial College and MRC, UK).
Results
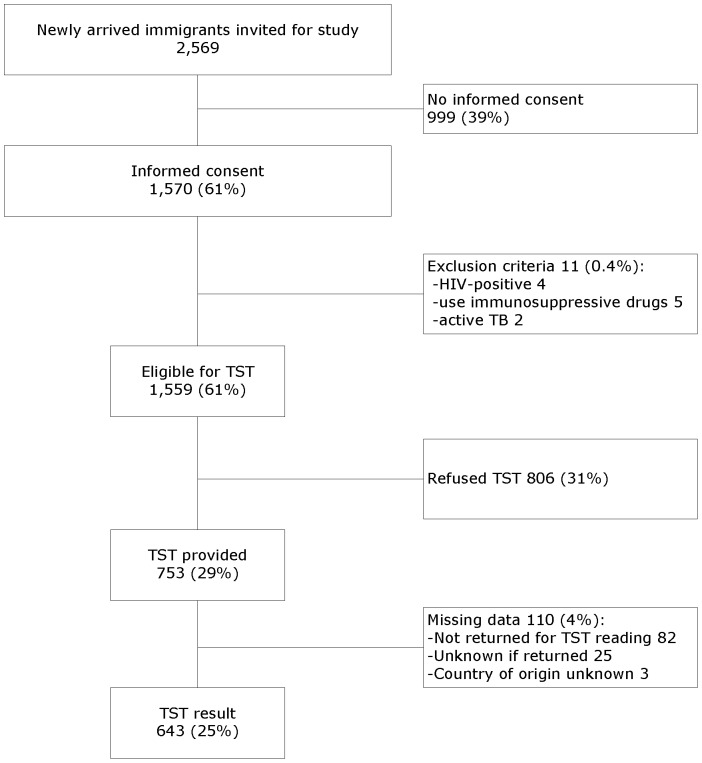
During the study period, 2,569 immigrants were approached for study enrolment at the seven PHSs. Out of 1,570 consenters, 1,559 were eligible for TST testing since 11 were excluded because they were HIV-positive (n = 4), used immunosuppressives (n = 5), or were diagnosed with active TB within 6 months (n = 2) (Figure 2). In 753 (48.3%) immigrants, out of those eligible, a TST was administered because others refused the TST due to a lack of time for the second visit. Out of these, 82 did not return for the reading of the TST, and in 25 the TST result was unknown because it was not registered. Three participants with no data on country of origin were excluded from all analyses, resulting in 643 (41%) immigrants with a TST result. The consenters were significantly more often from high-incidence countries than non-consenters (20% versus 14%, data not shown). Compared to the case source cohort, there were significantly more females (57% versus 52%), more immigrants from Asia (45% versus 41%), and more immigrants from high-incidence countries (23% versus 19%) in the base cohort (Table 1). The immigrants in the base cohort were significantly more often BCG-vaccinated (85% versus 76%) and from high incidence countries (23% versus 18%) than the immigrants without a TST result. Although the base cohort and the case source cohort differed in demographics a bit, we consider the base cohort representative for the case source cohort.
Figure 2. Flow diagram of study participants.
Table 1. Baseline characteristics for base cohort, case source cohort and participants without TST result.
| Base cohort† | Case source cohort* | Base cohort compared with case source cohort‡ | No TST result | Base cohort compared with no TST result‡ | |
| N (%) | N (%) | P-value | N (%) | P-value | |
| Total | 643 (100%) | 26,317 (100%) | – | 913 (100%) | – |
| Sex | |||||
| Female | 370 (57%) | 13,766 (52%) | 0.020 | 488 (54%) | 0.110 |
| Male | 273 (43%) | 12,504 (48%) | 425 (47%) | ||
| Unknown | – | 47 (0.2%) | – | ||
| Age | |||||
| 18 to 24 years | 180 (28%) | 7,877 (30%) | 0.173 | 284 (31%) | 0.072 |
| 25 to 34 years | 306 (48%) | 12,797 (49%) | 449 (49%) | ||
| ≥35 years | 157 (24%) | 5,643 (21%) | 180 (20%) | ||
| Region of birth | |||||
| Europe & the Americas | 156 (24%) | 7,647 (29%) | 0.014 | 245 (27%) | 0.048 |
| North Africa & Middle East | 93 (15%) | 3,680 (14%) | 133 (15%) | ||
| Asia | 291 (45%) | 10,849 (41%) | 428 (47%) | ||
| Sub-Saharan Africa | 103 (16%) | 3,894 (15%) | 102 (11%) | ||
| Unknown | – | 247 (1%) | 5 (0.5%) | ||
| Incidence in country of origin | |||||
| 0–99 | 302 (47%) | 13,799 (52%) | 0.001 | 470 (52%) | 0.040 |
| 100–199 | 195 (30%) | 7,231 (28%) | 277 (30%) | ||
| ≥200 | 146 (23%) | 5,040 (19%) | 161 (18%) | ||
| Unknown | – | 247 (1%) | 5 (0.5%) | ||
| BCG vaccination | |||||
| Yes | 549 (85%) | – | – | 695 (76%) | <0.001 |
| No | 94 (15%) | – | 59 (7%) | ||
| Unknown | – | 159 (18%) | |||
| Previous diagnose of TB | |||||
| Yes | 7 (1%) | – | – | 12 (1%) | 0.881 |
| No | 622 (97%) | – | 879 (96%) | ||
| Unknown | 14 (2%) | – | 22 (3%) | ||
| Ever smoked | |||||
| Yes | 190 (30%) | – | – | 262 (29%) | 0.390 |
| No | 450 (70%) | – | 641 (70%) | ||
| Unknown | 3 (0.5%) | – | 10 (1%) | ||
| Time in NL before screening ≤3 months | |||||
| Yes | 591 (92%) | – | – | 840 (92%) | 0.502 |
| No | 50 (9%) | – | 65 (7%) | ||
| Unknown | 2 (0.3%) | – | 8 (1%) | ||
BCG: bacille Calmette-Guérin; NL: the Netherlands, TST: tuberculin skin test.
Sample of newly arriving immigrants at seven Public Health Services (PHSs) between April 2009 and March 2011.
Three cohorts of immigrants who were screened at arrival in 2005, 2006 or 2007 and were registered in the Monitoring for Screening of Immigrants (MSI).
Chi2 test.
Overall, 273 out of 643 (42%) were TST positive when the cut-off value was set at 10 mm and 145 (23%) when the cut-off value was set at 15 mm (Table 2).
Table 2. Prevalence of TST positives for cut-off value ≥10 mm and ≥15 mm.
| Total | Number of TST positives if ≥10 mm(% of total) | Number of TST positives if ≥15 mm(% of total) | |
| Total | 643 | 273 (42) | 145 (23) |
| Sex | |||
| Female | 370 | 153 (41) | 83 (22) |
| Male | 273 | 120 (44) | 62 (23) |
| Age (yr) | |||
| 18–24 | 180 | 50 (28) | 20 (11) |
| 25–34 | 306 | 148 (48) | 86 (28) |
| ≥35 | 157 | 75 (48) | 39 (25) |
| Region of origin | |||
| Europe & Americas | 156 | 64 (41) | 30 (19) |
| North Africa & Middle East | 93 | 40 (43) | 25 (27) |
| Other Asia | 291 | 114 (39) | 55 (19) |
| Sub-Saharan Africa | 103 | 55 (53) | 35 (34) |
| Incidence in country of origin | |||
| <100 | 302 | 120 (40) | 58 (19) |
| 100–199 | 195 | 82 (42) | 44 (23) |
| ≥200 | 146 | 71 (49) | 43 (29) |
| BCG-vaccinated | |||
| Yes | 549 | 249 (45) | 128 (23) |
| No/unknown | 94 | 24 (26) | 17 (18) |
| Ever treated for TB | |||
| Yes | 7 | 5 (71) | 3 (43) |
| No/unknown | 636 | 268 (42) | 142 (22) |
| Ever smoked | |||
| Yes | 190 | 95 (50) | 45 (24) |
| No/unknown | 453 | 178 (39) | 100 (22) |
| ≤3 months in NL before screening | |||
| Yes | 591 | 255 (43) | 138 (23) |
| No/unknown | 52 | 18 (35) | 7 (14) |
BCG: bacille Calmette-Guérin; NL: the Netherlands; TST: tuberculin skin test.
In the case source cohort we identified 30 immigrants who developed active TB within two years. Ten cases were clustered, but none were epidemiologically linked to a TB patient in the Netherlands according to the registries. When the TST cut-off value was set at 10 mm, the expected number of TST positives in the case source cohort based on the prevalence found among the base cohort, was 11,173 (42%) (Table 3). The median sensitivity of the TST was estimated at 90% (95% CI 72%–100%). The overall risk of progression to TB for TST positives was 238 per 100,000 population (95% CI 151–343) (Table 3). For TST negatives, this risk was 19 per 100,000 population (95% CI 0–59).
Table 3. Risk of progression to TB within two years after entry screening for TST ≥10 mm.
| Case source cohort | TB within two years* | Incidence of TBwithin two years per100.000 population | Expected TST positive at entry† | Estimated risk of progression toTB per 100.000 population‡ | ||
| N | N | N (%) | TST-positive | TST-negative | ||
| Overall | 26,317 | 30 | 114 | 11,173 (42) | 238 (151–343) | 19 (0–59) |
| Sex | ||||||
| Female | 13,766 | 15 | 109 | 5,692 (41) | 233 (120–373) | 17 (0–57) |
| Male | 12,504 | 15 | 120 | 5,621 (45) | 241 (122–388) | 20 (0–66) |
| Age (yr) | ||||||
| 18–24 | 7,877 | 6 | 76 | 2,188 (28) | 237 (75–472) | 9 (0–36) |
| 25–34 | 12,797 | 16 | 125 | 6,189 (48) | 228 (122–364) | 22 (0–74) |
| ≥35 | 5,643 | 8 | 142 | 2,696 (48) | 258 (99–476) | 24 (0–89) |
| TB incidence in country of origin | ||||||
| <100 | 13,799 | 12 | 87 | 5,483 (40) | 193 (90–322) | 13 (0–46) |
| 100–199 | 7,231 | 12 | 166 | 3,041 (42) | 346 (163–581) | 26 (0–89) |
| ≥200 | 5,040 | 6 | 119 | 2,451 (49) | 212 (68–418) | 20 (0–79) |
TB: tuberculosis; TST: tuberculin skin test.
Based on surveillance data from the MSI & NTR.
Number estimated based on prevalence positive TST ≥10 mm in base cohort.
Based on a median (95% CI) sensitivity for TST≥10 mm of 90% (72–100%).
For the cut-off value of 15 mm we estimated a sensitivity of 59% (95% CI 37%–82%). The overall risk of progression to TB for TST positives was 295 per 100.000 population (95% CI 161–473) (Table 4) and 58 per 100,000 population (95% CI 25–103) for TST negatives.
Table 4. Risk of progression to TB within two years after entry screening for TST ≥15 mm.
| Case source cohort | TB within two years* | Incidence of TBwithin two years per100.000 population | Expected TST positive at entry† | Estimated risk of progression toTB per 100.000 population‡ | ||
| N | N | N (%) | TST-positive | TST-negative | ||
| Overall | 26,317 | 30 | 114 | 5,934 (23) | 295 (161–473) | 58 (25–103) |
| Sex | ||||||
| Female | 13,766 | 15 | 109 | 3,088 (22) | 281 (130–492) | 55 (21–105) |
| Male | 12,504 | 15 | 120 | 2,840 (23) | 306 (139–538) | 61 (23–116) |
| Age (yr) | ||||||
| 18–24 | 7,877 | 6 | 76 | 875 (11) | 388 (108–830) | 33 (7–75) |
| 25–34 | 12,797 | 16 | 125 | 3,057 (24) | 257 (122–447) | 68 (26–130) |
| ≥35 | 5,643 | 8 | 142 | 1,402 (25) | 325 (114–643) | 72 (21–157) |
| TB incidence in country of origin | ||||||
| <100 | 13,799 | 12 | 87 | 2,650 (19) | 261 (110–475) | 42 (15–83) |
| 100–199 | 7,231 | 12 | 166 | 1,632 (23) | 423 (178–773) | 83 (30–166) |
| ≥200 | 5,040 | 6 | 119 | 1,483 (29) | 228 (64–486) | 64 (14–149) |
TB: tuberculosis; TST: tuberculin skin test.
Based on surveillance data from the MSI & NTR.
Number estimated based on prevalence positive TST ≥15 mm in base cohort.
Based on a median (95% CI) sensitivity for TST≥15 mm of 59% (37–82%).
For both cut-off values of the TST, no marked differences in risk of progression to TB were estimated between the males and females., while minor differences were seen with respect to strata of age (15 mm) or TB-incidence in country of origin (10 mm and 15 mm)s (Tables 3, 4).
The NNT and NNS to prevent one TB patient within two years after entry for a TST cut-off value of 10 mm were 700 and 1649, respectively. The corresponding NNT and NNS for a TST cut-off value of 15 mm were 565 and 2505, respectively.
Discussion
This study shows that the TST had the discriminatory ability to identify high and low risk groups for progression to active TB among newly arriving immigrants. This ability was irrespective of the cut-off value of 10 mm or 15 mm, but did not apply for the highest age group (if cut-off value 15 mm) and highest incident category with respect to country of origin (for both cut-off values). The estimated risks for TST positives to progress to TB within two years were considerably higher than the incidence of 50/100,000 which is used in the Netherlands as a cut-off value to define a risk group. Appropriate preventive interventions to lower the incidence of TB among newly arriving immigrants should therefore be undertaken.
In a previous study [5] we found that the risk of progression to active TB within two years per 100,000 population was 467 (95% CI 314–603) among QFT-GIT positive individuals whereas the risk of progression to active TB among QFT-GIT negatives was 25 (95% CI 0–64). It appears therefore that the discriminatory ability of QFT-GIT is slightly better than that of the TST using either a 10 mm or 15 mm cut-off value. The discriminatory ability of TST was somewhat lower than of QFT-GIT, probably because of the, in general, lower specificity (cross reactions attributable to BCG and environmental mycobacteria) and, when using a 15 mm cut-off value, a lower sensitivity to identify LTBI. However, because most credibility intervals overlap these differences between the tests should be interpreted carefully.
Screening immigrants at entry will only be effective if positive test results are followed by adequate interventions like prescribing preventive treatment. The Dutch preventive treatment regimen is three months daily isoniazid plus rifampicin, or six months daily isoniazid. The duration and the risk of severe side effects, such as hepatotoxicity, influence adherence and could harm the efficacy of the treatment [16]. The NNT to prevent one TB patient within two years after entry we have presented when screening with the TST was considerably higher (for both cut-off values) than the NNT of 350 we have reported previously for the QFT-GIT [5]. The corresponding NNS when screening with TST was also higher (for cut-off value 15 mm) than the NNS of 1800 we have reported for the QFT-GIT. These findings suggest that screening newly arriving immigrants for LTBI by QFT-GIT would be more effective than screening with TST. It has been proposed that screening individuals may be most cost-effective if TST positives are subsequently tested with QFT-GIT [17], [18], [19], but more cost-effectiveness studies are needed in screening immigrants, especially by comparing strategies including screening with QFT-GIT, TST, or a combination of these diagnostics. Even if screening immigrants with TST is cost-effective, it will be logistically challenging to have all immigrants visit the PHS twice for placing and reading of the TST.
We did not observe marked differences in the risk of progression to disease for both TST-positives and TST-negatives between the strata of sex, age and incidence in country of origin. The overlapping credibility intervals are a result of the small number of immigrants who progressed to disease.
There were several limitations of this study. Firstly, more than half of the immigrants eligible for TST testing refused. However, there were no marked differences in baseline characteristics between the participants and the ones who refused. The main reason for refusing was a lack of time for a second visit and the fact that it was not mandatory. This highlights that the willingness of immigrants to become screened by TST is limited. There is some controversy whether physician’s and patient’s adherence to guidelines is determined by the diagnostics used. Grinsdale et al. showed that contacts of culture-confirmed TB patients were more likely to complete evaluation and to complete isoniazid preventive therapy when tested with IGRA compared to the TST [20], whereas Shah et al. observed no differences in treatment initiation or completion between the period pre- and post implementation of the IGRA [21]. Secondly, although we aimed to measure the prevalence of TST-positives in a representative cohort, we observed, be it relatively small, demographic differences between the base cohort and the case source cohort. A consequence of having a slight overrepresentation of immigrants originating from high incidence countries in the base cohort might be that we have overestimated the overall prevalence of TST positives, and thereby underestimated the overall risk of progression to disease. Thirdly, we had to assume that the immigrants who progressed to disease within two years were already infected at entry. The absence of clustering and the absence of confirmed contact among clustered cases are consistent with reactivation of LTBI. Fourthly, we could not adjust for potential bias as a result of differential drop-out of immigrants during the two year passive follow-up. In other words, from the surveillance data we could not determine whether diseased individuals were more prone to (re)migrate or stay in the host country. Finally, our findings cannot be translated to newly arriving immigrant children (<18 years).
In conclusion, in this study we found that newly arriving immigrants with a positive TST result at entry were at considerable risk of progression to active TB, whereas for TST negatives this risk was limited. Nevertheless, the discriminatory ability of the TST was somewhat lower than that of the QFT-GIT. This suggests that the QFT-GIT, either used alone or in combination with the TST, is the favorable diagnostic tool for screening newly arriving immigrants coming to low-incidence countries. Further study is needed with respect to the cost-effectiveness for the TST and the QFT-GIT in the immigrant screening program to gain further evidence for considering screening immigrants for LTBI.
Supporting Information
Calculation of the risk of progression to tuberculosis.
(DOC)
Acknowledgments
We would like to acknowledge all participants, person and institutions involved during the data collection. All staff from the seven participating Public Health Services (GGD Amsterdam, GGD The Hague, GGD Regio Twente, GG&GD Utrecht, GGD Brabant Zuidoost, GGD Regio Nijmegen, Hulpverlenersdienst GGD Groningen) these include Ria Dubbink, Wende de Haan, Caroline Runhaar Compper, Myriam Olsthoorn, Khadija Abdulkadir, Soumya Bairi, Marie-Claire Engelen, Athiná Kougioumtzoglou and Dorothee van Trier. We like to thank Job van Rest, Henrieke Schimmel and Erika Slump for preparing the data from the NTR and the MSI for the analyses.
Funding Statement
This work and its open access publication were supported by the Netherlands Organization for Health Research and Development (ZonMw, Grant number: 125010011). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.KNCV Tuberculosis Foundation (2010) Tuberculose in Nederland 2010 [Tuberculosis in The Netherlands 2010]
- 2. Greenaway C, Sandoe A, Vissandjee B, Kitai I, Gruner D, et al. (2011) Tuberculosis: evidence review for newly arriving immigrants and refugees. Cmaj 183: E939–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bwire R, Nagelkerke N, Keizer ST, Annee-van Bavel J, Sijbrant J, et al. (2000) Tuberculosis screening among immigrants in The Netherlands: what is its contribution to public health? Neth J Med 56: 63–71. [DOI] [PubMed] [Google Scholar]
- 4. Pai M, Dheda K, Cunningham J, Scano F, O’Brien R (2007) T-cell assays for the diagnosis of latent tuberculosis infection: moving the research agenda forward. Lancet Infect Dis 7: 428–438. [DOI] [PubMed] [Google Scholar]
- 5. Mulder C, van Deutekom H, Huisman EM, Toumanian S, Koster BF, et al. (2012) Role of Quantiferon-TB Gold In-Tube in screening new immigrants for tuberculosis infection. Eur Respir J 40: 1443–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kik SV, Franken WP, Mensen M, Cobelens FG, Kamphorst M, et al. (2010) Predictive value for progression to tuberculosis by IGRA and TST in immigrant contacts. Eur Respir J 35: 1346–1353. [DOI] [PubMed] [Google Scholar]
- 7. Mahomed H, Hawkridge T, Verver S, Abrahams D, Geiter L, et al. (2011) The tuberculin skin test versus QuantiFERON TB Gold(R) in predicting tuberculosis disease in an adolescent cohort study in South Africa. PLoS One 6: e17984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prentice RL (1986) A case-cohort design for epidemiologic studies and disease prevention trials. Biometrika 73: 1–11. [Google Scholar]
- 9. Vynnycky E, Fine PE (2000) Lifetime risks, incubation period, and serial interval of tuberculosis. Am J Epidemiol 152: 247–263. [DOI] [PubMed] [Google Scholar]
- 10. Diel R, Loddenkemper R, Meywald-Walter K, Niemann S, Nienhaus A (2008) Predictive value of a whole blood IFN-gamma assay for the development of active tuberculosis disease after recent infection with Mycobacterium tuberculosis. Am J Respir Crit Care Med 177: 1164–1170. [DOI] [PubMed] [Google Scholar]
- 11. Winje BA, Oftung F, Korsvold GE, Mannsaker T, Jeppesen AS, et al. (2008) Screening for tuberculosis infection among newly arrived asylum seekers: comparison of QuantiFERONTB Gold with tuberculin skin test. BMC Infect Dis 8: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hill PC, Jackson-Sillah DJ, Fox A, Brookes RH, de Jong BC, et al. (2008) Incidence of tuberculosis and the predictive value of ELISPOT and Mantoux tests in Gambian case contacts. PLoS One 3: e1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Torres Costa J, Silva R, Sa R, Cardoso MJ, Nienhaus A (2010) Serial testing with the interferon-gamma release assay in Portuguese healthcare workers. Int Arch Occup Environ Health 84: 461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harstad I, Jacobsen GW, Heldal E, Winje BA, Vahedi S, et al. (2010) The role of entry screening in case finding of tuberculosis among asylum seekers in Norway. BMC Public Health 10: 670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smieja MJ, Marchetti CA, Cook DJ, Smaill FM (2000) Isoniazid for preventing tuberculosis in non-HIV infected persons. Cochrane Database Syst Rev: CD001363. [DOI] [PMC free article] [PubMed]
- 16. Lobue P, Menzies D Treatment of latent tuberculosis infection: An update. Respirology 15: 603–622. [DOI] [PubMed] [Google Scholar]
- 17. Nienhaus A, Schablon A, Torres Costa J, Diel R (2011) Systematic review of cost and cost-effectiveness of different TB-screening strategies. BMC Health Serv Res 11: 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pooran A, Booth H, Miller RF, Scott G, Badri M, et al. (2010) Different screening strategies (single or dual) for the diagnosis of suspected latent tuberculosis: a cost effectiveness analysis. BMC Pulm Med 10: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abdalhamid B, Hinrichs SH, Garrett JL, O’Neill JM, Hansen-Cain KM, et al. (2010) Utilization of the QuantiFERON-TB Gold Test in a 2-Step Process with the Tuberculin Skin Test to Evaluate Healthcare Workers for Latent Tuberculosis. J Clin Microbiol 48: 2955–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grinsdale JA, Ho CS, Banouvong H, Kawamura LM (2011) Programmatic impact of using QuantiFERON((R)) -TB Gold in routine contact investigation activities. Int J Tuberc Lung Dis 15: 1614–1620. [DOI] [PubMed] [Google Scholar]
- 21. Shah M, Dipietro D, Greenbaum A, Ketemepi S, Martins-Evora M, et al. (2012) Programmatic Impact of QuantiFERON-TB Gold In-Tube Implementation on Latent Tuberculosis Diagnosis and Treatment in a Public Health Clinic. PLoS One 7: e36551. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Calculation of the risk of progression to tuberculosis.
(DOC)