Abstract
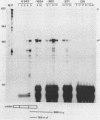
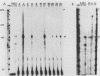
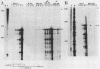
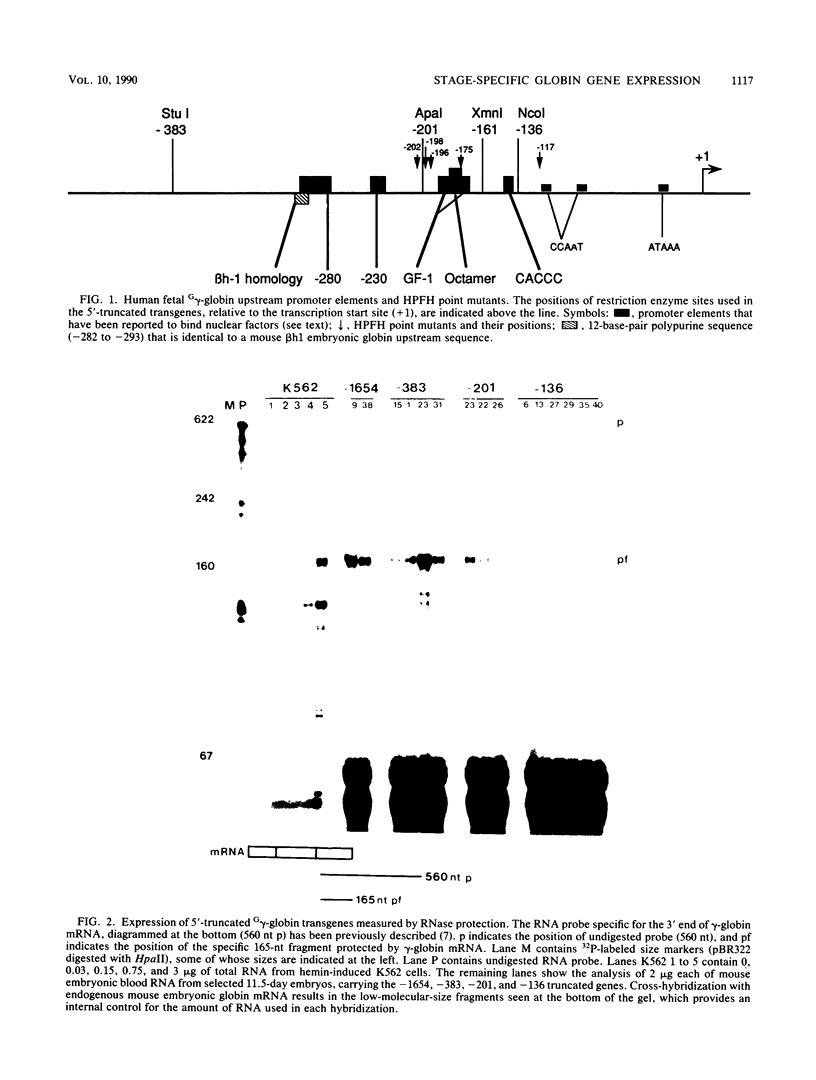
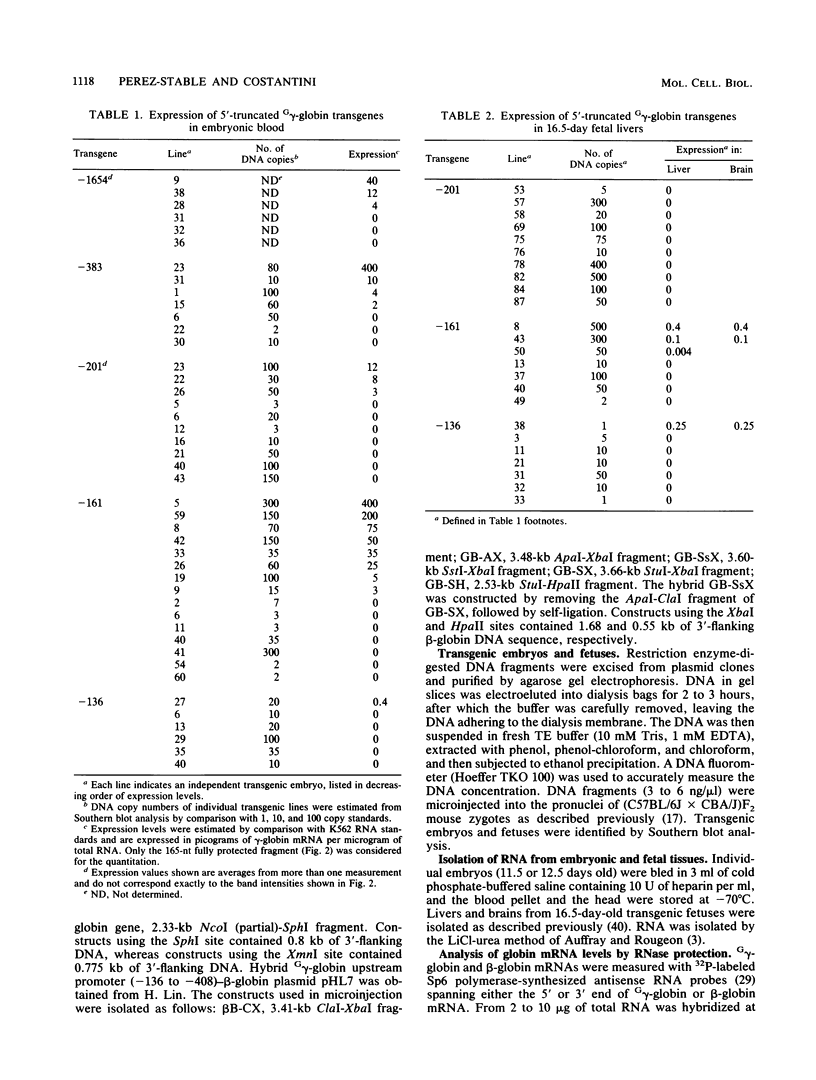
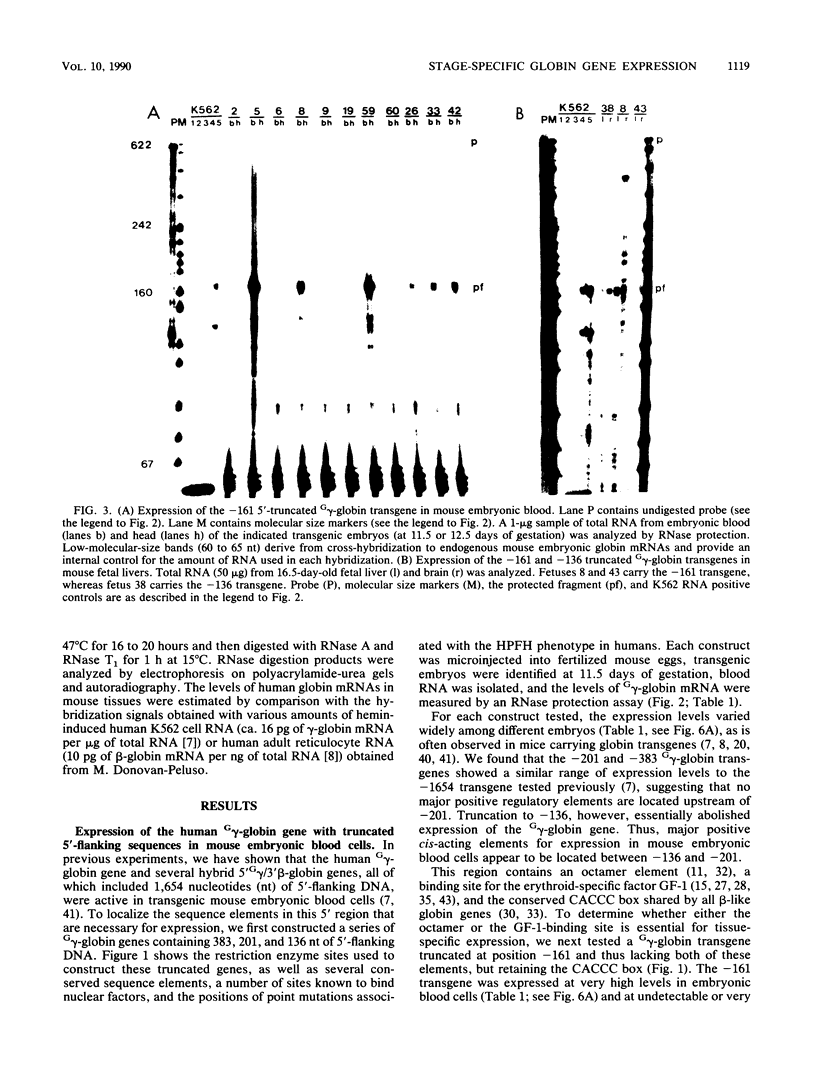
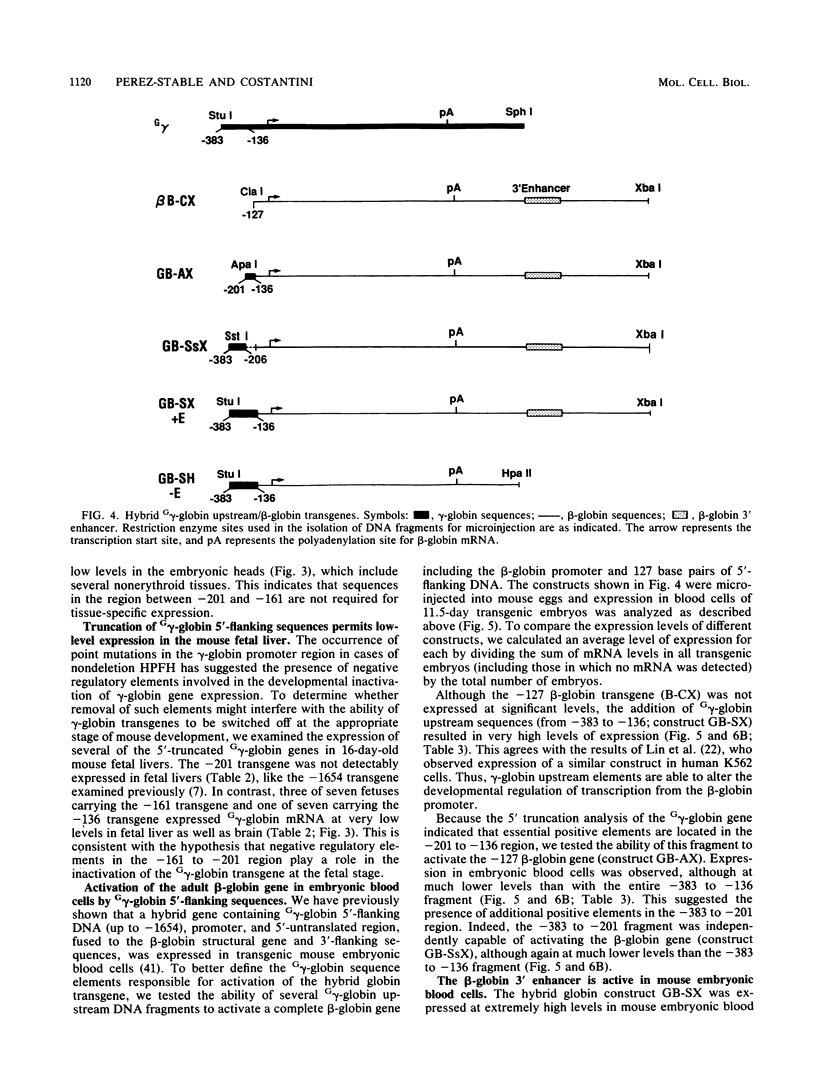
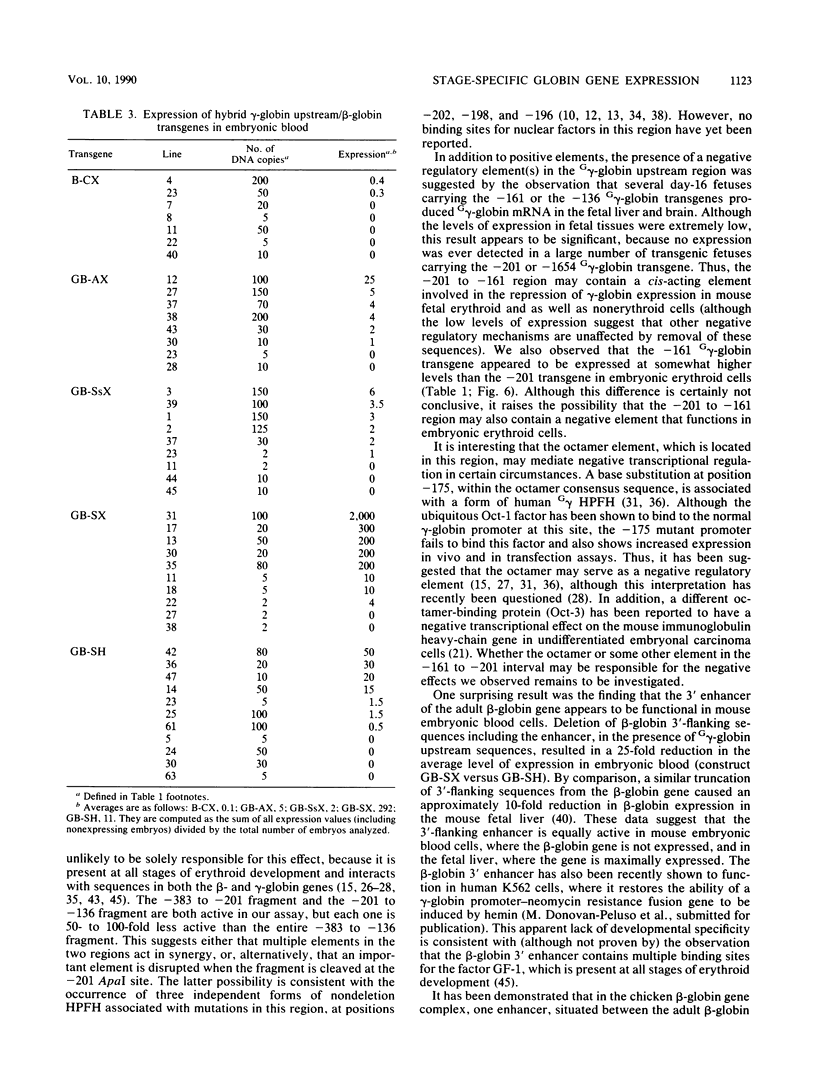
The human fetal G gamma-globin and adult beta-globin genes are expressed in a tissue- and developmental stage-specific pattern in transgenic mice: the G gamma gene in embryonic cells and the beta gene in fetal and adult erythroid cells. Several of the cis-acting DNA sequences thought to be responsible for these patterns of expression are located 5' to the G gamma-globin gene and 3' to the beta-globin gene. To further define the locations and functional roles of these elements, we examined the effects of 5' truncations on the expression of the G gamma-globin gene, as well as the ability of G gamma-globin upstream sequences to alter the developmental regulation of a beta-globin gene, as well as the ability of G gamma-globin upstream sequences to alter the developmental regulation of a beta-globin gene. We found that sequences between -201 and -136 are essential for expression of the G gamma-globin gene, whereas those upstream of -201 have little effect on the level or tissue or stage specificity of G gamma-globin expression. The G gamma-globin upstream sequences from -201 to -136 were, furthermore, capable of activating a linked beta-globin gene in embryonic blood cells; however, a G gamma-globin fragment from -383 to -206 was similarly active in this assay, and the complete fragment from -383 to -136 was considerably more active than either of the smaller fragments, suggesting the presence of multiple cis-acting elements for embryonic blood cells. Our data also suggested the possibility of a negative regulatory element between -201 and -136. These results are discussed in relation to several DNA elements in the G gamma-globin upstream region, which have been shown to bind nuclear factors in erythroid cells. Finally, we observed that removal of the beta-globin 3'-flanking sequences, including the 3' enhancer, from the G gamma-globin upstream-beta-globin hybrid gene resulted in a 25-fold reduction in expression in embryonic blood cells. This suggests that the beta-globin 3' enhancer is potentially active at the embryonic stage and thus cannot be solely responsible for the fetal or adult specificity of the beta-globin gene.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acuto S., Donovan-Peluso M., Giambona N., Bank A. The role of human globin gene promoters in the expression of hybrid genes in erythroid and non-erythroid cells. Biochem Biophys Res Commun. 1987 Mar 30;143(3):1099–1106. doi: 10.1016/0006-291x(87)90365-2. [DOI] [PubMed] [Google Scholar]
- Antoniou M., deBoer E., Habets G., Grosveld F. The human beta-globin gene contains multiple regulatory regions: identification of one promoter and two downstream enhancers. EMBO J. 1988 Feb;7(2):377–384. doi: 10.1002/j.1460-2075.1988.tb02824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Behringer R. R., Hammer R. E., Brinster R. L., Palmiter R. D., Townes T. M. Two 3' sequences direct adult erythroid-specific expression of human beta-globin genes in transgenic mice. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7056–7060. doi: 10.1073/pnas.84.20.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom van Assendelft G., Hanscombe O., Grosveld F., Greaves D. R. The beta-globin dominant control region activates homologous and heterologous promoters in a tissue-specific manner. Cell. 1989 Mar 24;56(6):969–977. doi: 10.1016/0092-8674(89)90630-2. [DOI] [PubMed] [Google Scholar]
- Bodine D. M., Ley T. J. An enhancer element lies 3' to the human A gamma globin gene. EMBO J. 1987 Oct;6(10):2997–3004. doi: 10.1002/j.1460-2075.1987.tb02605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catala F., deBoer E., Habets G., Grosveld F. Nuclear protein factors and erythroid transcription of the human A gamma-globin gene. Nucleic Acids Res. 1989 May 25;17(10):3811–3827. doi: 10.1093/nar/17.10.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chada K., Magram J., Costantini F. An embryonic pattern of expression of a human fetal globin gene in transgenic mice. Nature. 1986 Feb 20;319(6055):685–689. doi: 10.1038/319685a0. [DOI] [PubMed] [Google Scholar]
- Chada K., Magram J., Raphael K., Radice G., Lacy E., Costantini F. Specific expression of a foreign beta-globin gene in erythroid cells of transgenic mice. 1985 Mar 28-Apr 3Nature. 314(6009):377–380. doi: 10.1038/314377a0. [DOI] [PubMed] [Google Scholar]
- Choi O. R., Engel J. D. Developmental regulation of beta-globin gene switching. Cell. 1988 Oct 7;55(1):17–26. doi: 10.1016/0092-8674(88)90005-0. [DOI] [PubMed] [Google Scholar]
- Collins F. S., Stoeckert C. J., Jr, Serjeant G. R., Forget B. G., Weissman S. M. G gamma beta+ hereditary persistence of fetal hemoglobin: cosmid cloning and identification of a specific mutation 5' to the G gamma gene. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4894–4898. doi: 10.1073/pnas.81.15.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner F. G., Zachau H. G. Correct transcription of an immunoglobulin kappa gene requires an upstream fragment containing conserved sequence elements. Nature. 1984 Jul 5;310(5972):71–74. doi: 10.1038/310071a0. [DOI] [PubMed] [Google Scholar]
- Gelinas R., Bender M., Lotshaw C., Waber P., Kazazian H., Jr, Stamatoyannopoulos G. Chinese A gamma fetal hemoglobin: C to T substitution at position-196 of the A gamma gene promoter. Blood. 1986 Jun;67(6):1777–1779. [PubMed] [Google Scholar]
- Giglioni B., Casini C., Mantovani R., Merli S., Comi P., Ottolenghi S., Saglio G., Camaschella C., Mazza U. A molecular study of a family with Greek hereditary persistence of fetal hemoglobin and beta-thalassemia. EMBO J. 1984 Nov;3(11):2641–2645. doi: 10.1002/j.1460-2075.1984.tb02187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Gumucio D. L., Rood K. L., Gray T. A., Riordan M. F., Sartor C. I., Collins F. S. Nuclear proteins that bind the human gamma-globin gene promoter: alterations in binding produced by point mutations associated with hereditary persistence of fetal hemoglobin. Mol Cell Biol. 1988 Dec;8(12):5310–5322. doi: 10.1128/mcb.8.12.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A., Hardies S. C., Phillips S. J., Davis M. G., Hutchison C. A., 3rd, Edgell M. H. Two mouse early embryonic beta-globin gene sequences. Evolution of the nonadult beta-globins. J Biol Chem. 1984 Mar 25;259(6):3739–3747. [PubMed] [Google Scholar]
- Kioussis D., Wilson F., Khazaie K., Grosveld F. Differential expression of human globin genes introduced in K562 cells. EMBO J. 1985 Apr;4(4):927–931. doi: 10.1002/j.1460-2075.1985.tb03720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollias G., Hurst J., deBoer E., Grosveld F. The human beta-globin gene contains a downstream developmental specific enhancer. Nucleic Acids Res. 1987 Jul 24;15(14):5739–5747. doi: 10.1093/nar/15.14.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollias G., Wrighton N., Hurst J., Grosveld F. Regulated expression of human A gamma-, beta-, and hybrid gamma beta-globin genes in transgenic mice: manipulation of the developmental expression patterns. Cell. 1986 Jul 4;46(1):89–94. doi: 10.1016/0092-8674(86)90862-7. [DOI] [PubMed] [Google Scholar]
- Lenardo M. J., Staudt L., Robbins P., Kuang A., Mulligan R. C., Baltimore D. Repression of the IgH enhancer in teratocarcinoma cells associated with a novel octamer factor. Science. 1989 Jan 27;243(4890):544–546. doi: 10.1126/science.2536195. [DOI] [PubMed] [Google Scholar]
- Lin H. J., Anagnou N. P., Rutherford T. R., Shimada T., Nienhuis A. W. Activation of the human beta-globin promoter in K562 cells by DNA sequences 5' to the fetal gamma- or embryonic zeta-globin genes. J Clin Invest. 1987 Aug;80(2):374–380. doi: 10.1172/JCI113082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd J. A., Lee R. F., Lingrel J. B. Mutations in two regions upstream of the A gamma globin gene canonical promoter affect gene expression. Nucleic Acids Res. 1989 Jun 12;17(11):4339–4352. doi: 10.1093/nar/17.11.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magram J., Chada K., Costantini F. Developmental regulation of a cloned adult beta-globin gene in transgenic mice. Nature. 1985 May 23;315(6017):338–340. doi: 10.1038/315338a0. [DOI] [PubMed] [Google Scholar]
- Magram J., Niederreither K., Costantini F. Beta-globin enhancers target expression of a heterologous gene to erythroid tissues of transgenic mice. Mol Cell Biol. 1989 Oct;9(10):4581–4584. doi: 10.1128/mcb.9.10.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani R., Malgaretti N., Giglioni B., Comi P., Cappellini N., Nicolis S., Ottolenghi S. A protein factor binding to an octamer motif in the gamma-globin promoter disappears upon induction of differentiation and hemoglobin synthesis in K562 cells. Nucleic Acids Res. 1987 Nov 25;15(22):9349–9364. doi: 10.1093/nar/15.22.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani R., Malgaretti N., Nicolis S., Ronchi A., Giglioni B., Ottolenghi S. The effects of HPFH mutations in the human gamma-globin promoter on binding of ubiquitous and erythroid specific nuclear factors. Nucleic Acids Res. 1988 Aug 25;16(16):7783–7797. doi: 10.1093/nar/16.16.7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. I., Tsai S. F., Orkin S. H. Increased gamma-globin expression in a nondeletion HPFH mediated by an erythroid-specific DNA-binding factor. Nature. 1989 Mar 30;338(6214):435–438. doi: 10.1038/338435a0. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Tilly K., Maniatis T. Fine structure genetic analysis of a beta-globin promoter. Science. 1986 May 2;232(4750):613–618. doi: 10.1126/science.3457470. [DOI] [PubMed] [Google Scholar]
- Parslow T. G., Blair D. L., Murphy W. J., Granner D. K. Structure of the 5' ends of immunoglobulin genes: a novel conserved sequence. Proc Natl Acad Sci U S A. 1984 May;81(9):2650–2654. doi: 10.1073/pnas.81.9.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüle R., Muller M., Otsuka-Murakami H., Renkawitz R. Cooperativity of the glucocorticoid receptor and the CACCC-box binding factor. Nature. 1988 Mar 3;332(6159):87–90. doi: 10.1038/332087a0. [DOI] [PubMed] [Google Scholar]
- Superti-Furga G., Barberis A., Schaffner G., Busslinger M. The -117 mutation in Greek HPFH affects the binding of three nuclear factors to the CCAAT region of the gamma-globin gene. EMBO J. 1988 Oct;7(10):3099–3107. doi: 10.1002/j.1460-2075.1988.tb03176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surrey S., Delgrosso K., Malladi P., Schwartz E. A single-base change at position -175 in the 5'-flanking region of the G gamma-globin gene from a black with G gamma-beta+ HPFH. Blood. 1988 Mar;71(3):807–810. [PubMed] [Google Scholar]
- Surrey S., Delgrosso K., Malladi P., Schwartz E. A single-base change at position -175 in the 5'-flanking region of the G gamma-globin gene from a black with G gamma-beta+ HPFH. Blood. 1988 Mar;71(3):807–810. [PubMed] [Google Scholar]
- Talbot D., Collis P., Antoniou M., Vidal M., Grosveld F., Greaves D. R. A dominant control region from the human beta-globin locus conferring integration site-independent gene expression. Nature. 1989 Mar 23;338(6213):352–355. doi: 10.1038/338352a0. [DOI] [PubMed] [Google Scholar]
- Tate V. E., Wood W. G., Weatherall D. J. The British form of hereditary persistence of fetal hemoglobin results from a single base mutation adjacent to an S1 hypersensitive site 5' to the A gamma globin gene. Blood. 1986 Dec;68(6):1389–1393. [PubMed] [Google Scholar]
- Townes T. M., Lingrel J. B., Chen H. Y., Brinster R. L., Palmiter R. D. Erythroid-specific expression of human beta-globin genes in transgenic mice. EMBO J. 1985 Jul;4(7):1715–1723. doi: 10.1002/j.1460-2075.1985.tb03841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel M., Costantini F. A 3' enhancer contributes to the stage-specific expression of the human beta-globin gene. Genes Dev. 1987 Nov;1(9):954–961. doi: 10.1101/gad.1.9.954. [DOI] [PubMed] [Google Scholar]
- Trudel M., Magram J., Bruckner L., Costantini F. Upstream G gamma-globin and downstream beta-globin sequences required for stage-specific expression in transgenic mice. Mol Cell Biol. 1987 Nov;7(11):4024–4029. doi: 10.1128/mcb.7.11.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. F., Martin D. I., Zon L. I., D'Andrea A. D., Wong G. G., Orkin S. H. Cloning of cDNA for the major DNA-binding protein of the erythroid lineage through expression in mammalian cells. Nature. 1989 Jun 8;339(6224):446–451. doi: 10.1038/339446a0. [DOI] [PubMed] [Google Scholar]
- Tuan D., Solomon W., Li Q., London I. M. The "beta-like-globin" gene domain in human erythroid cells. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6384–6388. doi: 10.1073/pnas.82.19.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall L., deBoer E., Grosveld F. The human beta-globin gene 3' enhancer contains multiple binding sites for an erythroid-specific protein. Genes Dev. 1988 Sep;2(9):1089–1100. doi: 10.1101/gad.2.9.1089. [DOI] [PubMed] [Google Scholar]
- Wang X. F., Calame K. The endogenous immunoglobulin heavy chain enhancer can activate tandem VH promoters separated by a large distance. Cell. 1985 Dec;43(3 Pt 2):659–665. doi: 10.1016/0092-8674(85)90238-7. [DOI] [PubMed] [Google Scholar]