Abstract
Within the species Astyanax mexicanus, there are several inter-fertile populations of river-dwelling sighted fish and cave-dwelling blind fish which have evolved morphological and behavioral adaptations. We have recently reported a developmental and neurophysiological basis for the loss of aggressive behavior in the blind cavefish morph of Astyanax. Using an appropriate behavioral assay, we have shown that surface Astyanax show intense dominance-related aggressiveness. The expression of this behavior is inversely correlated with the serotonin (5HT) levels in their hindbrain raphe nucleus. Moreover this behavior is not solely visually-evoked and has a genetic component. Conversely in cavefish, there is no raphe-driven dominance aggressiveness. Instead, the embryonic Sonic Hedgehog–dependent modification of the size of a serotonergic neuronal group localized in their hypothalamus causes a shift in their behavioral pattern: instead of fighting, they search for food. Here we further discuss the origin and nature of this behavioral shift.
Keywords: Astyanax mexicanus, evolution, brain development, Sonic Hedgehog, serotonin, aggressive behaviour, social behaviour, foraging behaviour, hypothalamus, raphe
Experimental Evidences for Loss and Gain of Behaviors in Cavefish
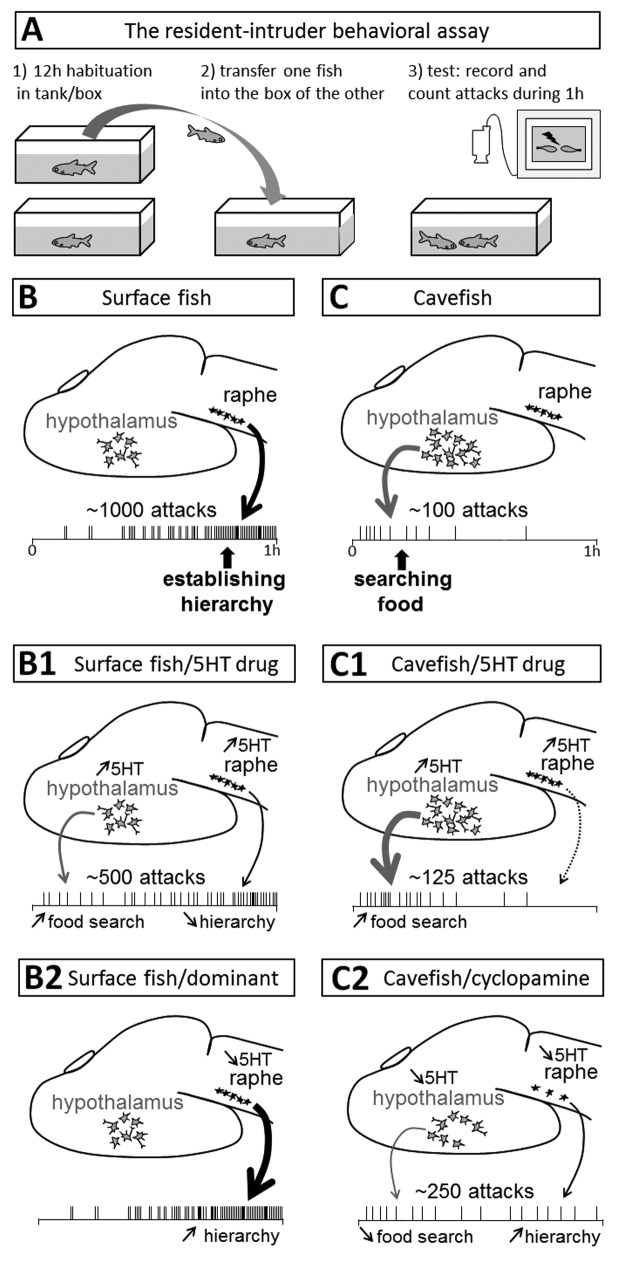
Because cavefish are blind, it was impossible to use the classical “mirror test” that most researchers working on agonistic behavior use; so we used a “resident-intruder assay” modified from Espinasa and colleagues1 (Fig. 1A). With this set-up, we counted the number of attacks between the two fish, and the patterns of attacks along the one hour test were also thoroughly analyzed (Fig. 1BC).Quantitatively, surface fish are about ten times more aggressive than cavefish. Qualitatively, attacks between two surface fish occur in the second half of the test, whereas attacks between two cavefish occur at the beginning of the test. This later result gave us a hint that the attacks recorded between two surface fish or between two cavefish may not have the same signification at all (Fig. 1B and C).
Figure 1. Link between aggressive behavior, food searching behavior, and serotonergic neurotransmission in surface fish and cavefish brains. (A) Depicts the behavioral test used to measure Astyanax aggressive behavior. (B–C) Correlation between brain neuroanatomy and recorded behavioral pattern. The top drawing shows a schematic brain in lateral view with anterior to the left, on which serotonergic neurons of the hypothalamus (gray) and the raphe nucleus (black) are indicated. The bottom schema shows attacks (vertical lines) distributed over time during the one hour behavioral test. (B) In surface fish, the experience-dependent regulation of serotonin levels in the raphe drives attacks which increase progressively in frequency during the test, which are very numerous, and which correspond to establishment of dominance between the two fish. (C) In blind cavefish, there is apparently no such regulation of raphe serotonin levels. Rather, the developmental evolution of a serotonergic hypothalamic neuronal group drives attacks which occur only at the beginning of the test, which are very few, and which correspond to foraging behavior. (B1–C2) correlation between brain 5HT circuits and behavioral patterns in various experimental conditions. (B1) In surface fish withpharmacologically-increased 5HT levels (after fluoxetine or deprenyl treatment) early attacks are slightly increased due to increased 5HT in the hypothalamus, and late attacks are strongly diminished, due to increased 5HT in the raphe. The total number of attacks is about 50% of a control surface fish test. (C1) In cavefish with pharmacologically-increased 5HT levels, food searching at the beginning of the test is further increased when compared with control cavefish, leading to a slight increase in the total number of attacks. There is no raphe-driven effect. (B2) In dominant surface fish, whose raphe 5HT levels are low as measured by HPLC, there are only late and numerous “hierarchy-type” attacks. (C2) In cavefish that have been treated with the Shh signaling inhibitor cyclopamine early in development, when 5HT neurons differentiate, the number of 5HT neurons is reduced, both in the hypothalamus and in the raphe. The behavioral correlate is a decrease in food searching at the beginning of the test, due to decreased 5HT transmission in the hypothalamus; and an increase in late attacks, because the 5HT raphe levels are low and therefore drive some “dominance-like” behavior. Accordingly, the global number of attacks is increased when compared with a control cavefish test.
We propose that surface fish attacks, driven by low levels of 5HT in the raphe, correspond to the establishment of hierarchical order between the two opponents. The attacks therefore increase in frequency as the test progresses, with dominance being established. For cavefish, we propose instead that “attacks” correspond to food searching attempts, these blind animals sensing vibrations that may indicate a prey in the environment when the intruder is transferred into the resident box.2 This foraging behavior is controlled by hypothalamic 5HT neurons, whose number is increased in cavefish due to increased Sonic Hedgehog signaling during early embryogenesis3—a signaling modification that also leads them to lose their eyes.4,5 Our model is well supported by the results observed in various experimental conditions, after pharmacological or embryonic perturbations (Fig. 1, panels B1−C2 and legends). In particular, the use of compounds such as fluoxetine or deprenyl which increase 5HT levels show that aggressiveness and raphe 5HT levels are inversely correlated. Moreover, strong support for cavefish “attacks” actually being food searching behavior come from our finding that starved cavefish are75% more “aggressive” than normal-fed or over-fed cavefish, with the attacks always occurring at the beginning of the test.3
Loss of Aggressiveness
We would like to propose that the loss of aggressiveness in cavefish is related to a general loss of social behaviors during their evolution in the subterranean environment (Fig. 2). Strikingly, surface fish school or shoal intensely, with a dominant individual (usually a female) leading the group, at the top of a hierarchy pyramid including moderately and severely subordinate individuals.3 It is thought that this type of collective behavior protects from predation6 and helps finding food sources. During reproductive behavior also, we have observed coordinated and collective behaviors in surface fish (personal observations). Cavefish on the contrary do not school: they swim independently from each other, both in their natural ponds and in a laboratory aquarium. Their reproductive behavior is also different, as they only have very rapid contacts when the male fertilizes the eggs spawn by the female (personal observations). Certainly, cavefish do not lack social behavior because they do not see: blind fish can school,7,8 and in the case of Astyanax cavefish, their increased lateral line probably confers them with ample navigational capabilities. However, we propose that it is probably advantageous to lack collective swimming in caves. Indeed, the subterranean environment is characterized by no or rare predators, food scarcity, and total darkness. There, isolated swim is probably more efficient to find the rare food particles that drop onto the water, and which are precisely detected by the lateral line system through the VAB, the vibration attraction behavior.2,9 It will be very interesting to analyze whether a re-wiring of the neuromasts sensory circuits occurred during cavefish evolution in the dark.

Figure 2. Loss of collective behaviors in cavefish. The visual sense is depicted by dotted lines. The lateral line, neuromast-mediated sense is depicted by thunderstorm arrows. (A) Surface fish shoal or school. This collective behavior is dependent on the lateral line system.8 The dominant fish in the school has low raphe 5HT levels (gray fish). Prey detection and escape from predators also depend on the lateral line, but relies heavily as well on the visual sense in a lighted environment. (B) Blind cavefish do not shoal or school, they perform isolated swim. Their increased lateral line helps them navigate in the dark and finding food through the VAB (vibration attraction behavior),2 in the absence of visual sense. Their motivation to search and find food is high, probably governed by hypothalamic circuits, including a large 5HT neuronal group.
Gain of Feeding Behavior
In the dark, cavefish are four times more efficient at finding food than surface fish.10 This excellent ability to find food is certainly one of the reasons for their success in cave colonization, and probably results from several subtle modifications in their brains, governing such a complex trait. For example they feed on the bottom of the substrate with a fixed feeding angle that is different from surface fish and that is more efficient,11 and they show an increased and continuous swimming/exploratory behavior.3 Both constitute motor control changes. They use the VAB to locate food,2 which constitutes a sensory change. We also propose that one of their hypothalamic cell groups controls the intensity of foraging behavior, therefore potentially representing a regulatory change in motivation for food or in mechanisms controlling hunger and satiety.3 Interestingly and in the same line, modifications in transcription levels of receptors for neurotransmitter such as glutamate or cannabinoids were recently discussed in terms of their potential enhancement of cavefish behavioral drive toward food and reproduction.12 The neurophysiological bases of these motor, sensory, and regulatory changes in the cavefish brain are not known, and their developmental bases begin to be deciphered. Besides the role of Shh in cavefish eye degeneration,4 Yamamoto et al. have also shown the Shh-dependence of the increase in tastebud number in cavefish,13 and we have demonstrated the Shh-dependence of cell migration to their olfactory bulbs,14 of the size of their hypothalamus as a whole5,14 and on the size of a particular serotonergic cell group in their hypothalamus.3 Therefore, the embryonic modifications in early Shh morphogen signaling seems to pleiotropically affect the developmental evolution of the cavefish brain (discussed in ref. 15).
Acknowledgments
Our work is supported by an ANR grant [ASTYCO] to S.R.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/23166
References
- 1.Espinasa L, Yamamoto Y, Jeffery WR. Non-optical releasers for aggressive behavior in blind and blinded Astyanax (Teleostei, Characidae) Behav Processes. 2005;70:144–8. doi: 10.1016/j.beproc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Yoshizawa M, Goricki S, Soares D, Jeffery WR. Evolution of a behavioral shift mediated by superficial neuromasts helps cavefish find food in darkness. Curr Biol. 2010;20:1631–6. doi: 10.1016/j.cub.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elipot Y, Hinaux H, Callebert J, Rétaux S. Evolutionary Shift from Fighting to Foraging in Blind Cavefish through Changes in the Serotonin Network. Curr Biol. 2012;23:1–10. doi: 10.1016/j.cub.2012.10.044. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto Y, Stock DW, Jeffery WR. Hedgehog signalling controls eye degeneration in blind cavefish. Nature. 2004;431:844–7. doi: 10.1038/nature02864. [DOI] [PubMed] [Google Scholar]
- 5.Pottin K, Hinaux H, Rétaux S. Restoring eye size in Astyanax mexicanus blind cavefish embryos through modulation of the Shh and Fgf8 forebrain organising centres. Development. 2011;138:2467–76. doi: 10.1242/dev.054106. [DOI] [PubMed] [Google Scholar]
- 6.Handegard NO, Boswell KM, Ioannou CC, Leblanc SP, Tjøstheim DB, Couzin ID. The dynamics of coordinated group hunting and collective information transfer among schooling prey. Curr Biol. 2012;22:1213–7. doi: 10.1016/j.cub.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 7.Pitcher TJ, Partridge BL, Wardle CS. A blind fish can school. Science. 1976;194:963–5. doi: 10.1126/science.982056. [DOI] [PubMed] [Google Scholar]
- 8.Partridge BL, Pitcher TJ. The sensory basis for fish schools: relative roles of lateral line and vision. J Comp Physiol. 1980;135:315–25. doi: 10.1007/BF00657647. [DOI] [Google Scholar]
- 9.Yoshizawa M, Jeffery WR. Evolutionary tuning of an adaptive behavior requires enhancement of the neuromast sensory system. Commun Integr Biol. 2011;4:89–91. doi: 10.4161/cib.4.1.14118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hüppop K. Food-finding ability in cave fish (Astyanax fasciatus) Int J Speleol. 1987;16:59–66. [Google Scholar]
- 11.Wilkens H. Evolution and genetics of epigean and cave Astyanax fasciatus (Characidae, Pisces). Support for the neutral mutation theory. In: Hecht, MK, Wallace, B (Eds) Evolutionary Biology 1988 vol 23 Plenum, New York and London, 271-367. [Google Scholar]
- 12.Strickler AG, Soares D. Comparative genetics of the central nervous system in epigean and hypogean Astyanax mexicanus. Genetica. 2011;139:383–91. doi: 10.1007/s10709-011-9557-1. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto Y, Byerly MS, Jackman WR, Jeffery WR. Pleiotropic functions of embryonic sonic hedgehog expression link jaw and taste bud amplification with eye loss during cavefish evolution. Dev Biol. 2009;330:200–11. doi: 10.1016/j.ydbio.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menuet A, Alunni A, Joly JS, Jeffery WR, Rétaux S. Expanded expression of Sonic Hedgehog in Astyanax cavefish: multiple consequences on forebrain development and evolution. Development. 2007;134:845–55. doi: 10.1242/dev.02780. [DOI] [PubMed] [Google Scholar]
- 15.Jeffery WR. Pleiotropy and eye degeneration in cavefish. Heredity (Edinb) 2010;105:495–6. doi: 10.1038/hdy.2010.7. [DOI] [PubMed] [Google Scholar]