Abstract
Our increased understanding of host pathogen interactions shows that pathogens could capitalize on host cell pathways to favor entry and disease establishment. One such pathway used by Leishmania mexicana to enter into neutrophils and macrophages is the PI3Kγ signaling pathway. We recently showed that the use of the PI3Kγ inhibitor AS-605240 for the treatment of experimental L. mexicana infection in mice resulted in significantly lower parasite burdens and lesion sizes than WT untreated mice. Further, AS-605240 was found to be as effective as Sodium Stibogluconate, the drug of choice for treatment of L. mexicana infection, in reducing parasite burdens in mice. Here, we provide potential mechanisms of PI3Kγ blockade in promoting resistance to L. mexicana infection in mice. As a proof of principle, we propose that targeting host cell signaling pathways used in the establishment of infection could be a possible therapeutic option in the management of obligate intracellular pathogens.
Keywords: Leishmania, AS-605240, PI3Kγ, cell migration, macrophages, neutrophils, phagocytosis
PI3Kinase belongs to a large family of enzymes that phosphorylate phosphoinositol containing lipids at the 3′ hydroxyl of the inositol head group, generating 3′ phosphoinositides which serve as second messengers involved in signal transduction.1 These lipid kinases are involved in a host of cellular homeostatic processes including growth, cell survival, cell cycle regulation and apoptosis. PI3Kγ, a class IB isoform, has received much attention because it is predominantly expressed in immune cells and inactivation of this enzyme genetically or pharmacologically protects against various mouse models of inflammatory diseases such as SLE, rheumatoid arthritis, COPD and atherosclerosis.2,3 In these cases, inhibiting PI3Kγ activity attenuates innate and adaptive immune responses thereby significantly reducing the severity of disease.
While such studies are very exciting and offer a therapeutic alternative to the use of corticosteroids in the treatment of autoimmune diseases, the application of PI3Kγ inhibitors to the treatment of infectious diseases have not been fully exploited. Our increased understanding of host-pathogen interactions reveal that pathogens could capitalize on host cell signaling pathways aimed at eliminating the pathogen to favor its establishment.4 This is especially true in the case of the intracellular parasite Leishmania mexicana.
L. mexicana belongs to a group of intracellular parasites of the genus Leishmania which cause diseases that have long been considered a major public health concern in many regions of the world.5 These obligate intracellular parasites are transmitted by a phlebotomine sand fly vector and cause cutaneous, mucocutaneous or visceral leishmaniasis in patients. According to the World Health Organization, it is estimated that about 350 million people currently suffer from leishmaniasis in about 88 countries (www.who.int). L. mexicana causes chronic localized infections on the skin of mice and humans.
Current approaches to the treatment of Leishmania involve the use of drugs that target the pathogen itself or metabolic pathways employed by the parasite to establish infection. However, increasing reports of multidrug resistant strains as well as problems associated with toxicity and patient compliance of antileishmanial drugs make alternative approaches to the management of Leishmania and other intracellular pathogens a viable, attractive and even necessary option.
In a recent study, we demonstrated that PI3Kγ mediates the entry of L. mexicana into phagocytic host cells and that blockade of this enzyme significantly lowers parasite entry into macrophages and neutrophils in vitro and in vivo.6 Neutrophils not only serve as ‘Trojan Horses’ for the establishment of Leishmania, but may also protect the parasite from extracellular destruction.7-10 And although macrophages are the principal immune cells required for the eradication of Leishmania, these cells are targeted by the parasite for its growth and propagation. Since PI3Kγ is involved in cellular trafficking and phagocytosis of immune cells, we investigated the effects of PI3Kγ blockade in L. mexicana infection. We showed that genetic deletion of PI3Kγ or selective inhibition using the PI3Kγ inhibitor AS-605240 significantly reduced parasite entry into neutrophils and macrophages. Further, lesion growths and parasite burdens were lower in PI3Kγ-/- mice and in mice treated with AS-605240 than in WT C57BL/6 mice. AS-605240 was also found to be as effective as Sodium Stibogluconate (the drug of choice for treatment of L. mexicana infection) in reducing parasite burdens in mice. We therefore showed PI3Kγ as a possible drug target for the management of L. mexicana and potentially other obligate intracellular pathogens. We also demonstrate that targeting host cell signaling pathways exploited by pathogens provide a viable alternative to conventional therapeutic approaches which tend to focus on the pathogen alone. This expanded approach to the management of infectious diseases is beginning to gain wide attention in the scientific community owing to an increased understanding of host-pathogen interactions, and a combination of therapeutic approaches could very well be the future of effective disease control and eradication.
Mechanisms involved in PI3Kγ mediated susceptibility to intracellular pathogens are not fully understood, but seem to primarily involve phagocytosis and phagocytic cell recruitment to infected tissues. PI3Kγ has been shown to initiate F-actin polymerization and cytoskeletal rearrangement, mechanisms involved in migration and phagocytosis.1 PI3Kγ is also a mediator in G-protein coupled receptor signaling and therefore is involved in the directed migration of neutrophils and macrophages in response to chemokines.11,12 This suggests that interfering with PI3Kγ signaling prevents pathogen entry and establishment by selective inhibition of macrophage recruitment and phagocytic mechanisms.
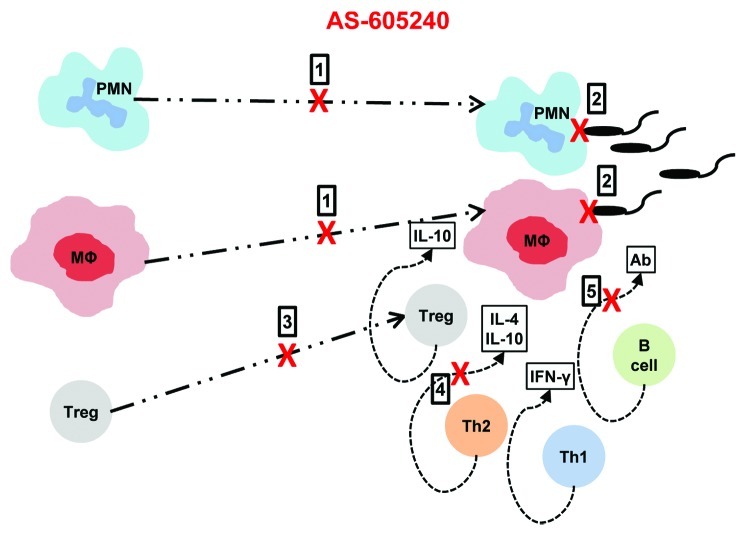
PI3Kγ is part of a complex signaling network associated with a wide range of immune receptors in a variety of cells.13 Inhibition of PI3Kγ therefore affects other immune cells and these effects in the context of intracellular infections are still being investigated. In L. mexicana infection, we observed significantly decreased levels of serum IgG1 and IgG2a in PI3Kγ-/- mice compared with WT mice (unpublished data) which suggests possible B cell defects. Interestingly, some researchers have shown that in the absence of circulating antibody, Fc receptor mediated internalization of L. mexicana in mouse phagocytes is compromised, leading to increased host protection.14 Other possible mechanisms of PI3Kγ blockade based resistance of L. mexicana include suppression of migration of IL-10 producing regulatory T cells (Tregs) to the site of infection as well as impaired production of Th2 associated cytokines, IL-4 and IL-10.6 However, suppression of Th2 cytokine production in PI3Kγ inhibitor treated mice infected with L. mexicana seems to be an indirect effect. A summary of the effects of PI3Kγ inhibition in the resistance to L. mexicana infection in mice is presented in Figure 1.
Figure 1. Possible mechanisms of action of PI3Kγ inhibitor AS-605240 in mediating resistance to L. mexicana infection: (1) prevent migration of neutrophils and macrophages to L mexicana infected sites;, (2) block phagocytosis of L mexicana promastigotes by neutrophils and macrophages; (3) inhibit migration of IL-10 producing regulatory T cells to infected areas which would otherwise dampen the immune response; (4) indirectly prevent production of Th-2 associated cytokines IL-4 and IL-10; and (5) directly or indirectly suppress the production of serum antibodies which could otherwise contribute to Fc receptor mediated internalization of L. mexicana parasites.
Studies that define mechanisms of Leishmania pathogenesis, host immune evasion and exploitation have been particularly useful in designing efficient therapeutic strategies. Successful approaches do not always directly target the pathogen, but could capitalize on host immune response pathways exploited by the pathogen to facilitate entry and establishment of disease. Although the use of PI3Kγ inhibitor AS-605240 for the management of intracellular pathogens like Leishmania in humans still requires additional research, it does show a lot of promise. When used in combination with current treatment options for cutaneous Leishmania infections, PI3Kγ inhibitors like AS-605240 present a viable alternative.
Disclosure of Conflicts of Interest
There were no potential conflicts of interest to disclose.
Acknowledgments
Research in ARS lab is supported by the grants from the NIH. S.O. is supported by NIDCR training grant T32DE014320.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/23360
References
- 1.Deane JA, Fruman DA. Phosphoinositide 3-kinase: diverse roles in immune cell activation. Annu Rev Immunol. 2004;22:563–98. doi: 10.1146/annurev.immunol.22.012703.104721. [DOI] [PubMed] [Google Scholar]
- 2.Ghigo A, Damilano F, Braccini L, Hirsch E. PI3K inhibition in inflammation: Toward tailored therapies for specific diseases. Bioessays. 2010;32:185–96. doi: 10.1002/bies.200900150. [DOI] [PubMed] [Google Scholar]
- 3.Barberis L, Hirsch E. Targeting phosphoinositide 3-kinase gamma to fight inflammation and more. Thromb Haemost. 2008;99:279–85. doi: 10.1160/TH07-10-0632. [DOI] [PubMed] [Google Scholar]
- 4.Schwegmann A, Brombacher F. Host-directed drug targeting of factors hijacked by pathogens. Sci Signal. 2008;1:re8. doi: 10.1126/scisignal.129re8. [DOI] [PubMed] [Google Scholar]
- 5.Alexander J, Satoskar AR, Russell DG. Leishmania species: models of intracellular parasitism. J Cell Sci. 1999;112:2993–3002. doi: 10.1242/jcs.112.18.2993. [DOI] [PubMed] [Google Scholar]
- 6.Cummings HE, Barbi J, Reville P, Oghumu S, Zorko N, Sarkar A, et al. Critical role for phosphoinositide 3-kinase gamma in parasite invasion and disease progression of cutaneous leishmaniasis. Proc Natl Acad Sci U S A. 2012;109:1251–6. doi: 10.1073/pnas.1110339109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laufs H, Müller K, Fleischer J, Reiling N, Jahnke N, Jensenius JC, et al. Intracellular survival of Leishmania major in neutrophil granulocytes after uptake in the absence of heat-labile serum factors. Infect Immun. 2002;70:826–35. doi: 10.1128/IAI.70.2.826-835.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Zandbergen G, Klinger M, Mueller A, Dannenberg S, Gebert A, Solbach W, et al. Cutting edge: neutrophil granulocyte serves as a vector for Leishmania entry into macrophages. J Immunol. 2004;173:6521–5. doi: 10.4049/jimmunol.173.11.6521. [DOI] [PubMed] [Google Scholar]
- 9.Peters NC, Egen JG, Secundino N, Debrabant A, Kimblin N, Kamhawi S, et al. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science. 2008;321:970–4. doi: 10.1126/science.1159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laskay T, van Zandbergen G, Solbach W. Neutrophil granulocytes--Trojan horses for Leishmania major and other intracellular microbes? Trends Microbiol. 2003;11:210–4. doi: 10.1016/S0966-842X(03)00075-1. [DOI] [PubMed] [Google Scholar]
- 11.Rickert P, Weiner OD, Wang F, Bourne HR, Servant G. Leukocytes navigate by compass: roles of PI3Kgamma and its lipid products. Trends Cell Biol. 2000;10:466–73. doi: 10.1016/S0962-8924(00)01841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones GE, Prigmore E, Calvez R, Hogan C, Dunn GA, Hirsch E, et al. Requirement for PI 3-kinase gamma in macrophage migration to MCP-1 and CSF-1. Exp Cell Res. 2003;290:120–31. doi: 10.1016/S0014-4827(03)00318-5. [DOI] [PubMed] [Google Scholar]
- 13.Fruman DA, Cantley LC. Phosphoinositide 3-kinase in immunological systems. Semin Immunol. 2002;14:7–18. doi: 10.1006/smim.2001.0337. [DOI] [PubMed] [Google Scholar]
- 14.Kima PE, Constant SL, Hannum L, Colmenares M, Lee KS, Haberman AM, et al. Internalization of Leishmania mexicana complex amastigotes via the Fc receptor is required to sustain infection in murine cutaneous leishmaniasis. J Exp Med. 2000;191:1063–8. doi: 10.1084/jem.191.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]