Abstract
One of our major research interests is to define BCL-2 family function in the cellular decision to induce mitochondrial outer membrane permeabilization and apoptosis. Curiosity in BCL-2 family - mitochondrial interactions led to the identification that the sphingolipid pathway plays a crucial role in BCL-2 family function. For approximately 20 years, sphingolipid biology and apoptotic mechanisms have criss-crossed, but remained distinct, as neither literature could explain the observable interplay between these pathways. We recently identified that two products within the sphingolipid pathway, sphingosine-1-PO4 and hexadecenal, directly regulate BAK and BAX activation, respectively. Furthermore, our work suggests that mitochondrial communication with heterotypic membrane and/or organelles may be an important regulatory mechanism for apoptotic sensitivity.
Keywords: apoptosis, BCL-2 family, cytochrome c release, endoplasmic reticulum, mitochondria, organelles, sphingolipids
Throughout the decades of BCL-2 (B-cell lymphoma 2) family research, the majority of effort focused on defining the individual BCL-2 family members.1 As such, the BCL-2 family has been parsed into anti-apoptotic (e.g., BCL-2) and pro-apoptotic proteins (e.g., BAX, BCL-2 associated protein X). Furthermore, the functional cooperation between pro-apoptotic members has also been defined via elegant biochemical and cellular studies that revealed the mechanisms leading to apoptosis.2-8
Pro-apoptotic BCL-2 family members are divided into two subclasses: the “effector” proteins (e.g., BAX and BAK, BCL-2 antagonist killer) and the “BH3-only” proteins (e.g., BID, BH3 interacting-domain death agonist).1 The effector proteins are responsible for targeting the outer mitochondrial membrane (OMM), leading to its permeabilization and the release of pro-apoptotic factors in a process referred to as mitochondrial outer membrane permeabilization (MOMP).2 Once MOMP happens, the apoptotic proteases are activated and cell death rapidly ensues.9
As suggested above, the pro-apoptotic BCL-2 family members activate at the OMM leading to its disruption, but the mechanisms that allow for mitochondrial targeting are highly debated. There are suggestions that OMM proteins allow for specificity, along with evidence that mitochondrial lipids contribute to BAK/BAX activation and MOMP.10-12 Using an unbiased approach, we biochemically isolated and defined an activity that supported the activation of pro-apoptotic BCL-2 proteins at the OMM.13 Our experiments identified that a neutral sphingomyelinase activity was responsible to maintain mitochondrial responses to BID-induced BAK/BAX activation and apoptosis.13 The identified pathway was no stranger to the apoptosis field as an extensive literature supports a role for sphingolipids in the cell death pathways, but our work provided a novel interpretation of how the BCL-2 family and sphingolipid pathways mechanistically intersect to engage MOMP and apoptosis.14-16
Our recent studies highlighted a role for two products within a sphingomyelin metabolic pathway as essential regulators of BAK/BAX-mediated MOMP: sphingosine-1-PO4 and its degradation product, hexadecenal (Fig. 1A). Through a series of biochemical approaches, we determined that S1P and hexadecenal promoted the activation of BAK and BAX, respectively, and that these sphingolipid products could specifically cooperate with pro-apoptotic BH3-only proteins to coordinate MOMP and cytochrome c release. As purified full length, functional BAX can be examined in vitro, we demonstrated that high micromolar concentrations of hexadecenal directly activated BAX leading to conformational changes within BAX monomers that supported oligomerization and the permeabilization of liver mitochondria and defined liposomes.17 We speculate that cellular signaling events that lead to increased hexadecenal production (or decreased hexadecenal degradation/metabolism) could promote BAX activation, MOMP and apoptosis.
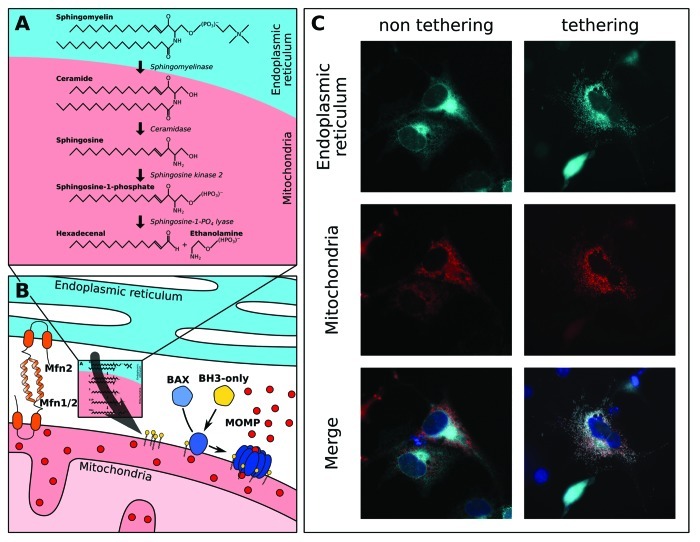
Figure 1. Inter-organellar sphingolipid metabolism with mitochondria regulates both the intrinsic and extrinsic pathways of apoptosis. (A) The relevant sphingolipid substrates, products and enzymes (italics), are shown. Our work suggests that sphingomyelin originates from a heterotypic membrane (e.g., the endoplasmic reticulum), and its hydrolysis product, ceramide, is transferred to mitochondria.13 (B) Communication between the ER and mitochondria is suggested to be regulated by proteins that tether the two organelles, such as mitofusins 1 and 2 (Mfn1/2).28 Biochemical data show that mitochondria contain numerous sphingolipid metabolism enzymes allowing for the generation of sphingosine-1-PO4 and hexadecenal to support BH3-only protein induced BAK and BAX activation, respectively, and MOMP.13 (C) Recombinant tethers have been generated that lead to marked increases in ER - mitochondrial communication.30 For example, here we show that recombinant tethers expressed in mouse embryonic fibroblasts cause reorganization of the ER (cyan, top) and mitochondrial (red, middle) networks and induce marked co-localization (merge, bottom). As control, non-tethering controls maintain distinct ER and mitochondrial networks.
Importantly, we were able to demonstrate that pharmacological inhibitors to sphingolipid metabolism that prevent sphingosine-1-PO4 and hexadecenal production block the mitochondrial pathway of apoptosis, along with death receptor mediated apoptosis that depends on mitochondrial contributions.13 Moreover, the exogenous expression of catalytically active neutral sphingomyelinases in cells could sensitize to both the mitochondrial pathway of apoptosis (e.g., induced by BCL-2 interacting mediator of cell of death, short isoform; BIM-S) and death receptor-induced apoptosis (e.g., tumor necrosis factor α, TNFα).13 These cellular data could be recapitulated biochemically by the addition of bacterial neutral sphingomyelinase or recombinant human neutral sphingomyelinase to isolated mitochondria, which also caused marked sensitization to pro-apoptotic stimuli.13
The novelty in our work is focused on the notion that sphingolipid metabolism directly regulates individual pro-apoptotic BCL-2 members to allow for MOMP. The majority of literature suggests that pro-apoptotic sphingolipids, such as ceramide, are necessary and sufficient to engage cell death; and a more recent literature provides evidence that sphingolipids allow for pro-apoptotic BCL-2 function by creating membrane environments that also support and synergize with BAX activation.16,18-21 Our studies provide evidence that sphingolipid metabolic products directly engage pro-apoptotic proteins like BAK and BAX to coordinate appropriate sensitivity to BH3-only proteins.13
Directly upstream of sphingosine-1-PO4 and hexadecenal production is two enzymes that coordinate metabolism: the sphingosine kinases (SPHK1 and SPHK2) and sphingosine-1-PO4 lyase (Fig. 1A and B). We investigated the localization of these enzymes and provided evidence that SPHK2 and lyase activities were biochemically detected in mitochondrial fractions.13 We find these results of importance as it suggests that mitochondria regulate the production of sphingosine-1-PO4 and hexadecenal, which directly impacts on sensitivity to pro-apoptotic BCL-2 family function and subsequent apoptosis (Fig. 1B). There is also intriguing evidence that both of these enzymes impact on cellular sensitivity to apoptosis via changes in the expression of various BCL-2 family, and vice versa22-25; and interesting data suggesting that mitochondrially localized pro-apoptotic BAK is required for stress-induced production of long chain ceramide through ceramide synthase.26 Moreover, the addition of hexadecenal has recently been implicated within the mitochondrial pathway of apoptosis via BIM- and BAX-dependent apoptosis.27
Our observations suggested that mitochondria contain many sphingolipid-metabolizing enzymes, yet they appear to lack significant neutral sphingomyelinase activity compared with other cellular compartments (Fig. 1A). This focused our efforts to investigate how the mitochondrial sphingolipid environment is actively maintained, which lead to identify that a protease-sensitive mitochondrial-associated organelle or compartment likely contributes to the sphingolipid environment of the OMM (Fig. 1B and C).13 Indeed, the majority of mitochondria are actively tethered to the endoplasmic reticulum, and this may be responsible for sphingolipid metabolism and/or transfer to the OMM.28-30 At present, we are investigating the influence of mitochondrial tethering to the endoplasmic reticulum on sphingolipid metabolism, mitochondrial composition and cellular sensitivity to pro-apoptotic stimulation (Fig. 1C); all of which will surely provide compelling data to highlight the marked influence of inter-organellar communication on cell fate decisions. These results may reveal unique pharmacological opportunities to enhance or inhibit cell death in various human pathologies.
Acknowledgments
This work was supported by: NIH CA157740 (to J.E.C.), the JJR Foundation (to J.E.C.), the William A. Spivak Fund (to J.E.C.), and the Fridolin Charitable Trust (to J.E.C.). This work was also supported in part by a Research Grant 5-FY11-74 from the March of Dimes Foundation (to J.E.C.). We would like to thank the members of the Chipuk laboratory for their support, and Drs. György Csordás and György Hajnóczky from the Thomas Jefferson University for kindly providing us with the tethering constructs. This work was supported by: NIH CA157740 (to J.E.C.), the JJR Foundation (to J.E.C.), the William A. Spivak Fund (to J.E.C.), and the Fridolin Charitable Trust (to J.E.C.). This work was also supported in part by a Research Grant 5-FY11-74 from the March of Dimes Foundation (to J.E.C.)
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/22872
References
- 1.Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–64. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, et al. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14:2060–71. [PMC free article] [PubMed] [Google Scholar]
- 4.Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, et al. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–81. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dewson G, Kratina T, Czabotar P, Day CL, Adams JM, Kluck RM. Bak activation for apoptosis involves oligomerization of dimers via their alpha6 helices. Mol Cell. 2009;36:696–703. doi: 10.1016/j.molcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Dewson G, Kratina T, Sim HW, Puthalakath H, Adams JM, Colman PM, et al. To trigger apoptosis, Bak exposes its BH3 domain and homodimerizes via BH3:groove interactions. Mol Cell. 2008;30:369–80. doi: 10.1016/j.molcel.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Gavathiotis E, Reyna DE, Davis ML, Bird GH, Walensky LD. BH3-triggered structural reorganization drives the activation of proapoptotic BAX. Mol Cell. 2010;40:481–92. doi: 10.1016/j.molcel.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–92. doi: 10.1016/S1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 9.Pop C, Salvesen GS. Human caspases: activation, specificity, and regulation. J Biol Chem. 2009;284:21777–81. doi: 10.1074/jbc.R800084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, et al. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–42. doi: 10.1016/S0092-8674(02)01036-X. [DOI] [PubMed] [Google Scholar]
- 11.Zaltsman Y, Shachnai L, Yivgi-Ohana N, Schwarz M, Maryanovich M, Houtkooper RH, et al. MTCH2/MIMP is a major facilitator of tBID recruitment to mitochondria. Nat Cell Biol. 2010;12:553–62. doi: 10.1038/ncb2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ott M, Norberg E, Walter KM, Schreiner P, Kemper C, Rapaport D, et al. The mitochondrial TOM complex is required for tBid/Bax-induced cytochrome c release. J Biol Chem. 2007;282:27633–9. doi: 10.1074/jbc.M703155200. [DOI] [PubMed] [Google Scholar]
- 13.Chipuk JE, McStay GP, Bharti A, Kuwana T, Clarke CJ, Siskind LJ, et al. Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell. 2012;148:988–1000. doi: 10.1016/j.cell.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Brocklyn JR, Williams JB. The control of the balance between ceramide and sphingosine-1-phosphate by sphingosine kinase: oxidative stress and the seesaw of cell survival and death. Comp Biochem Physiol B Biochem Mol Biol. 2012;163:26–36. doi: 10.1016/j.cbpb.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Bartke N, Hannun YA. Bioactive sphingolipids: metabolism and function. J Lipid Res. 2009;50(Suppl):S91–6. doi: 10.1194/jlr.R800080-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science. 1993;259:1769–71. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki M, Youle RJ, Tjandra N. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell. 2000;103:645–54. doi: 10.1016/S0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]
- 18.Lee H, Rotolo JA, Mesicek J, Penate-Medina T, Rimner A, Liao WC, et al. Mitochondrial ceramide-rich macrodomains functionalize Bax upon irradiation. PLoS ONE. 2011;6:e19783. doi: 10.1371/journal.pone.0019783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smyth MJ, Obeid LM, Hannun YA. Ceramide: a novel lipid mediator of apoptosis. Adv Pharmacol. 1997;41:133–54. doi: 10.1016/S1054-3589(08)61057-1. [DOI] [PubMed] [Google Scholar]
- 20.Siskind LJ, Colombini M. The lipids C2- and C16-ceramide form large stable channels. Implications for apoptosis. J Biol Chem. 2000;275:38640–4. doi: 10.1074/jbc.C000587200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganesan V, Perera MN, Colombini D, Datskovskiy D, Chadha K, Colombini M. Ceramide and activated Bax act synergistically to permeabilize the mitochondrial outer membrane. Apoptosis. 2010;15:553–62. doi: 10.1007/s10495-009-0449-0. [DOI] [PubMed] [Google Scholar]
- 22.Li G, Alexander H, Schneider N, Alexander S. Molecular basis for resistance to the anticancer drug cisplatin in Dictyostelium. Microbiology. 2000;146:2219–27. doi: 10.1099/00221287-146-9-2219. [DOI] [PubMed] [Google Scholar]
- 23.Limaye V, Li X, Hahn C, Xia P, Berndt MC, Vadas MA, et al. Sphingosine kinase-1 enhances endothelial cell survival through a PECAM-1-dependent activation of PI-3K/Akt and regulation of Bcl-2 family members. Blood. 2005;105:3169–77. doi: 10.1182/blood-2004-02-0452. [DOI] [PubMed] [Google Scholar]
- 24.Bektas M, Jolly PS, Müller C, Eberle J, Spiegel S, Geilen CC. Sphingosine kinase activity counteracts ceramide-mediated cell death in human melanoma cells: role of Bcl-2 expression. Oncogene. 2005;24:178–87. doi: 10.1038/sj.onc.1208019. [DOI] [PubMed] [Google Scholar]
- 25.Bonhoure E, Pchejetski D, Aouali N, Morjani H, Levade T, Kohama T, et al. Overcoming MDR-associated chemoresistance in HL-60 acute myeloid leukemia cells by targeting sphingosine kinase-1. Leukemia. 2006;20:95–102. doi: 10.1038/sj.leu.2404023. [DOI] [PubMed] [Google Scholar]
- 26.Siskind LJ, Mullen TD, Romero Rosales K, Clarke CJ, Hernandez-Corbacho MJ, Edinger AL, et al. The BCL-2 protein BAK is required for long-chain ceramide generation during apoptosis. J Biol Chem. 2010;285:11818–26. doi: 10.1074/jbc.M109.078121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar A, Byun HS, Bittman R, Saba JD. The sphingolipid degradation product trans-2-hexadecenal induces cytoskeletal reorganization and apoptosis in a JNK-dependent manner. Cell Signal. 2011;23:1144–52. doi: 10.1016/j.cellsig.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–10. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 29.Csordás G, Renken C, Várnai P, Walter L, Weaver D, Buttle KF, et al. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174:915–21. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Csordás G, Várnai P, Golenár T, Roy S, Purkins G, Schneider TG, et al. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol Cell. 2010;39:121–32. doi: 10.1016/j.molcel.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]