Abstract
Bacterial leaf streak caused by Xanthomonas oryzae pv. oryzicola (Xoc) is one of the most important diseases in rice. However, little is known about the pathogenicity mechanisms of Xoc. Here we have investigated the function of three HD-GYP domain regulatory proteins in biofilm formation, the synthesis of virulence factors and virulence of Xoc. Deletion of rpfG resulted in altered production of extracellular polysaccharides (EPS), abolished virulence on rice and enhanced biofilm formation, but had little effect on the secretion of proteases and motility. In contrast, mutational analysis showed that the other two HD-GYP domain proteins had no effect on virulence factor synthesis and tested phenotypes. Mutation of rpfG led to up-regulation of the type III secretion system and altered expression of three putative glycosyltransferase genes gumD, pgaC and xagB, which are part of operons directing the synthesis of different extracellular polysaccharides. The pgaABCD and xagABCD operons were greatly up-regulated in the Xoc ΔrpfG mutant, whereas the expression of the gum genes was unaltered or slightly enhanced. The elevated biofilm formation of the Xoc ΔrpfG mutant was dramatically reduced upon deletion of gumD, xagA and xagB, but not when pgaA and pgaC were deleted. Interestingly, only the ΔgumD mutant, among these single gene mutants, exhibits multiple phenotype alterations including reduced biofilm and EPS production and attenuated virulence on rice. These data indicate that RpfG is a global regulator that controls biofilm formation, EPS production and bacterial virulence in Xoc and that both gumD- and xagB-dependent EPS contribute to biofilm formation under different conditions.
Introduction
Xanthomonas oryzae pv. oryzicola (Xoc) causes bacterial leaf streak (BLS) in rice, one of the most important bacterial diseases in tropical and subtropical Asia, some parts of Africa, as well as rice-growing regions of northern Australia. The BLS disease can cause yield loss up to 30% in epidemic years [1], [2], [3]. Xoc invades rice leaves mainly through stomata, and sometimes through wounds. The pathogen colonizes in the intercellular spaces of the parenchyma and is restricted to the apoplast of the mesophyll tissue. Xoc does not invade the xylem, which is in contrast to another rice bacterial pathogen Xanthomonas oryzae pv. oryzae (Xoo) that causes bacterial blight by invading vascular tissues [2]. Interactions between Xoo/Xoc and rice have become models for understanding fundamental aspects of bacterial pathogenesis in host plants and plant disease resistance, as well as functional and comparative genomics in microbial biology [4].
Several categories of genes have been identified to contribute to Xoc virulence; these include genes encoding functions involved in type III secretion, lipopolysaccharide synthesis, type IV pilus and twitching motility, carbohydrate synthesis and two-component regulation [5], [6]. Further to this, comparative genomic studies have revealed the conservation of functions or genes with an established role in virulence in other Xanthomonas species within the Xoc genome. Of particular interest here are proteins implicated in intracellular signaling involving the nucleotide second messenger cyclic diguanosine monophosphate (c-di-GMP), which has been implicated in virulence of a number of xanthomonads as well as a diverse range of unrelated bacterial pathogens [7], [8], [9], [10].
c-di-GMP was first identified in Gluconacetobacter xylinus as an activator of cellulose synthesis [11]. The molecule was later identified to be a widely conserved second messenger that is implicated in the regulation of various biological functions in bacteria, such as cellulose biosynthesis [11], bacterial motility [12], biofilm formation [13], the production of extracellular polysaccharide and the secretion of extracellular hydrolytic enzymes such as proteases and endoglucanases [14], [15], [16], [17]. The level of c-di-GMP in bacteria is regulated by at least three categories of proteins containing GGDEF, EAL or HD-GYP domains, respectively [8], [18], [19]. GGDEF domain-containing proteins function as diguanylate cyclases (DGCs) that synthesize c-di-GMP [18], while the HD-GYP and EAL domain-containing proteins act as phosphodiesterases (PDEs) that degrade c-di-GMP [7], [8], [19], [20]. All three domains are broadly distributed in many bacterial species [21].
A total of 37 proteins with HD-GYP, GGDEF and/or EAL domains were identified in the genome of Xcc 8004 [22]; Xoo PXO99 and Xoc BLS256 genomes encode at least 27 and 32 such proteins, respectively [23], [24]. The contribution of all 37 proteins to Xcc virulence has been examined by a functional genomic approach [22]. The findings showed that many proteins with GGDEF and/or EAL domains in addition to the HD-GYP domain protein RpfG contributed to Xcc virulence in Chinese radish. In Xoo, two groups have reported the involvement of proteins carrying GGDEF and EAL domains in motility, biofilm formation and/or virulence [10], [25]. To our knowledge, however, no functional studies for GGDEF, EAL or HD-GYP domain proteins have been reported in Xoc so far. A greater understanding of c-di-GMP signaling system in Xoc and its role in the interactions between the pathogen and host rice plants could have substantial implications for new approaches for disease control.
All Xanthomonas spp. genomes encode three conserved HD-GYP domain proteins of which the best studied is RpfG of Xcc. The RpfG regulator comprises a CheY-like receiver domain and an HD-GYP domain and acts together with the sensor kinase RpfC in a two-component system implicated in sensing and transduction of the diffusible signal factor DSF [26], [27]. The synthesis of DSF is dependent on RpfF, which belongs to the crotonase family and is encoded by a linked gene [17], [22], [26]. RpfG, which is required for full virulence of Xcc, positively regulates motility and the synthesis of virulence determinants such as extracellular polysaccharide (EPS) and extracellular enzymes but negatively regulates biofilm formation [17], [22], [26], [27], [28]. The regulatory action of this protein on extracellular enzyme synthesis depends upon the c-di-GMP phosphodiesterase of the HD-GYP domain whereas the influence on motility depends upon the interaction of RpfG with two GGDEF domain proteins, directed by the GYP motif of the HD-GYP domain [8], [29], [30]. The two other HD-GYP domain proteins in Xcc 8004 are XC0362 and XC1755. Deletion of XC1755 attenuated virulence of Xcc on Chinese radish but had no effect on the secretion of extracellular enzymes whereas mutation of XC0362 had no effect on virulence or extracellular enzyme production [22], indicating that HD-GYP domain proteins have diverse actions. The Xoc BLS256 genome encodes three HD-GYP domain proteins; XOC2264 (RpfG), XOC1984, and XOC4564 share 95.2%, 86.0% and 82.6% sequence identity to Xcc HD-GYP proteins XC2335 (RpfG), XC1755 and XC0362, respectively. Although RpfG is well studied in Xcc, little is known for the function of HD-GYP domain proteins in other phytopathogenic bacteria.
Here we describe experiments to address the function and regulatory role of HD-GYP domain proteins in Xoc by examination of the effects of deletion of the encoding genes. We directly tested virulence to rice as well as effects on production of a range of virulence factors including biofilm formation, extracellular enzyme production and expression of type III secretion systems (T3SS). We show that RpfG is essential for full virulence in Xoc and has a substantial influence on biofilm formation and EPS production, although only a minor effect on the secretion of extracellular proteases and swimming motility. Subsequent expression and functional analyses demonstrated that expression of three putative glycosyltransferase genes gumD, xagB and pgaC was differentially regulated by RpfG and that GumD and XagB are important factors for biofilm formation in Xoc.
Results
The Xoc Genome Encodes three HD-GYP Domain Proteins
The complete genome sequence of Xoc strain BLS256 allowed us to identify the HD-GYP domain proteins in Xoc through bioinformatic analysis [23]. BLAST searches revealed that three genes encode HD-GYP domain proteins in Xoc BLS256; these are XOC2264 (rpfG ), XOC1984, and XOC4564. The latter two proteins were designated as HgdA and HgdC (HD-GYP domain-containing proteins). RpfG and HgdA have a CheY-like response receiver (REC) regulatory domain at N-terminus, whereas in HgdC, the HD-GYP domain comprises the central region of the un-characterized protein (Figure S1). To determine the effect of these HD-GYP domain proteins on bacterial behaviors and in vivo virulence of Xoc, the single, double and triple mutants involving rpfG, hgdA and hgdC genes were constructed as described in Materials and Methods and confirmed by Southern blot analyses (Figure S2).
Mutations of hgdA, rpfG and hgdC Genes have Distinct Effects on Biofilm Formation in Xoc
RpfG and c-di-GMP are important regulatory factors in biofilm formation in those xanthomonad bacteria tested thus far [22], [30], [31]. To evaluate the function of HD-GYP proteins in biofilm formation in Xoc, the wild-type and mutant strains were quantified for biofilm production at the air-media interface in glass tubes in L medium using crystal violet (CV) staining (see Methods). The ΔrpfG mutant produced approximately twice the amount of biofilm as the wild-type strain, whereas the hgdA, hgdC or the double hgdA/hgdC mutant strains produced wild-type levels of biofilm in L-medium (Figure 1A). The rpfG double and triple mutants involving hgdA and/or hgdC were not significantly different from the single rpfG mutant in biofilm production. Complementation analysis, which was performed by transforming the pVSP61 plasmid with the full-length rpfG gene into ΔrpfG, restored the phenotype of ΔrpfG in biofilm formation to the wild-type level (Figure 1A). The results suggest that under the conditions used, RpfG negatively regulates biofilm formation in Xoc but that HgdA and HgdC have no influence. Work in Xcc has shown that the diffusible signal molecule DSF also negatively regulates aggregation and biofilm formation through a pathway involving RpfG [27], [32]. Similarly, the ΔrpfF mutant of Xoc, which cannot synthesize DSF, produced much more biofilm when cultured in L-medium (Figure S3). The Xoc rpfF complementation strain restored the wild-type phenotype in biofilm formation (Figure S3).
Figure 1. Effects of hgdA, rpfG and hgdC mutations on biofilm formation and the production of extracellular polysaccharide (EPS) in X. oryzae pv.oryzicola.

(A) Increased biofilm formation was observed after mutation of rpfG, but not hgdA and hgdC deletion. Introducing the full-length rpfG gene into ΔrpfG reduced biofilm production to the wild-type level. Upper panel: The glass-bound biofilm was stained with crystal violet; Lower panel: The crystal violet in the stained biofilm on glass tubes was dissolved with 90% ethanol and detected spectrophotometrically at 590 nm. (B) EPS production was reduced when rpfG, but not hgdA and hgdC, was deleted in Xoc. The complemented strain ΔrpfG(rpfG) had a level of EPS production similar to the wild-type.
The Effects of hgdA, rpfG and hgdC Gene Deletions on the Production of Extracellular Polysaccharide, Protease Secretion and Motility in Xoc
Extracellular polysaccharide (EPS) is well known as one of important virulence factors in phytopathogenic bacteria [33]. To determine if RpfG and other HD-GYP domain proteins play a role in EPS production in Xoc, we quantified EPS produced in single, double and triple mutant strains with hgdA, rpfG and/or hgdC gene deletions. It was observed that the ΔrpfG mutant generated 53±9% of the EPS produced by the wild-type Xoc. However, the ΔhgdA, ΔhgdC and ΔhgdA/hgdC mutants produced similar amount of EPS to the wild-type strain (Figure 1B). The ΔrpfG/hgdA, ΔrpfG/hgdC and ΔrpfG/hgdA/hgdC mutant strains also produced much less EPS than the wild-type strain, but the levels in these strains were not significantly different from the ΔrpfG strain (Figure 1B). The ability to produce EPS of the ΔrpfG mutant was restored when a plasmid-borne full length rpfG gene was introduced into ΔrpfG.
Mutation of rpfG in Xcc caused a substantial reduction in the secretion of the extracellular enzymes endoglucanase, endomannanase and proteases [22]. Xoc secreted very low levels of endoglucanase and endomannanase after cell cultures were grown in NB and OB medium [34] (data not shown). By contrast, the secretion of proteases in Xoc can be easily detected by observing clearing zones around bacterial cultures in skim-milk-containing agar plates. As shown in Figure S4A, all single, double and triple deletion mutant strains exhibited no significant difference on the diameter of clearing zones, indicating that the ability to synthesize and secrete proteases is not altered in these mutants. Similar results were observed when these Xoc strains were cultured on the skim-milk NYGA plates (data not shown).
The swimming motility of the wild-type and different mutant strains was determined after inoculation of bacteria onto semi-solid plates. No mutant showed substantial alteration in its swimming motility from the wild-type (Figure S4B). These findings established that all of these HD-GYP domain proteins in Xoc are not involved in the synthesis and secretion of extracellular proteases and swimming motility. Taken together, the results indicate that RpfG positively regulates EPS production in Xoc, but has little or no influence on protease production or motility. The hgdA and hgdC genes have no effect on EPS production, motility or protease secretion when deleted either singly or in combination.
Deletion of rpfG, but not of hgdA and hgdC Genes Reduces Xoc Virulence on Rice
To investigate the role of HD-GYP proteins in Xoc virulence on rice, the wild-type and mutant strains were pressure-inoculated into the leaves of six-week-old rice plants (Oryza sativa cvs. Nipponbare and Jingang 30). Virulence of each mutant was determined by measuring the length of disease lesions 2 weeks after inoculation. The deletion of rpfG caused nearly complete loss of Xoc virulence on rice, but the hgdA and hgdC gene deletions had no influence on Xoc virulence (Figure 2A). Complementation restored virulence of the ΔrpfG mutant towards the wild-type level. Enumeration of bacteria isolated from the inoculated rice leaves clearly showed that the in planta population size of ΔrpfG was much smaller than that of the wild-type and complemented strains (Figure 2B). The data indicate that RpfG plays an essential role in the virulence and colonization ability of Xoc on rice.
Figure 2. Virulence assays of the wild-type and rpfG-, hgdA- and hgdC-related single, double and triple mutant strains on rice cv. Jingang 30.
(A) The length of disease lesions was measured at 14 days after pressure inoculation of the wild-type (WT), ΔrpfG, complemented ΔrpfG (rpfG), ΔhgdA, ΔhgdA/hgdC, ΔhgdA/rpfG, ΔrpfG/hgdC, ΔhgdA/rpfG/hgdC strains, respectively. Ten to 15 leaves were scored for each strain; means ± standard error (SE) are shown. (B) In planta bacterial populations of Xoc RS105, ΔrpfG and ΔrpfG(rpfG) at the specific time points after inoculation. Data are presented as means ± SE.
The PDE Activity of RpfG is Required for Regulation of Virulence Factor Synthesis and Virulence to Rice
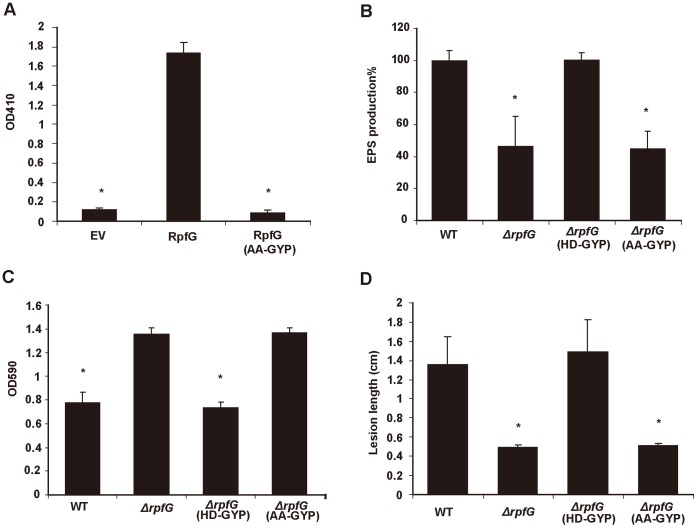
The action of the HD-GYP domain as a PDE active against c-di-GMP has been demonstrated in proteins from several bacteria including Xcc, Pseudomonas aeruginosa and Borrelia burgdorferi [8], [9], [35]. To test whether the regulatory influence on virulence and virulence factor synthesis in Xoc depended upon the c-di-GMP PDE activity, our approach was to examine the effects of introducing alanine substitutions in the presumed HD catalytic diad [8], [36], [37], [38] on both the enzymatic and regulatory activities of the protein. RpfG was expressed in E. coli as N-terminal His6-tagged fusions and then purified using nickel columns (see Methods). The purified protein had the PDE activity against the model substrate bis(p- nitrophenyl) phosphate and could degrade c-di-GMP into two products identified by LC-MS as pGpG and GMP (Figure S5). The AA-GYP variant of RpfG, in which the residues of the HD diad were substituted by alanine, was also purified as an N-terminal His6-tag protein. As expected, this alteration of RpfG protein completely abolished its PDE activity as measured by the hydrolysis of bis-(p-nitrophenyl) phosphate (Figure 3A).
Figure 3. Mutation of H231 and D232 residues in the HD-GYP domain of Xoc RpfG disrupts the PDE activity and regulatory function.
(A) Purified RpfGAA-GYP completely lost its PDE activity detected by colorimetric assays. (B–D) RpfGAA-GYP lost the ability to restore EPS production (B), biofilm formation (C) and virulence to rice (D) of ΔrpfG to the wild-type level. WT: wild-type, EV: empty vector.
Then the importance of the active site mutation for RpfG regulatory function was assessed through comparative phenotype analyses of the rpfG mutant expressing either the wild-type (HD-GYP) protein or variant (AA-GYP) protein from the pVSP61 plasmid. The results showed that RpfG AA-GYP variant lost the ability to restore the mutant phenotypes of EPS production, biofilm formation and virulence on rice to the wild-type level (Figure 3B, 3C and 3D). The findings indicate that the regulatory influence of RpfG on virulence and virulence factor synthesis in Xoc depends upon the enzymatic activity against c-di-GMP, consistent with previous work on Xcc [8].
RpfG Negatively Regulates T3SS Expression
The T3SS in most of plant pathogenic bacteria is up-regulated during host infection and essential for virulence [39], [40]. In Dickeya dadantii, expression of the T3SS genes hrpA and hrpN was dramatically reduced in ΔecpB and ΔecpC, suggesting a potential role of c-di-GMP in T3SS regulation [7]. To investigate if RpfG is also involved in the T3SS regulation in Xoc, the expression of three key hrp regulatory genes, hrpG, hrpX and hrpA in the wild-type and ΔrpfG mutant strains was examined using quantitative real-time polymerase chain reaction (qRT-PCR) (Figure 4A). A significant increase of hrpG, hrpX and hrpA mRNA expression was detected in ΔrpfG, compared to the wild-type and complementation strains. Furthermore, the expression of these genes in the ΔrpfG mutant was evaluated using the promotor-β-glucuronidase (GUS) fusions. It was demonstrated that the GUS activity driven by the hrpX, hrpG and hrpA promoter was up-regulated in ΔrpfG by about 2.5-fold, five-fold and 1.5-fold compared to the wild-type strain, respectively (Figure 4B). Complementation of ΔrpfG with full-length rpfG reduced expression of these genes towards the wild-type level (Figure 4). Thus both sets of expression data from qRT-PCR and gusA fusions indicate that RpfG negatively regulates the expression of these hrp regulatory genes. Notably, these gene expression analyses were investigated in XOM3 minimal medium [41], where the growth rate of ΔrpfG is similar to that of wild-type Xoc strain (Figure S6).
Figure 4. The effect of RpfG on the expression of hrp-related genes in Xoc.
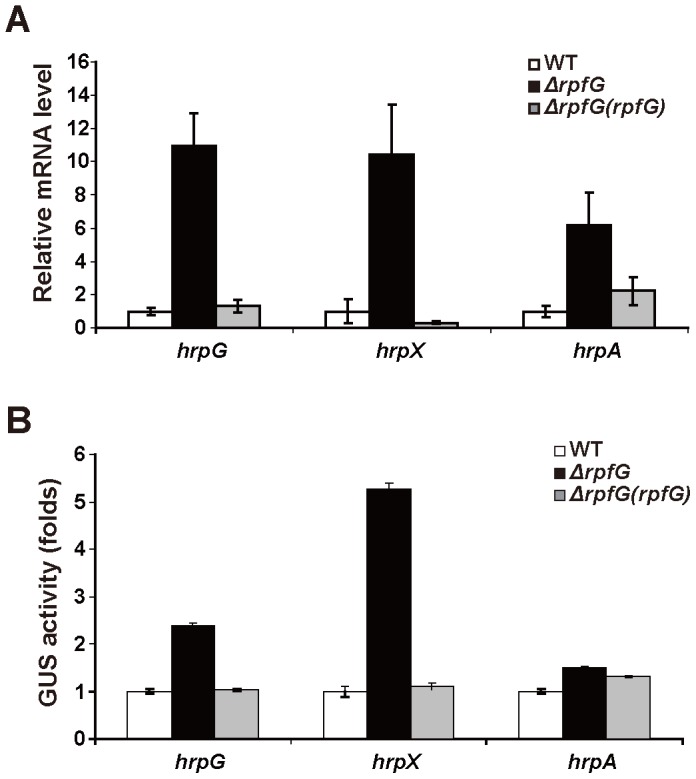
(A) Gene expression of hrpG, hrpX and hrpA in wild-type Xoc (RS105), ΔrpfG and complemented strain ΔrpfG(rpfG) was detected by qRT-PCR. 16S RNA was used as an internal control for data analyses. (B) Gene expression of hrpG, hrpX and hrpA in RS105, ΔrpfG and ΔrpfG(rpfG) strains was examined by GUS activities of appropriate promoter-GUS fusions. WT: wild-type.
RpfG has Divergent Effects on Expression of Genes Encoding Glycosyl Transferases
The Xoc genome encodes a number of putative glycosyltransferases, several of which have been demonstrated to be involved in biofilm formation in other bacteria. The gum operon, which is responsible for the synthesis of xanthan gum, has been shown to be involved in biofilm production in Xcc [42]. A modified gum cluster is required for biofilm formation in Xylella fastidiosa [43]. Deletion of a distinct gene cluster named xag in Xcc also resulted in decreased extracellular polysaccharide production and abolished biofilm formation [32]. In addition to gum and xag genes, the Xoc genome carries the pgaABCD operon, which is not found in Xcc. The pga operon directs the synthesis of poly-β-1,6-N-acetyl-D-glucosamine-like polysaccharide (β-1,6-GlcNAc; PGA), an extracellular polysaccharide that has been shown to serve as an adhesin and is required for biofilm formation in bacteria such as Staphylococcus epidermidis and Escherichia coli [44], [45], [46]. The effects of mutation of rpfG on biofilm formation, described above, prompted us to explore which of the three EPSs putatively produced by Xoc are important for biofilm formation.
As a first step towards this, the expression of xag, pga and gum operons in the wild-type and rpfG mutant backgrounds was quantified by qRT-PCR. The expression of pga and xag genes were up-regulated by 3 to 14 fold in ΔrpfG compared to the wild-type and complemented strains (Figure 5A and 5B). In contrast, the expression of all four tested gum genes was not dramatically altered or moderately up-regulated in the Xoc ΔrpfG mutant (Figure 5C). These data implied that RpfG has separate effects on expression of the pgaABCD and xagABCD operons and the gum cluster in Xoc.
Figure 5. The effect of rpfG deletion on the expression of three putative glycosyltransferase gene operons, pgaABCD, xagABCD and the gum cluster in Xoc.
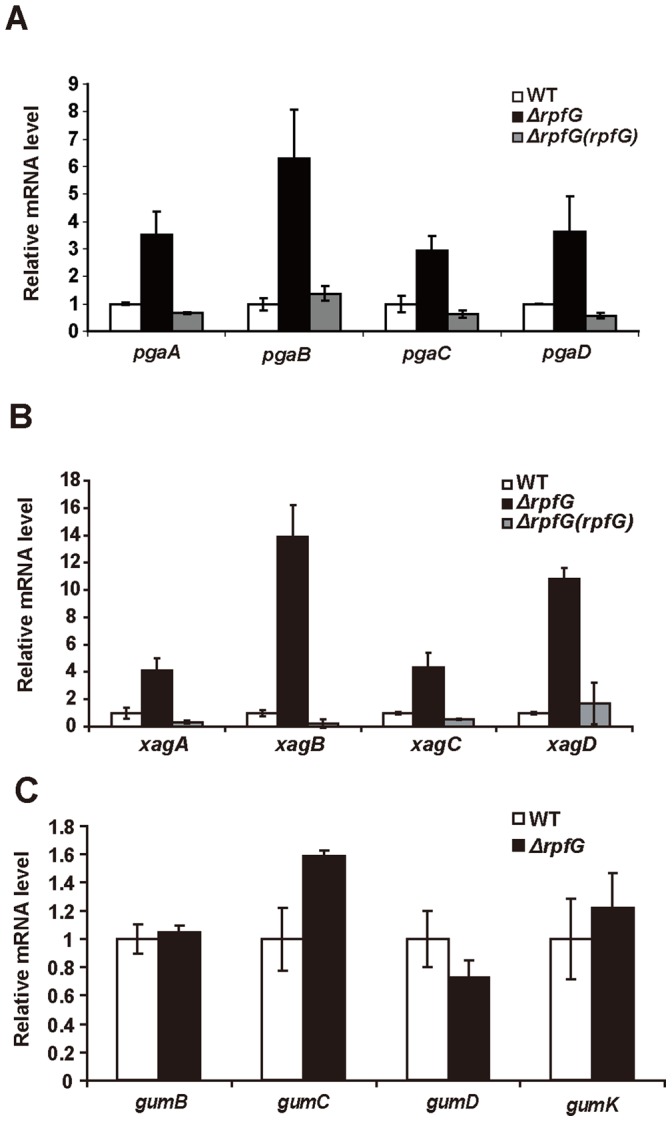
(A–C) Gene expression of pgaABCD (A), xagABCD (B) and gumB, gumC, gumD, gumK (C) in wild-type Xoc RS105, ΔrpfG and complemented ΔrpfG(rpfG) strains was detected by qRT-PCR. 16S RNA was used as an internal control for data analyses.
GumD and XagB, but not PgaC Contribute to Biofilm Formation in the Xoc ΔrpfG Mutant
To determine which EPSs are essential for biofilm formation in Xoc, the genes pgaA and pgaC in the pga operon, xagA and xagB in the xag operon and gumD in the gum operon were deleted from the Xoc wild-type and rpfG mutant strains using homologous recombination. The ability to produce biofilm and EPS was investigated in all these mutant strains. As shown in Figure 6A, the ΔrpfG/gumD, ΔrpfG/xagA and ΔrpfG/xagB double mutants formed much less biofilm than the ΔrpfG single mutant, while the ability of ΔrpfG/pgaA and ΔrpfG/pgaC double mutants to produce biofilm is not altered compared to ΔrpfG mutant in L-medium. In a consistent fashion, the complemented strains in which the full-length gumD and xagA were introduced into the respective double mutants ΔrpfG/gumD and ΔrpfG/xagA restored the production of biofilm towards that seen in the single rpfG mutant (Figure 6A). These data indicate that the GumD- and XagB-dependent EPSs but not PGA contribute to elevated biofilm formation in the rpfG mutant.
Figure 6. Effects of mutations of genes encoding several putative glycosyltransferases on biofilm formation and EPS production in Xoc wild-type and ΔrpfG mutant backgrounds.
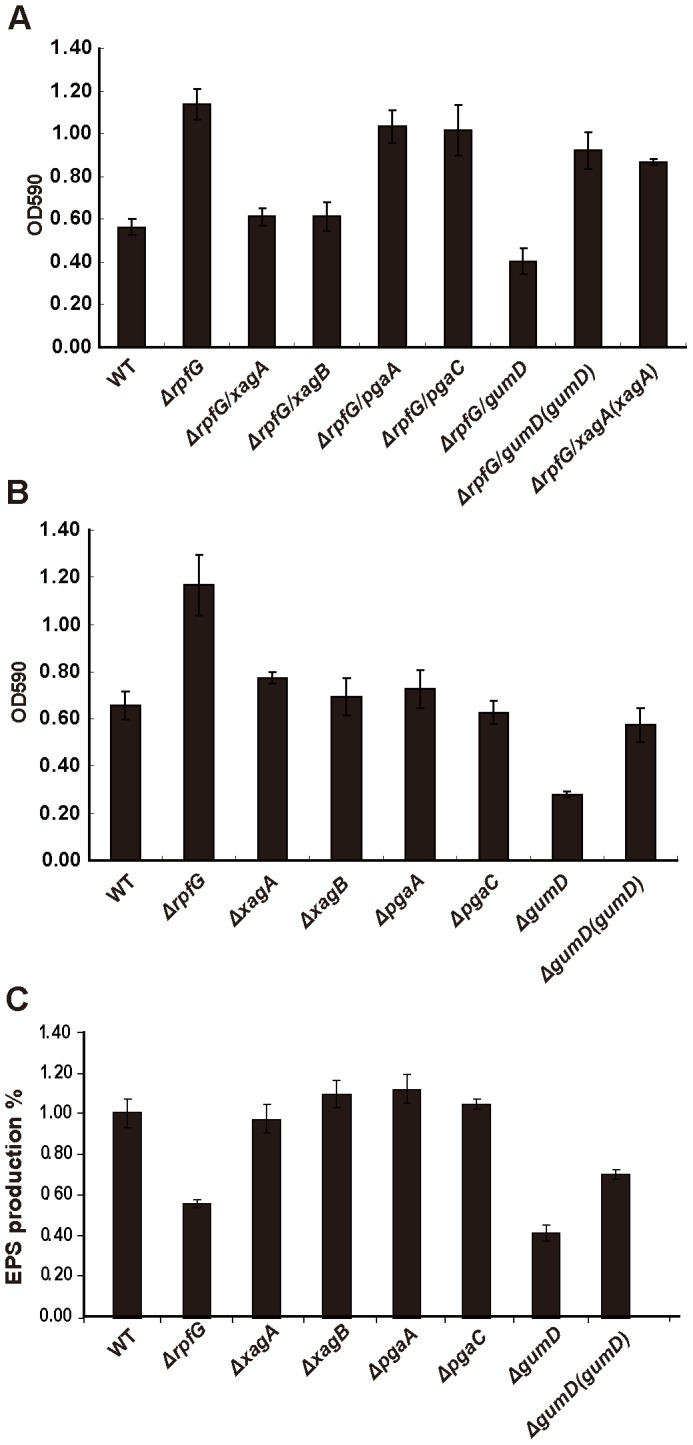
(A) Elevated biofilm formation in Xoc ΔrpfG was reduced to the wild-type level when xagA, xagB and gumD, but not pgaA or pgaC was deleted singly in the ΔrpfG genotype. The full length xagA and gumD genes restored the ability of biofilm formation in ΔrpfG/xagA and ΔrpfG/gumD mutants, respectively. (B) The ability of ΔgumD to form biofilm was greatly attenuated in the wild-type background, but was restored by complementation. Biofilm formation in the ΔpgaA, ΔpgaC, ΔxagA and ΔxagB single mutants was not altered compared to the wild-type strain. (C) EPS production was significantly reduced in the ΔgumD mutant, but not in ΔpgaA, ΔpgaC, ΔxagA and ΔxagB mutants compared to the wild-type. These experiments were repeated at least three times with similar results.
To investigate the function of these three putative glycosyltransferases in biofilm formation in the wild-type background, we measured biofilm formed by the wild-type Xoc and single mutants in L-medium (Figure 6B). The single gene mutants ΔpgaA, ΔpgaC, ΔxagA and ΔxagB produced similar amount of biofilm to the wild-type strain, while the ΔgumD mutant exhibited much less adhesion to glass than the wild-type strain when cultured in L-medium (Figure 6B) and in NB medium (data not shown). Complementation to generate the ΔgumD(gumD) strain restored the phenotype near to the wild-type level. These data indicate that GumD-dependent xanthan plays an essential role in biofilm formation in both wild-type and rpfG mutant backgrounds.
Swimming motility of these single gene-deletion mutants was also tested and was not significantly altered compared to the wild-type (Figure S7). The contribution of the different polysaccharides to total EPS production was further investigated for Xoc strains grown in M210 medium that contains sucrose (see Methods). EPS produced in the ΔpgaA, ΔpgaC, ΔxagA, ΔxagB and ΔgumD single mutants was quantified and compared to the wild-type. The findings (Figure 6C) showed that only deletion of gumD greatly disrupted the ability of Xoc to produce EPS, although this ability can be partially restored by complementation (Figure 6C). EPS produced by the ΔgumD mutant is even less than that by the ΔrpfG mutant. This suggests that xanthan is by far the major EPS produced in Xoc under the conditions used for this experiment.
GumD, XagB and PgaC have Differing Contributions to Xoc Virulence
Besides the effect on EPS production and biofilm formation, we investigated the effect of deletions of the putative glycosyltransferase genes gumD, xagB, and pgaC on virulence to rice (Figure 7). Virulence of these deletion mutants was determined using pressure inoculation into rice leaves. Independent repeated experiments showed that virulence of the gumD mutant was greatly attenuated while virulence of other mutants including xagA, xagB, pgaA and pgaC was not altered (Figure 7).
Figure 7. The effect of gumD deletion on bacterial virulence in Xoc.
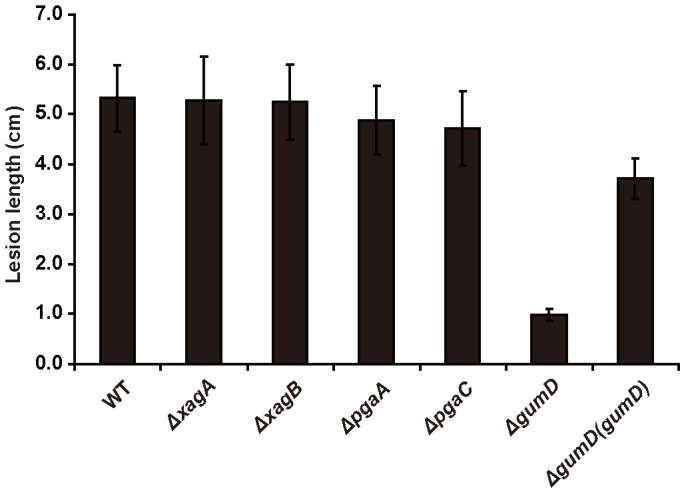
Xoc virulence to rice was greatly attenuated when gumD was deleted, but was unaltered in the ΔpgaA, ΔpgaC, ΔxagA and ΔxagB mutant strains. Virulence of the ΔgumD mutant was restored by the introduction of full-length gumD into the ΔgumD(gumD) strain. The length of disease lesions was measured at 20 days after pressure inoculation. Ten to 15 leaves were scored for each strain; means ± SE are shown. The experiments were repeated three times with similar results.
Discussion
Although more than 590 HD-GYP domain proteins in over 140 bacterial genomes have been reported, only a few have been characterized so far [8], [9], [47], [48]. RpfG in Xcc, which is one of the best-studied HD-GYP proteins, functions as a phosphodiesterase to break the signal molecule c-di-GMP and subsequently regulates various biological processes and bacterial virulence [8], [26], [27]. In the present study, we investigated the function of the three HD-GYP domain proteins (HgdA, RpfG and HgdC) in the important rice pathogen Xoc.
Deletion of hgdA and hgdC in Xoc had no effect on virulence to rice or on any of the phenotypes tested under our experimental conditions. In contrast, deletion of rpfG in Xoc resulted in decreased bacterial virulence to rice, increased biofilm formation and alterations in the expression of T3SS genes and genes encoding enzymes involved in the synthesis of different EPSs. The effects of rpfG mutation on virulence, synthesis of EPS and expression of xag and gum genes are similar to those seen in Xcc. However, the deletion of rpfG has no influence on swimming motility and the secretion of extracellular proteases in Xoc, while RpfG in Xcc positively regulates pilus-dependent motility and the secretion of extracellular enzymes including endoglucanase, endomannase and proteases [26], [27]. RpfG proteins in Xoc and Xcc are highly conserved (95.2% amino acid identical), both are active in c-di-GMP degradation and their regulatory activity depends upon their enzymatic activity. Thus differences in the regulatory functions of RpfG may reflect differences in the complement or action of c-di-GMP effectors in the two bacteria. Similar considerations may explain recent observations that DSF signaling system might regulate virulence-associated traits in a completely contrasting pattern in Xoo and Xcc [22], [27], [49].
Mutation of rpfG led to different effects on expression of three operons directing the synthesis of different EPSs. The expression of xag and pga genes was significantly up-regulated in ΔrpfG mutant, whereas the expression of gum genes was slightly increased or unaltered in the mutant (Figure 5). Consistent with our observations, comparisons of RpfF, RpfC and RpfG regulons in X. citri subsp. citri revealed that XAC3522-XAC3525 genes that share high sequence identities with xagABCD in Xoc were up-regulated in ΔrpfG mutant [40]. Deletions of rpfF, rpfC or rpfG genes in Xcc led to drastically increased expression of xagABCD [32]. It is interesting to note that distribution of these different operons in different Xanthomonas spp. Comparative genomics reveals that although the gum, xagABCD and pgaABCD operons are all found in the Xoc genome, xagABCD is not found in Xoo and pgaABCD is not found in Xcc. Wider distribution of the gum operon implies that the gum-dependent EPS is likely more important to biofilm formation and other virulence-associated traits. Extensive phenotype analyses of multiple single and double mutants confirmed this hypothesis; gumD is critical for biofilm formation, EPS production and virulence under the wild-type and ΔrpfG mutant background (Figure 6 and 7). Further functional analyses showed that the deletion of xagA and xagB had different effects on biofilm formation under the ΔrpfG mutant and wild-type background (Figure 6A and 6B). The ΔrpfG/pgaA and ΔrpfG/pgaC double mutants exhibited the phenotype of ΔrpfG mutant in biofilm formation. The results indicate that both gum- and xag-dependent EPSs contribute to elevated adhesion in the Xoc ΔrpfG mutant. In Pseudomonas aeruginosa, at least three exopolysaccharides (alginate, Psl, and Pel) contribute to the formation of biofilms [50]. In E. coli cells, the glycosyltransferase PgaC is required for the synthesis of PGA that functions as an adhesin for biofilm formation [46]. Gene deletion analyses demonstrated that PgaC plays a minor role in biofilm formation in Xoc, suggesting that other adhesins present in this pathogen might have a role.
The regulatory activity of RpfG on biofilm formation and virulence factor synthesis in both Xoc and Xcc depends upon its action against c-di-GMP (Figure 3) and mutation of rpfG leads to an increase in the level of the nucleotide in Xcc [8]. The enzymatic action of RpfG is modulated by phosphorylation during sensing and transduction of the DSF signal. We speculate that similar changes in c-di-GMP level that are seen in the rpfG mutant can occur in the wild-type as a response to the presence (or absence) of different environmental cues that include DSF.
Deletion of rpfG in Xoc resulted in nearly complete loss of bacterial virulence despite unaltered bacterial motility and protease secretion and enhanced biofilm formation. A previous study in D. dadantii 3937 showed that both c-di-GMP PDEs, EcpB and EcpC, were required for the expression of the type III secretion system (T3SS) that is essential for bacterial virulence [7]. Reduced expression of T3SS is hypothesized to be one of the factors responsible for reduced bacterial virulence in the ecpB and ecpC mutants of D. dadantii 3937. Therefore, we investigated the expression of T3SS regulatory genes in Xoc wild-type and ΔrpfG mutant strains. HrpG, an OmpR family response regulator, controls the expression of hrpA to hrpF and hrpX in X. campestris pv. vesicatoria [51], [52] or only hrpX in Xcc [53]. HrpX, an AraC-type regulator, activates the expression of the hrpB operon and several effector genes [52], [54], [55]. Both GUS-fusion transcriptional assays and qRT-PCR results showed hrpG, hrpX and hrpA genes were all up-regulated in the Xoc rpfG mutant when cultured in minimal media XOM3. Paradoxically, although the Xoc rpfG mutant had elevated expression of T3SS, its virulence to rice was attenuated. We speculate that RpfG has such a broad regulatory influence on the cell that up-regulation of some factors such as T3SS cannot compensate for the down-regulation of others or override the negative effect of sustained biofilm formation. In Xcc, the expression of some hrp genes was also down-regulated by RpfF and diffusible siganl factor (DSF) under in vitro culture conditions [39]. In contrast, transcriptome analysis of RpfG regulon indicated that a few genes encoding the T3SS translocon and effectors were up-regulated by RpfG in X. citri subsp. citri [40]. However, these experiments were performed in rich medium where hrp genes are not fully expressed therefore it is comparison with the work described here on Xoc grown in XOM3 minimal medium should be made cautiously.
Identification of some aspects of the function of RpfG in Xoc, to include effects on EPS production and biofilm formation, increases our understanding of the c-di-GMP signaling in the regulation of virulence and virulence-associated traits. Nevertheless our picture of the roles of RpfG in regulation of bacterial virulence in Xoc is still far from complete.
Materials and Methods
Plant Materials, Bacterial Strains, Plasmids and Culture Conditions
Rice plants (Oryza sativa cvs. Nipponbare and Jingang 30) were grown in greenhouse. Bacterial strains and plasmids used in this study are listed in Table 1. The Xoc RS105 wild-type and mutant strains were grown in NB medium (beef extract, 3 g/L; yeast extract, 1 g/L; tryptone, 5 g/L; sucrose, 10 g/L ), NYG medium or in XOM3, T3SS-inducing minimal medium [56] at 28°C. Antibiotics were used at the following concentrations: ampicillin, 100 µg/ml; kanamycin, 50 µg/ml; rifampin (Rif), 25 µg/ml. All the experiments were repeated at least three times with similar results unless noted.
Table 1. Bacterial strains and plasmids.
| Strains/plasmids | Characteristics | References or source |
| E. coli | ||
| DH5α | High efficiency transformation | |
| pRK600 | Helper strain in tri-parental mating | |
| X. oryzae | ||
| RS105 | Wild-type, RifR | |
| ΔhgdA | In frame deletion of XOC_1984, RifR | This Study |
| ΔrpfG | In frame deletion of rpfG, RifR | This Study |
| ΔhgdC | In frame deletion of XOC_4564, RifR | This Study |
| ΔhgdA/rpfG | In frame deletion of XOC_1984 and rpfG, RifR | This Study |
| ΔhgdA/hgdC | In frame deletion of XOC_1984 and XOC_4564, RifR | This Study |
| ΔrpfG/hgdC | In frame deletion of rpfG and XOC_4564, RifR | This Study |
| ΔhgdA/rpfG/hgdC | In frame deletion of XOC_1984, rpfG and XOC_4564, RifR | This Study |
| ΔgumD | In frame deletion of gumD, RifR | This Study |
| ΔpgaA | In frame deletion of XOC_0767, RifR | This Study |
| ΔpgaC | In frame deletion of XOC_0765, RifR | This Study |
| ΔxagA | In frame deletion of XOC_3785, RifR | This Study |
| ΔxagB | In frame deletion of XOC_3784, RifR | This Study |
| ΔrpfG/gumD | In frame deletion of rpfG and gumD, RifR | This Study |
| ΔrpfG/pgaA | In frame deletion of rpfG and XOC_0767, RifR | This Study |
| ΔrpfG/pgaC | In frame deletion of rpfG and XOC_0765, RifR | This Study |
| ΔrpfG/xagA | In frame deletion of rpfG and XOC_3785, RifR | This Study |
| ΔrpfG/xagB | In frame deletion of rpfG and XOC_3784, RifR | This Study |
| Plasmids | ||
| pUFR80 | Suicide vector for homologous recombination, KmR | [60] |
| pVSP61 | Expression vector, KmR | [61] |
| pMD18-T | High efficiency cloning vector, AmpR | Takara |
| pVSP61-rpfGxoc | Complementation, rpfGxoc cloned in pVSP61, KmR | This study |
| pVSP61-rpfFxoc | Complementation, rpfFxoc cloned in pVSP61, KmR | This study |
| pVSP61-xagAxoc | Complementation, xagAxoc cloned in pVSP61, KmR | This study |
| pQE30 | In-vitro expression vector, AmpR | This study |
| pQE30-rpfG | rpfG cloned in pQE30 for RpfG purification, AmpR | This study |
Construction of Xoc Mutant Strains using Non-marker Homologous Recombination
Construction of Xoc mutant strains was performed following the procedures described by Sun et al with minor modifications [57], [58]. DNA was isolated from the Xoc wild-type strain RS105 using a genomic DNA isolation kit (New Industry Company, Beijing, China) following provided instructions. Two fragments approximately 800 bp to 1 kb long, upstream and downstream close to the start and stop codons of rpfG, were amplified separately via PCR from Xoc genomic DNA using Pfu polymerase. The used primer sets rpfG-XhoI-F/rpfG-del-R and rpfG-del-F/rpfG-HindIII-R are listed in Table S1 with underlined XhoI and HindIII restriction sites, respectively. PCR products were gel purified and added together into a fusion PCR reaction. The resultant PCR fragment carrying flanking regions of the rpfG gene but lacking the rpfG open reading frame was cloned into the pUFR80 sacB suicide vector [59], [60]. The pUFR80-ΔrpfG plasmid was transferred into Xoc RS105 by triparental mating and subjected to kanamycin selection. Single transformation colonies of Xoc with kanamycin resistance were picked and cultured overnight in NB medium without kanamycin and sucrose, then spread onto NA plates with 5% sucrose to screen sucrose-insensitive clones. The gene-deletion genotype of kanamycin-sensitive/sucrose-insensitive Xoc colonies was confirmed by colony PCR and sequencing PCR products, as well as by Southern blot analyses. The same strategy was applied to construct other gene-deletion strains including ΔhgdA, ΔhgdC, ΔgumD, ΔpgaA, ΔpgaC, ΔxagA and ΔxagB except that different restriction enzyme sites were created by PCR for deletion fragments (Table S1). Single and double unmarked mutants were used to construct the second and third gene deletion, respectively.
Construction of Complementation Strains for Xoc Mutant Strains
For complementation, the full-length rpfG gene including the 5′- and 3′- regulatory sequences (687 bp and 225 bp respectively) were amplified by PCR using the respective primer sets rpfG-XhoI-F/rpfG-HindIII-R (Table S1). The resultant PCR fragments were cloned into the wide host range vector pVSP61 [61] and mated into the specified Xoc strains. Hence the rpfG complementation construct carried short segments of adjacent open reading frames from separate operons but no other full-length genes. All other complementation strains were constructed using the same procedure with the primer sets listed in Table S1. All constructs were subjected to sequencing.
Southern Blot Analysis
Southern blot analysis was performed using standard molecular biology methods unless noted [62]. Briefly, genomic DNA was isolated from Xoc strains as described above and then digested with appropriate restriction enzymes. After separated with agarose gel, genomic DNA was blotted onto nylon membrane and probed with a 32P-labeled PCR product generated with the primer sets rpfG-probe-F/rpfG-probe-R, hgdA-probe-F/hgdA-probe-R and hgdC-probe-F/hgdC-probe-R, respectively (Table S1).
Site-directed Mutagenesis
Site-directed mutagenesis for changing HD residues to AA residues in HD-GYP domain proteins was performed by two-step fusion PCR [57]. In the first round of PCR, two separate reactions were carried out using the primer sets, rpfG-XhoI-F/rpfG-MutHD-R and rpfG-MutHD-F/rpfG-HindIII-R, respectively. The primers rpfG-MutHD-R and rpfG-MutHD-F were intentionally designed to be partially complementary to each other and to change the His-Asp codons to Ala-Ala codons (Table S1). DNA fragments amplified from the first round of PCR were added together in a fusion PCR with the primer set rpfG-XhoI-F and rpfG-HindIII-R. The resultant PCR products were then subcloned into the pVSP61 expression vector for functional studies.
Biofilm Assays
The protocol for measuring biofilm formation was adapted from the method described by O’Toole and Kolter [63]. Briefly, overnight bacterial cultures were inoculated into 5 ml L medium (tryptone, 10 g/L; yeast extract, 5 g/L; NaCl, 5 g/L; glucose, 1 g/L) with 1∶1000 dilution and incubated in the borosilicate glass tubes without shaking at 28°C for 1 week. The cultured cells were then stained with crystal violet (CV) for 15 min. The unbound dye was removed by rinsing with H2O. The glass-bound dye was solubilized in 90% ethanol and quantified by spectrophotometry at 590 nm.
Quantitative Determination of EPS
The quantity of EPS produced in Xoc strains was determined using the method as described [64], [65]. Briefly, overnight cultures of the Xoc wild-type and mutant strains were collected and re-suspended in sterile water to an OD600 of 1.0. The cells were then diluted at 1∶1000 in M210 medium (casein enzymatic hydrolysates, 8 g/L; yeast extract, 4 g/L; sucrose, 5 g/L; KH2PO4, 3 g/L; MgSO4.7H2O, 0.3 g/L) and cultured overnight to cell density of OD600≈2. The cell cultures (10 ml) were collected by centrifugation at 12,000 rpm for 10 min. The supernatants were mixed with two volumes of absolute ethanol and incubated at –20°C overnight to precipitate EPS. The pellet was then collected by centrifugation at 10,000 rpm for 5 min and fully dried at 55°C before weighing.
Protease Assays
The secretion of proteases in Xoc strains was evaluated on the plates with skimmed milk [66]. Overnight cultures of Xoc were collected by centrifugation and re-suspended in sterile water to cell density of 109 cfu/ml. Five microliter of cells were spotted onto nutrient agar (NYGA) or water agar plates containing 1% (w/v) skimmed milk and incubated at 28°C for 4 days. The proteolytic activity of Xoc strains was quantified by measuring the diameter of clearing zones around the colonies that were formed after proteolytic degradation of milk proteins.
Motility Assays
Swimming motility of Xoc strains was investigated on semisolid medium plates with 0.3% noble agar as described by DiLuzio et al [67]. All Xoc strains were inoculated into the center of the plates by pipetting. After incubating at 28°C for 4 days, the colony diameter was measured.
Virulence Assays of Xoc Strains on Rice
Virulence on rice of different Xoc strains was investigated by pressure inoculation [5]. Overnight Xoc cultures were diluted to an OD600 of 0.3 and injected into the leaves of 6-week-old rice plants with needleless syringes. The length of disease lesion on the leaves was measured at 14 to 20 days after inoculation. At least 10 leaves were inoculated and scored for each tested Xoc strain. For establishing growth curves, inoculated rice leaves were harvested at four time points (0, 5, 10, 15 days after inoculation), immediately sliced into small pieces, incubated in 1 ml sterile water including 25 µg/ml of rifampicin with shaking for 1 h, and then filtered through two layers of sterilization gauze. The filtrates were diluted and then plated onto NA agar plates with antibiotics. Colonies on the plates were counted after 3 days of incubation at 28°C [68].
In vitro Protein Expression and Purification
The open reading frame of rpfG was amplified from Xoc RS105 genome by PCR using primers rpfG-BamHI-F and rpfG-HindIII-R (seen in Table S1). The PCR fragment was subcloned into the pQE30 expression vector (Qiagen) after digestion with BamHI and HindIII. The construct was transformed into E. coli XL1-blue cells and sequenced to confirm no nucleotide changes. Cells were grown in 5 ml of LB medium containing ampicillin overnight at 37°C. The culture was 1∶50 diluted and grown further until it reached to an OD600 of 0.5∼0.7. Isopropyl β-D-thiogalactopyranoside (IPTG) was then added to a final concentration of 1 mM to induce the expression of proteins at 28°C. After 3 h of incubation, the cells were collected by centrifugation, re-suspended in lysis buffer (50 mM NaH2PO4, 300 mM NaCl, and 10 mM imidazole, pH 8.0), and then sonicated with 10 s pauses at 200∼300 W for 6 times. The lysates were centrifuged at 10,000 g for 30 min and the supernatant was then loaded onto nickel-nitrilotriacetic acid agarose superflow columns (Qiagen), which were subsequently rinsed with wash buffer (50 mM NaH2PO4, 300 mM NaCl, and 20 mM imidazole, pH 8.0). The bound His6-tagged proteins were eluted with elution buffer (50 mM NaH2PO4, 300 mM NaCl, and 250 mM imidazole, pH 8.0) and dialyzed extensively in PBS (pH 7.4). The concentration of proteins was determined using the BCA protein assay kit (Pierce).
PDE Colorimetric Assays
The PDE activity of in vitro purified proteins were assayed by incubation with bis(p-nitrophenyl) phosphate [69]. Purified proteins (20 µg) were incubated with 5 mM bis(p-nitrophenyl) phosphate at 37°C for 1.5 h in assay buffers (50 mM Tris-HCl, 1 mM MnCl2, pH 8.5). The release of p-nitrophenol was then quantified at OD410 using spectrophotometer.
PDE Enzyme Assay by HPLC and Mass Spectrometry
The PDE activity of purified proteins was also assayed by detecting the degradation of c-di-GMP as described [7], [8]. The reaction assay mix included 20 µg purified protein, 100 µM c-di-GMP, 50 mM Tris-HCl (pH 7.6), 10 mM MgCl2, 10 mM MnCl2, 0.5 mM EDTA and 50 mM NaCl in a total volume of 600 µl. After incubated in 37°C for 6 h, the reaction mix was boiled for 3 min to stop the reaction. The supernatant was collected by centrifugation at 15,000 g for 2 min and filtered through a 0.22 µm filter. The HPLC analysis was performed on a reversed phase C18 column (250×4.60 mm; Phenomenex, USA) with an Agilent 1100 series. The samples were separated at a flow rate of 1 ml/min under isocratic condition in eluent A (20 mM potassium phosphate buffer, pH 5.8, containing 1% methanol) at the first three minutes and then on a linear gradient from 0–20% methanol in the next 20 minutes.
Separation of nucleotides for mass spectrometry was performed on a reversed phase C18 column in a liner gradient from 0–20% buffer B (acetonitrile containing 0.01% formic acid) in buffer A (0.1% ammonium formate, pH 3.7). Mass spectrometry was operated at negative ion mode with Agilent 1100 series LC/MSD Trap (VL). The GMP and c-di-GMP standards were purchased from Sigma (USA) and Biolog (Germany), respectively.
RNA Isolation and qRT-PCR
Overnight Xoc cultures were diluted in XOM3 medium to an OD600 of 0.08 and grown till OD600 = 0.6, and then harvested for RNA isolation. RNA was isolated using PureYield™ RNA midiprep System (Promega) according to the manufacturer’s instructions. The isolated RNA was used as a template in a PCR reaction with the primer set 16S-RNA-F/16S-RNA-R (seen in Table S1) to confirm no DNA contamination. RNA (60 ng) was then used to synthesize cDNA with the TransScript II first-strand cDNA synthesis supermix (Transgen, Beijing, China). The SYBR Green premix ExTaq (Takara) was used in qRT-PCR reactions to quantify the transcript levels. 16S rRNA was used as the internal reference for data analysis.
Promoter-GUS Fusion
The promoter regions of hrpG, hrpX and hrpA were amplified from Xoc RS105 genome by PCR using primers hrpG-pro-F/hrpG-pro-R, hrpX-pro-F/hrpX-pro-R and hrpA-pro-F/hrpA-pro-R, respectively (Table S1). PCR products were subcloned into the pJY-Tn7T-GUS vector after digestion with appropriate restriction enzymes [70]. After verified by sequencing, constructed plasmids and the transposase pTNS-1 helper plasmid were co-transferred into Xoc strains by parental mating [71]. The insertion-containing transformants were screened on the NA plates supplemented with 25 µg/ml rifampicin, 50 µg/ml streptomycin, 100 µg/ml spectinomycin. Colony PCR with primers GUS-1/GUS-2 (Table S1) was used to confirm the promoter-GUS fusion.
Bacterial cells were collected by centrifugation at 12,000 rpm for 3 min. Total soluble proteins were prepared by sonication in lysis buffer (20 mM Tris-HCl, pH 7.4; 5 mM EDTA, 10 mM mercaptoethanol, 1% Triton X-100). The GUS activity of soluble proteins was assessed using 4-methylumbelliferyl-β-D-glucuronide(MUG) as substrates according to the standard protocol [72], [73]. Fluorescence was measured on an Infinite F200 microplate reader (TECAN, Austria) with excitation at 360 nm and emission at 485 nm.
Statistical Analysis
Means and standard errors of experimental data were calculated using Microsoft Office Excel. All statistical analyses were performed by Duncan’s multiple range test using SAS software. P<0.05 was considered statistically significant.
Supporting Information
Predicted domain organizations of three HD-GYP domain proteins HgdA (XOC1984), RpfG (XOC2264) and HgdC (XOC4564). HgdA and RpfG have an HD-GYP domain in association with an N-terminal CheY-like response receiver (REC) regulatory domain. HgdC has an HD-GYP domain with additional, uncharacterized N-terminal and C-terminal domains. The numbers indicate amino acid residue positions.
(TIF)
Xoc rpfG -related mutants were verified by Southern blot analyses. Digested genomic DNA was separated, blotted onto membrane and then probed with the isotope-labelled rpfG-probe (A), hgdA-probe (B) and hgdC-probe (C) PCR fragments. A, Genome DNA from the wild-type (lane 1 and 2) and ΔrpfG (lane 3 and 4) strains digested by ApaI (lane 1 and lane3) and BamHI (lane 2 and 4) was hybridized with rpfG-probe. B, Genome DNA from the indicated mutant strains digested by KpnI and EcoRI/EcoRV was hybridized with hgdA-probe. C, Genome DNA from hgdC-related mutant strains digested by BamHI and SmaI was hybridized with hgdC-probe. The primers were designed to amplify DNA fragments as probes that do not hybridize with genome DNA of mutant strains because the fragments were deleted via homologous recombination. M: Marker.
(TIF)
The effect of rpfF mutation on biofilm formation in Xoc . Biofilm formation was dramatically increased in Xoc ΔrpfF mutant when cultured in L-medium. Complementation with introduction of the full-length rpfF gene to produce the ΔrpfF(rpfF) strain reduced biofilm formation to the wild-type level. WT: wild-type.
(TIF)
Effects of hgdA , rpfG and hgdC mutations on swimming motility and protease secretion in Xoc . (A) The amount of secreted proteases in Xoc was assessed by the diameter of clearing zones produced after the hydrolysis of skimmed milk on water agar plates. (B) Swimming motility of the Xoc wild-type and mutant strains was determined on semisolid plates with 0.3% noble agar. The motility was indicated by the diameter (cm) of the radial growth.
(TIF)
The phosphodiesterase activity of Xoc RpfG. Xoc RpfG was in vitro expressed as an N-terminal His6-tagged fusion and then purified using nickel columns under native conditions. The PDE activity of Xoc RpfG against c-di-GMP was assessed by reverse phase High Performance Liquid Chromatography (HPLC). (A-D) HPLC analyses of the RpfGXoc PDE activity using c-di-GMP as a substrate. (A) and (B) GMP and c-di-GMP standard. (C) The purified RpfGXoc had activity against standard cyclic di-GMP, generating two hydrolytic products with the retention time at 6.024 s and 12.922 s, respectively after purified RpfGXoc was incubated with c-di-GMP for 6 h. (D) Reaction control without RpfGXoc. C-di-GMP was stable and no degraded product but only c-di-GMP was detected. (E–F) Mass spectrometry, operated at negative ion mode, was used to confirm the identity of HPLC fractions in Figure S5C. (E) The GMP peak was detected by LC-MS at an m/z of 362.0. (F) The second peak was distinct from c-di-GMP and GMP with a [M-H]+ m/z at 707.1, which corresponds to the intermediate product pGpG.
(TIF)
The growth rate of ΔrpfG compared to those of the wild-type and complemented strains in XOM3 minimal medium. The bacterial population was determined by counting colony forming units after manual plating at the indicated time points. WT: wild-type.
(TIF)
Effects of xagA , xagB , pgaA , pgaC and gumD deletions on swimming motility in Xoc . Swimming motility of the Xoc wild-type, mutant and complementation strains was determined on semisolid plates with 0.3% noble agar. The motility was indicated by the diameter (cm) of the radial growth.
(TIF)
Primers used in this study.
(DOC)
Acknowledgments
We thank Zejian Guo at China Agricultural University, Yawen He at Shanghai Jiaotong University for helpful suggestions and discussion. We also thank Caitilyn Allen at University of Wisconsin-Madison, Congfeng Song at Nanjing Agricultural University, Chenyang He at Institute of Plant Protection, Chinese Academy of Agricultural Sciences for providing materials.
Funding Statement
The work was supported by the 973 program 2011CB100700, National Natural Science Foundation of China (NSFC) grant 31272007, PCSIRT Program IRT1042, NCET-09-0736 and Ph.D. Programs Foundation of Ministry of Education of China to WS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Awoderu V (1991) Rice yellow mottle virus in West Africa. Int J Pest Manage 37: 356–362. [Google Scholar]
- 2.Ou SH (1985) Rice diseases. Kew, Surrey: Commonwealth Agricultural Bureau.
- 3.Moffett M, Croft B (1983) Xanthomonas. In: Plant Bacterial Diseases (Fahy, P.C. and Persley, G.J., eds), p. 393. New York: Academic Press.
- 4. NiÑO-Liu DO, Ronald PC, Bogdanove AJ (2006) Xanthomonas oryzae pathovars: model pathogens of a model crop. Mol Plant Pathol 7: 303–324. [DOI] [PubMed] [Google Scholar]
- 5. Wang L, Makino S, Subedee A, Bogdanove AJ (2007) Novel candidate virulence factors in rice pathogen Xanthomonas oryzae pv. oryzicola as revealed by mutational analysis. Appl Environ Microbiol 73: 8023–8027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zou HS, Yuan L, Guo W, Li YR, Che YZ, et al. (2011) Construction of a Tn5-tagged mutant library of Xanthomonas oryzae pv. oryzicola as an invaluable resource for functional genomics. Curr Microbiol 62: 908–916. [DOI] [PubMed] [Google Scholar]
- 7. Yi X, Yamazaki A, Biddle E, Zeng Q, Yang C-H (2010) Genetic analysis of two phosphodiesterases reveals cyclic diguanylate regulation of virulence factors in Dickeya dadantii . Mol Microbiol 77: 787–800. [DOI] [PubMed] [Google Scholar]
- 8. Ryan RP, Fouhy Y, Lucey JF, Crossman LC, Spiro S, et al. (2006) Cell–cell signaling in Xanthomonas campestris involves an HD-GYP domain protein that functions in cyclic di-GMP turnover. Proc Natl Acad Sci U S A 103: 6712–6717. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9. Ryan RP, Lucey J, O’Donovan K, McCarthy Y, Yang L, et al. (2009) HD-GYP domain proteins regulate biofilm formation and virulence in Pseudomonas aeruginosa . Environ Microbiol 11: 1126–1136. [DOI] [PubMed] [Google Scholar]
- 10. Yang F, Tian F, Sun L, Chen H, Wu M, et al. (2012) A novel two-component system PdeK/PdeR regulates c-di-GMP turnover and virulence of Xanthomonas oryzae pv. oryzae . Mol Plant-Microbe Interact 25: 1361–1369. [DOI] [PubMed] [Google Scholar]
- 11. Ross P, Weinhouse H, Aloni Y, Michaeli D, Weinberger-Ohana P, et al. (1987) Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature 325: 279–81. [DOI] [PubMed] [Google Scholar]
- 12. Simm R, Morr M, Kader A, Nimtz M, Römling U (2004) GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol Microbiol 53: 1123–1134. [DOI] [PubMed] [Google Scholar]
- 13. Hickman JW, Tifrea DF, Harwood CS (2005) A chemosensory system that regulates biofilm formation through modulation of cyclic diguanylate levels. Proc Natl Acad Sci U S A 102: 14422–14427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Römling U (2005) Characterization of the rdar morphotype, a multicellular behaviour in Enterobacteriaceae. Cell Mol Life Sci 62: 1234–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jenal U, Malone J (2006) Mechanisms of cyclic-di-GMP signaling in bacteria. Annu Rev Genet 40: 385–407. [DOI] [PubMed] [Google Scholar]
- 16. Cotter PA, Stibitz S (2007) C-di-GMP-mediated regulation of virulence and biofilm formation. Curr Opin Microbiol 10: 17–23. [DOI] [PubMed] [Google Scholar]
- 17. Dow JM, Fouhy Y, Lucey JF, Ryan RP (2006) The HD-GYP domain, cyclic di-GMP signaling, and bacterial virulence to plants. Mol Plant-Microbe Interact 19: 1378–1384. [DOI] [PubMed] [Google Scholar]
- 18. Paul R, Weiser S, Amiot NC, Chan C, Schirmer T, et al. (2004) Cell cycle-dependent dynamic localization of a bacterial response regulator with a novel di-guanylate cyclase output domain. Gene Dev 18: 715–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schmidt AJ, Ryjenkov DA, Gomelsky M (2005) The ubiquitous protein domain EAL is a cyclic diguanylate-specific phosphodiesterase: enzymatically active and inactive EAL domains. J Bacteriol 187: 4774–4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Solano C, García B, Latasa C, Toledo-Arana A, Zorraquino V, et al. (2009) Genetic reductionist approach for dissecting individual roles of GGDEF proteins within the c-di-GMP signaling network in Salmonella . Proc Natl Acad Sci U S A 106: 7997–8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Galperin M (2005) A census of membrane-bound and intracellular signal transduction proteins in bacteria: bacterial IQ, extroverts and introverts. BMC Microbiol 5: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ryan RP, Fouhy Y, Lucey JF, Jiang BL, He YQ, et al. (2007) Cyclic di-GMP signalling in the virulence and environmental adaptation of Xanthomonas campestris . Mol Microbiol 63: 429–442. [DOI] [PubMed] [Google Scholar]
- 23. Bogdanove AJ, Koebnik R, Lu H, Furutani A, Angiuoli SV, et al. (2011) Two new complete genome sequences offer insight into host and tissue specificity of plant pathogenic Xanthomonas spp . J Bacteriol 193: 5450–5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Steven L, Daniel D, Michael C, Adam P, Pablo R, et al. (2008) Genome sequence and rapid evolution of the rice pathogen Xanthomonas oryzae pv. oryzae PXO99A. BMC Gen 9: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Han SW, Sriariyanun M, Lee SW, Sharma M, Bahar O, et al. (2011) Small protein-mediated quorum sensing in a gram-negative bacterium. PLoS One 6: e29192. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26. Slater H, Alvarez-Morales A, Barber CE, Daniels MJ, Dow JM (2000) A two-component system involving an HD-GYP domain protein links cell-cell signalling to pathogenicity gene expression in Xanthomonas campestris . Mol Microbiol 38: 986–1003. [DOI] [PubMed] [Google Scholar]
- 27. Dow JM, Crossman L, Findlay K, He YQ, Feng JX, et al. (2003) Biofilm dispersal in Xanthomonas campestris is controlled by cell–cell signaling and is required for full virulence to plants. Proc Natl Acad Sci U S A 100: 10995–11000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Crossman L, Dow JM (2004) Biofilm formation and dispersal in Xanthomonas campestris . Microbes Infect 6: 623–629. [DOI] [PubMed] [Google Scholar]
- 29. Andrade MO, Alegria MC, Guzzo CR, Docena C, Pareda Rosa MC, et al. (2006) The HD-GYP domain of RpfG mediates a direct linkage between the Rpf quorum-sensing pathway and a subset of diguanylate cyclase proteins in the phytopathogen Xanthomonas axonopodis pv citri . Mol Microbiol 62: 537–551. [DOI] [PubMed] [Google Scholar]
- 30. Ryan RP, McCarthy Y, Andrade M, Farah CS, Armitage JP, et al. (2010) Cell-cell signal-dependent dynamic interactions between HD-GYP and GGDEF domain proteins mediate virulence in Xanthomonas campestris . Proc Natl Acad Sci U S A 107: 5989–5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dow M (2008) Diversification of the function of cell-to-cell signaling in regulation of virulence within plant pathogenic xanthomonads. Science’s STKE 1: pe23. [DOI] [PubMed] [Google Scholar]
- 32. Tao F, Swarup S, Zhang LH (2010) Quorum sensing modulation of a putative glycosyltransferase gene cluster essential for Xanthomonas campestris biofilm formation. Environ Microbiol 12: 3159–3170. [DOI] [PubMed] [Google Scholar]
- 33. Dow J, Daniels M (1994) Pathogenicity determinants and global regulation of pathogenicity of Xanthomonas campestris pv. campestris . Curr Top Microbiol 192: 29–41. [DOI] [PubMed] [Google Scholar]
- 34. Feng JX, Song ZZ, Duan CJ, Zhao S, Wu YQ, et al. (2009) The xrvA gene of Xanthomonas oryzae pv. oryzae, encoding an H-NS-like protein, regulates virulence in rice. Microbiol 155: 3033–3044. [DOI] [PubMed] [Google Scholar]
- 35. Sultan SZ, Pitzer JE, Boquoi T, Hobbs G, Miller MR, et al. (2011) Analysis of the HD-GYP domain cyclic dimeric GMP phosphodiesterase reveals a role in motility and the enzootic life cycle of Borrelia burgdorferi . Infect and Immun 79: 3273–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Galperin MY, Natale DA, Aravind L, Koonin EV (1999) A specialized version of the HD hydrolase domain implicated in signal transduction. J Mol Microb Biotech 1: 303–305. [PMC free article] [PubMed] [Google Scholar]
- 37. Galperin MY, Nikolskaya AN, Koonin EV (2001) Novel domains of the prokaryotic two-component signal transduction systems. FEMS(Fed Eur Microbiol Soc) Microbiol Lett 203: 11–21. [DOI] [PubMed] [Google Scholar]
- 38. Galperin MY (2010) Diversity of structure and function of response regulator output domains. Curr Opin Microbiol 13: 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. He YW, Xu M, Lin K, Ng YJA, Wen CM, et al. (2006) Genome scale analysis of diffusible signal factor regulon in Xanthomonas campestris pv. campestris: identification of novel cell-cell communication-dependent genes and functions. Mol Microbiol 59: 610–622. [DOI] [PubMed] [Google Scholar]
- 40. Guo Y, Zhang Y, Li JL, Wang N (2012) Diffusible signal factor-mediated quorum sensing plays a central role in coordinating gene expression of Xanthomonas citri subsp. citri . Mol Plant-Microbe Interact 25: 165–179. [DOI] [PubMed] [Google Scholar]
- 41. Jiang J, Zou H, Li Y, Chen G (2009) Expression of the hrcC, hrpE and hpa3 genes is not regulated by the hrpG and hrpX genes in a rice pathogen Xanthomonas oryzae pv. oryzicola . Acta Microbiol Sinica 49: 1018–1025. [PubMed] [Google Scholar]
- 42. Harding N, Cleary J, Cabanas D, Rosen I, Kang K (1987) Genetic and physical analyses of a cluster of genes essential for xanthan gum biosynthesis in Xanthomonas campestris . J Bacteriol 169: 2854–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Souza LCA, Wulff NA, Gaurivaud P, Mariano AG, Virgílio ACD, et al. (2006) Disruption of Xylella fastidiosa CVC gumB and gumF genes affects biofilm formation without a detectable influence on exopolysaccharide production. FEMS(Fed Eur Microbiol Soc) Microbiol Lett 257: 236–242. [DOI] [PubMed] [Google Scholar]
- 44. Itoh Y, Rice JD, Goller C, Pannuri A, Taylor J, et al. (2008) Roles of pgaABCD genes in synthesis, modification, and export of the Escherichia coli biofilm adhesin poly-β-1, 6-N-acetyl-D-glucosamine. J Bacteriol 190: 3670–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Götz F (2002) Staphylococcus and biofilms. Mol Microbiol 43: 1367–1378. [DOI] [PubMed] [Google Scholar]
- 46. Wang X, Preston JF, Romeo T (2004) The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. J Bacteriol 186: 2724–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sultan SZ, Silva AJ, Benitez JA (2009) The PhoB regulatory system modulates biofilm formation and stress response in El Tor biotype Vibrio cholerae . FEMS(Fed Eur Microbiol Soc) Microbiol Lett 302: 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Plate L, Marletta MA (2012) Nitric oxide modulates bacterial biofilm formation through a multicomponent cyclic-di-GMP signaling network. Mol Cell 46: 449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rai R, Ranjan M, Pradhan BB, Chatterjee S (2012) Atypical regulation of virulence associated functions by a diffusible signal factor in Xanthomonas oryzae pv. oryzae . Mol Plant-Microbe Interact 25: 789–801. [DOI] [PubMed] [Google Scholar]
- 50. Ryder C, Byrd M, Wozniak DJ (2007) Role of polysaccharides in Pseudomonas aeruginosa biofilm development. Curr Opin Microbiol 10: 644–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wengelnik K, Rossier O, Bonas U (1999) Mutations in the regulatory gene hrpG of Xanthomonas campestris pv. vesicatoria result in constitutive expression of all hrp genes. J Bacteriol 181: 6828–6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wengelnik K, Van den Ackerveken G, Bonas U (1996) HrpG, a key hrp regulatory protein of Xanthomonas campestris pv. vesicatoria is homologous to two-component response regulators. Mol Plant-Microbe Interact 9: 704–712. [DOI] [PubMed] [Google Scholar]
- 53. Huang DL, Tang DJ, Liao Q, Li HC, Chen Q, et al. (2008) The Zur of Xanthomonas campestris functions as a repressor and an activator of putative zinc homeostasis genes via recognizing two distinct sequences within its target promoters. Nucleic Acids Res 36: 4295–4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Astua-Monge G, Minsavage GV, Stall RE, Davis MJ, Bonas U, et al. (2000) Resistance of tomato and pepper to T3 strains of Xanthomonas campestris pv. vesicatoria is specified by a plant-inducible avirulence gene. Mol Plant-Microbe Interact 13: 911–921. [DOI] [PubMed] [Google Scholar]
- 55. Koebnik R, Krüger A, Thieme F, Urban A, Bonas U (2006) Specific binding of the Xanthomonas campestris pv. vesicatoria AraC-type transcriptional activator HrpX to plant-inducible promoter boxes. J Bacteriol 188: 7652–7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li YR, Che YZ, Zou HS, Cui YP, Guo W, et al. (2011) Hpa2 required by HrpF to translocate Xanthomonas oryzae transcriptional activator-like effectors into rice for pathogenicity. Appl Environ Microbiol 77: 3809–3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sun W, Dunning FM, Pfund C, Weingarten R, Bent AF (2006) Within-species flagellin polymorphism in Xanthomonas campestris pv campestris and its impact on elicitation of Arabidopsis FLAGELLIN SENSING2–dependent defenses. Plant Cell 18: 764–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sun W, Liu L, Bent AF (2011) Type III secretion-dependent host defence elicitation and type III secretion-independent growth within leaves by Xanthomonas campestris pv. campestris . Mol Plant Pathol 12: 731–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Castañeda A, Reddy JD, El-Yacoubi B, Gabriel DW (2005) Mutagenesis of all eight avr genes in Xanthomonas campestris pv. campestris had no detected effect on pathogenicity, but one avr gene affected race specificity. Mol Plant-Microbe Interact 18: 1306–1317. [DOI] [PubMed] [Google Scholar]
- 60. Ried JL, Collmer A (1987) An nptI-sacB-sacR cartridge for constructing directed, unmarked mutations in gram-negative bacteria by marker exchange-eviction mutagenesis. Gene 57: 239–246. [DOI] [PubMed] [Google Scholar]
- 61. Loper JE, Lindow SE (1987) Lack of evidence for the in situ fluorescent pigment production by Pseudomonas syringae pv. syringae on bean leaf surfaces. Phytopathology 77: 1449–1454. [Google Scholar]
- 62. Cannon FC, Riedel GE, Ausubel FM (1979) Overlapping sequences of Klebsiella pneumoniae nifDNA cloned and characterised. Mol Gen Genet 174: 59–66. [DOI] [PubMed] [Google Scholar]
- 63. O’Toole GA, Kolter R (1998) Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol 30: 295–304. [DOI] [PubMed] [Google Scholar]
- 64. Boyer M, Chambost J, Magnan M, Cattaneo J (1984) Carboxymethyl-cellulase from Erwinia chrysanthemi. II. Purification and partial characterization of an endo-β-1, 4-glucanase. J Biotechnol 1: 241–252. [Google Scholar]
- 65. Swift S, Lynch MJ, Fish L, Kirke DF, Tomas JM, et al. (1999) Quorum sensing-dependent regulation and blockade of exoprotease production in Aeromonas hydrophila . Infect and Immun 67: 5192–5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tang JL, Liu YN, Barber C, Dow J, Wootton J, et al. (1991) Genetic and molecular analysis of a cluster of rpf genes involved in positive regulation of synthesis of extracellular enzymes and polysaccharide in Xanthomonas campestris pathovar campestris . Mol Gen Genet 226: 409–417. [DOI] [PubMed] [Google Scholar]
- 67. DiLuzio WR, Turner L, Mayer M, Garstecki P, Weibel DB, et al. (2005) Escherichia coli swim on the right-hand side. Nature 435: 1271–1274. [DOI] [PubMed] [Google Scholar]
- 68. Lee SW, Jeong KS, Han SW, Lee SE, Phee BK, et al. (2008) The Xanthomonas oryzae pv. oryzae PhoPQ two-component system is required for AvrXA21 activity, hrpG expression, and virulence. J Bacteriol 190: 2183–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bobrov AG, Kirillina O, Perry RD (2005) The phosphodiesterase activity of the HmsP EAL domain is required for negative regulation of biofilm formation in Yersinia pestis. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol Lett 247: 123–130. [DOI] [PubMed] [Google Scholar]
- 70. Meng F, Yao J, Allen C (2011) A MotN mutant of Ralstonia solanacearum is hypermotile and has reduced virulence. J. Bacteriol 193: 2477–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Choi KH, Schweizer HP (2006) mini-Tn7 insertion in bacteria with single attTn7 sites: example Pseudomonas aeruginosa . Nature Protocols 1: 153–161. [DOI] [PubMed] [Google Scholar]
- 72. Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tao F, He YW, Wu DH, Swarup S, Zhang LH (2010) The cyclic nucleotide monophosphate domain of Xanthomonas campestris global regulator Clp defines a new class of cyclic di-GMP effectors. J Bacteriol 192: 1020–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Predicted domain organizations of three HD-GYP domain proteins HgdA (XOC1984), RpfG (XOC2264) and HgdC (XOC4564). HgdA and RpfG have an HD-GYP domain in association with an N-terminal CheY-like response receiver (REC) regulatory domain. HgdC has an HD-GYP domain with additional, uncharacterized N-terminal and C-terminal domains. The numbers indicate amino acid residue positions.
(TIF)
Xoc rpfG -related mutants were verified by Southern blot analyses. Digested genomic DNA was separated, blotted onto membrane and then probed with the isotope-labelled rpfG-probe (A), hgdA-probe (B) and hgdC-probe (C) PCR fragments. A, Genome DNA from the wild-type (lane 1 and 2) and ΔrpfG (lane 3 and 4) strains digested by ApaI (lane 1 and lane3) and BamHI (lane 2 and 4) was hybridized with rpfG-probe. B, Genome DNA from the indicated mutant strains digested by KpnI and EcoRI/EcoRV was hybridized with hgdA-probe. C, Genome DNA from hgdC-related mutant strains digested by BamHI and SmaI was hybridized with hgdC-probe. The primers were designed to amplify DNA fragments as probes that do not hybridize with genome DNA of mutant strains because the fragments were deleted via homologous recombination. M: Marker.
(TIF)
The effect of rpfF mutation on biofilm formation in Xoc . Biofilm formation was dramatically increased in Xoc ΔrpfF mutant when cultured in L-medium. Complementation with introduction of the full-length rpfF gene to produce the ΔrpfF(rpfF) strain reduced biofilm formation to the wild-type level. WT: wild-type.
(TIF)
Effects of hgdA , rpfG and hgdC mutations on swimming motility and protease secretion in Xoc . (A) The amount of secreted proteases in Xoc was assessed by the diameter of clearing zones produced after the hydrolysis of skimmed milk on water agar plates. (B) Swimming motility of the Xoc wild-type and mutant strains was determined on semisolid plates with 0.3% noble agar. The motility was indicated by the diameter (cm) of the radial growth.
(TIF)
The phosphodiesterase activity of Xoc RpfG. Xoc RpfG was in vitro expressed as an N-terminal His6-tagged fusion and then purified using nickel columns under native conditions. The PDE activity of Xoc RpfG against c-di-GMP was assessed by reverse phase High Performance Liquid Chromatography (HPLC). (A-D) HPLC analyses of the RpfGXoc PDE activity using c-di-GMP as a substrate. (A) and (B) GMP and c-di-GMP standard. (C) The purified RpfGXoc had activity against standard cyclic di-GMP, generating two hydrolytic products with the retention time at 6.024 s and 12.922 s, respectively after purified RpfGXoc was incubated with c-di-GMP for 6 h. (D) Reaction control without RpfGXoc. C-di-GMP was stable and no degraded product but only c-di-GMP was detected. (E–F) Mass spectrometry, operated at negative ion mode, was used to confirm the identity of HPLC fractions in Figure S5C. (E) The GMP peak was detected by LC-MS at an m/z of 362.0. (F) The second peak was distinct from c-di-GMP and GMP with a [M-H]+ m/z at 707.1, which corresponds to the intermediate product pGpG.
(TIF)
The growth rate of ΔrpfG compared to those of the wild-type and complemented strains in XOM3 minimal medium. The bacterial population was determined by counting colony forming units after manual plating at the indicated time points. WT: wild-type.
(TIF)
Effects of xagA , xagB , pgaA , pgaC and gumD deletions on swimming motility in Xoc . Swimming motility of the Xoc wild-type, mutant and complementation strains was determined on semisolid plates with 0.3% noble agar. The motility was indicated by the diameter (cm) of the radial growth.
(TIF)
Primers used in this study.
(DOC)