Abstract
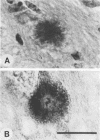
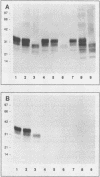
Given the critical role of the prion protein (PrP) in the transmission and pathogenesis of experimental scrapie, we investigated the PrP gene and its protein products in three hamster species, Chinese (CHa), Armenian (AHa), and Syrian (SHa), each of which were found to have distinctive scrapie incubation times. Passaging studies demonstrated that the host species, and not the source of scrapie prions, determined the incubation time for each species, and histochemical studies of hamsters with clinical signs of scrapie revealed characteristic patterns of neuropathology. Northern (RNA) analysis showed the size of PrP mRNA from CHa, AHa, and SHa hamsters to be 2.5, 2.4, and 2.1 kilobases, respectively. Immunoblotting demonstrated that the PrP isoforms were of similar size (33 to 35 kilodaltons); however, the monoclonal antibody 13A5 raised against SHa PrP did not react with the CHa or AHa PrP molecules. Comparison of the three predicted amino acid sequences revealed that each is distinct. Furthermore, differences within the PrP open reading frame that uniquely distinguish the three hamster species are within a hydrophilic segment of 11 amino acids that includes polymorphisms linked to scrapie incubation times in inbred mice and an inherited prion disease of humans. Single polymorphisms in this region correlate with the presence or absence of amyloid plaques for a given hamster species or mouse inbred strain. Our findings demonstrate distinctive molecular, pathological, and clinical characteristics of scrapie in three related species and are consistent with the hypothesis that molecular properties of the host PrP play a pivotal role in determining the incubation time and neuropathological features of scrapie.
Full text
PDF

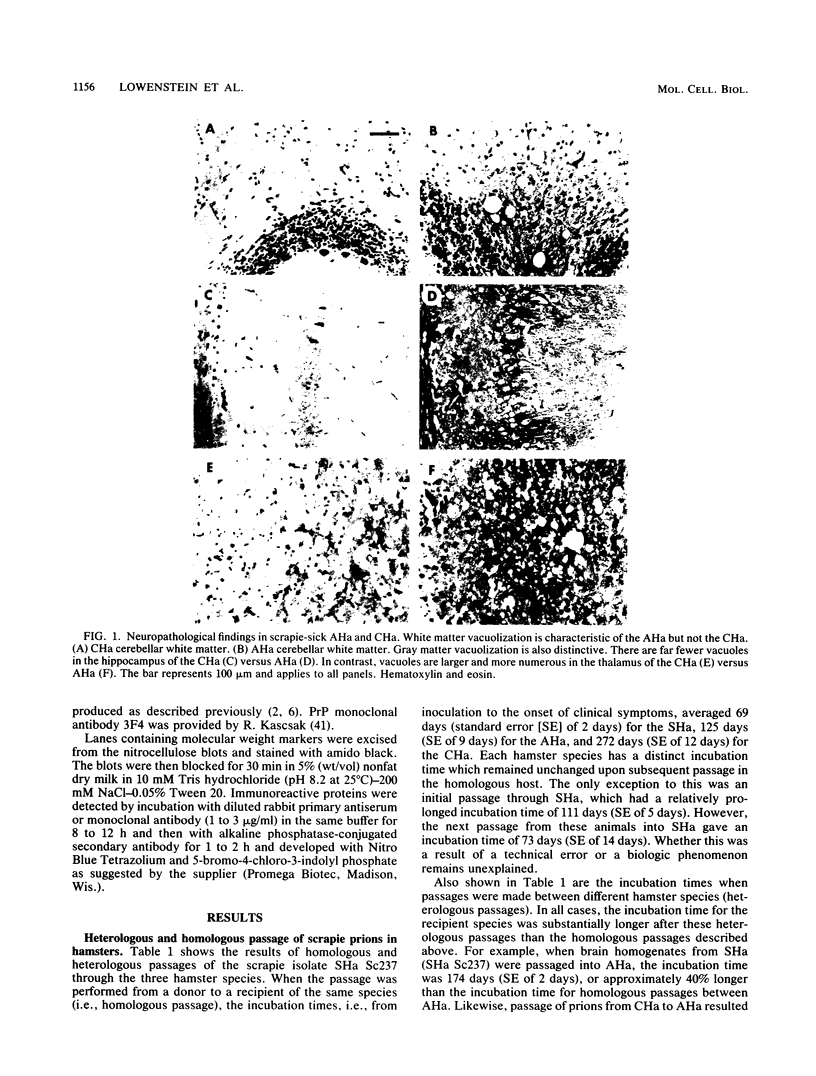



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry R. A., Prusiner S. B. Monoclonal antibodies to the cellular and scrapie prion proteins. J Infect Dis. 1986 Sep;154(3):518–521. doi: 10.1093/infdis/154.3.518. [DOI] [PubMed] [Google Scholar]
- Basler K., Oesch B., Scott M., Westaway D., Wälchli M., Groth D. F., McKinley M. P., Prusiner S. B., Weissmann C. Scrapie and cellular PrP isoforms are encoded by the same chromosomal gene. Cell. 1986 Aug 1;46(3):417–428. doi: 10.1016/0092-8674(86)90662-8. [DOI] [PubMed] [Google Scholar]
- Bazan J. F., Fletterick R. J., McKinley M. P., Prusiner S. B. Predicted secondary structure and membrane topology of the scrapie prion protein. Protein Eng. 1987 Feb-Mar;1(2):125–135. doi: 10.1093/protein/1.2.125. [DOI] [PubMed] [Google Scholar]
- Bendheim P. E., Barry R. A., DeArmond S. J., Stites D. P., Prusiner S. B. Antibodies to a scrapie prion protein. Nature. 1984 Aug 2;310(5976):418–421. doi: 10.1038/310418a0. [DOI] [PubMed] [Google Scholar]
- Bolton D. C., McKinley M. P., Prusiner S. B. Identification of a protein that purifies with the scrapie prion. Science. 1982 Dec 24;218(4579):1309–1311. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- Brown P., Cathala F., Castaigne P., Gajdusek D. C. Creutzfeldt-Jakob disease: clinical analysis of a consecutive series of 230 neuropathologically verified cases. Ann Neurol. 1986 Nov;20(5):597–602. doi: 10.1002/ana.410200507. [DOI] [PubMed] [Google Scholar]
- Bruce M. E., Dickinson A. G. Biological evidence that scrapie agent has an independent genome. J Gen Virol. 1987 Jan;68(Pt 1):79–89. doi: 10.1099/0022-1317-68-1-79. [DOI] [PubMed] [Google Scholar]
- Carlson G. A., Goodman P. A., Lovett M., Taylor B. A., Marshall S. T., Peterson-Torchia M., Westaway D., Prusiner S. B. Genetics and polymorphism of the mouse prion gene complex: control of scrapie incubation time. Mol Cell Biol. 1988 Dec;8(12):5528–5540. doi: 10.1128/mcb.8.12.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson G. A., Kingsbury D. T., Goodman P. A., Coleman S., Marshall S. T., DeArmond S., Westaway D., Prusiner S. B. Linkage of prion protein and scrapie incubation time genes. Cell. 1986 Aug 15;46(4):503–511. doi: 10.1016/0092-8674(86)90875-5. [DOI] [PubMed] [Google Scholar]
- Carlson G. A., Westaway D., DeArmond S. J., Peterson-Torchia M., Prusiner S. B. Primary structure of prion protein may modify scrapie isolate properties. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7475–7479. doi: 10.1073/pnas.86.19.7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Chandler R. L., Turfrey B. A. Inoculation of voles, Chinese hamsters, gerbils and guinea-pigs with scrapie brain material. Res Vet Sci. 1972 May;13(3):219–224. [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Collinge J., Harding A. E., Owen F., Poulter M., Lofthouse R., Boughey A. M., Shah T., Crow T. J. Diagnosis of Gerstmann-Sträussler syndrome in familial dementia with prion protein gene analysis. Lancet. 1989 Jul 1;2(8653):15–17. doi: 10.1016/s0140-6736(89)90256-0. [DOI] [PubMed] [Google Scholar]
- DeArmond S. J., McKinley M. P., Barry R. A., Braunfeld M. B., McColloch J. R., Prusiner S. B. Identification of prion amyloid filaments in scrapie-infected brain. Cell. 1985 May;41(1):221–235. doi: 10.1016/0092-8674(85)90076-5. [DOI] [PubMed] [Google Scholar]
- DeArmond S. J., Mobley W. C., DeMott D. L., Barry R. A., Beckstead J. H., Prusiner S. B. Changes in the localization of brain prion proteins during scrapie infection. Neurology. 1987 Aug;37(8):1271–1280. doi: 10.1212/wnl.37.8.1271. [DOI] [PubMed] [Google Scholar]
- Dickinson A. G., Meikle V. M., Fraser H. Identification of a gene which controls the incubation period of some strains of scrapie agent in mice. J Comp Pathol. 1968 Jul;78(3):293–299. doi: 10.1016/0021-9975(68)90005-4. [DOI] [PubMed] [Google Scholar]
- Doh-ura K., Tateishi J., Sasaki H., Kitamoto T., Sakaki Y. Pro----leu change at position 102 of prion protein is the most common but not the sole mutation related to Gerstmann-Sträussler syndrome. Biochem Biophys Res Commun. 1989 Sep 15;163(2):974–979. doi: 10.1016/0006-291x(89)92317-6. [DOI] [PubMed] [Google Scholar]
- Dunning A. M., Talmud P., Humphries S. E. Errors in the polymerase chain reaction. Nucleic Acids Res. 1988 Nov 11;16(21):10393–10393. doi: 10.1093/nar/16.21.10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Smart J. E., Burridge K., Helfman D. M., Thomas G. P. Co-existence of vinculin and a vinculin-like protein of higher molecular weight in smooth muscle. J Biol Chem. 1982 Sep 25;257(18):11024–11031. [PubMed] [Google Scholar]
- Gajdusek D. C. Unconventional viruses and the origin and disappearance of kuru. Science. 1977 Sep 2;197(4307):943–960. doi: 10.1126/science.142303. [DOI] [PubMed] [Google Scholar]
- Goldgaber D., Goldfarb L. G., Brown P., Asher D. M., Brown W. T., Lin S., Teener J. W., Feinstone S. M., Rubenstein R., Kascsak R. J. Mutations in familial Creutzfeldt-Jakob disease and Gerstmann-Sträussler-Scheinker's syndrome. Exp Neurol. 1989 Nov;106(2):204–206. doi: 10.1016/0014-4886(89)90095-2. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Grosveld F. G., Dahl H. H., de Boer E., Flavell R. A. Isolation of beta-globin-related genes from a human cosmid library. Gene. 1981 Apr;13(3):227–237. doi: 10.1016/0378-1119(81)90028-7. [DOI] [PubMed] [Google Scholar]
- Haraguchi T., Fisher S., Olofsson S., Endo T., Groth D., Tarentino A., Borchelt D. R., Teplow D., Hood L., Burlingame A. Asparagine-linked glycosylation of the scrapie and cellular prion proteins. Arch Biochem Biophys. 1989 Oct;274(1):1–13. doi: 10.1016/0003-9861(89)90409-8. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Hay B., Barry R. A., Lieberburg I., Prusiner S. B., Lingappa V. R. Biogenesis and transmembrane orientation of the cellular isoform of the scrapie prion protein [published errratum appears in Mol Cell Biol 1987 May;7(5):2035]. Mol Cell Biol. 1987 Feb;7(2):914–920. doi: 10.1128/mcb.7.2.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Murray K. Packaging recombinant DNA molecules into bacteriophage particles in vitro. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3259–3263. doi: 10.1073/pnas.74.8.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K., Baker H. F., Crow T. J., Poulter M., Owen F., Terwilliger J. D., Westaway D., Ott J., Prusiner S. B. Linkage of a prion protein missense variant to Gerstmann-Sträussler syndrome. Nature. 1989 Mar 23;338(6213):342–345. doi: 10.1038/338342a0. [DOI] [PubMed] [Google Scholar]
- Hunter N., Hope J., McConnell I., Dickinson A. G. Linkage of the scrapie-associated fibril protein (PrP) gene and Sinc using congenic mice and restriction fragment length polymorphism analysis. J Gen Virol. 1987 Oct;68(Pt 10):2711–2716. doi: 10.1099/0022-1317-68-10-2711. [DOI] [PubMed] [Google Scholar]
- Kascsak R. J., Rubenstein R., Merz P. A., Tonna-DeMasi M., Fersko R., Carp R. I., Wisniewski H. M., Diringer H. Mouse polyclonal and monoclonal antibody to scrapie-associated fibril proteins. J Virol. 1987 Dec;61(12):3688–3693. doi: 10.1128/jvi.61.12.3688-3693.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberlin R. H., Walker C. Characteristics of a short incubation model of scrapie in the golden hamster. J Gen Virol. 1977 Feb;34(2):295–304. doi: 10.1099/0022-1317-34-2-295. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. T., Kasper K. C., Stites D. P., Watson J. D., Hogan R. N., Prusiner S. B. Genetic control of scrapie and Creutzfeldt-Jakob disease in mice. J Immunol. 1983 Jul;131(1):491–496. [PubMed] [Google Scholar]
- Kretzschmar H. A., Stowring L. E., Westaway D., Stubblebine W. H., Prusiner S. B., Dearmond S. J. Molecular cloning of a human prion protein cDNA. DNA. 1986 Aug;5(4):315–324. doi: 10.1089/dna.1986.5.315. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lavappa K. S., Yerganian G. Spermatogonial and meiotic chromosomes of the Armenian hamster, Cricetulus migratorius. Exp Cell Res. 1970 Jul;61(1):159–172. doi: 10.1016/0014-4827(70)90270-3. [DOI] [PubMed] [Google Scholar]
- Liao Y. C., Tokes Z., Lim E., Lackey A., Woo C. H., Button J. D., Clawson G. A. Cloning of rat "prion-related protein" cDNA. Lab Invest. 1987 Oct;57(4):370–374. [PubMed] [Google Scholar]
- Marsh R. F., Kimberlin R. H. Comparison of scrapie and transmissible mink encephalopathy in hamsters. II. Clinical signs, pathology, and pathogenesis. J Infect Dis. 1975 Feb;131(2):104–110. doi: 10.1093/infdis/131.2.104. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKinley M. P., Bolton D. C., Prusiner S. B. A protease-resistant protein is a structural component of the scrapie prion. Cell. 1983 Nov;35(1):57–62. doi: 10.1016/0092-8674(83)90207-6. [DOI] [PubMed] [Google Scholar]
- McKinley M. P., Hay B., Lingappa V. R., Lieberburg I., Prusiner S. B. Developmental expression of prion protein gene in brain. Dev Biol. 1987 May;121(1):105–110. doi: 10.1016/0012-1606(87)90143-6. [DOI] [PubMed] [Google Scholar]
- Mobley W. C., Neve R. L., Prusiner S. B., McKinley M. P. Nerve growth factor increases mRNA levels for the prion protein and the beta-amyloid protein precursor in developing hamster brain. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9811–9815. doi: 10.1073/pnas.85.24.9811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton C. R., Kalsheker N., Graham A., Powell S., Gammack A., Riley J., Markham A. F. Diagnosis of alpha 1-antitrypsin deficiency by enzymatic amplification of human genomic DNA and direct sequencing of polymerase chain reaction products. Nucleic Acids Res. 1988 Sep 12;16(17):8233–8243. doi: 10.1093/nar/16.17.8233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch B., Westaway D., Wälchli M., McKinley M. P., Kent S. B., Aebersold R., Barry R. A., Tempst P., Teplow D. B., Hood L. E. A cellular gene encodes scrapie PrP 27-30 protein. Cell. 1985 Apr;40(4):735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- Owen F., Poulter M., Lofthouse R., Collinge J., Crow T. J., Risby D., Baker H. F., Ridley R. M., Hsiao K., Prusiner S. B. Insertion in prion protein gene in familial Creutzfeldt-Jakob disease. Lancet. 1989 Jan 7;1(8628):51–52. doi: 10.1016/s0140-6736(89)91713-3. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Courtneidge S. A., Crittenden L. B., Fadly A. M., Bishop J. M., Varmus H. E. Analysis of avian leukosis virus DNA and RNA in bursal tumours: viral gene expression is not required for maintenance of the tumor state. Cell. 1981 Feb;23(2):311–322. doi: 10.1016/0092-8674(81)90127-6. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Bolton D. C., Groth D. F., Bowman K. A., Cochran S. P., McKinley M. P. Further purification and characterization of scrapie prions. Biochemistry. 1982 Dec 21;21(26):6942–6950. doi: 10.1021/bi00269a050. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Cochran S. P., Groth D. F., Downey D. E., Bowman K. A., Martinez H. M. Measurement of the scrapie agent using an incubation time interval assay. Ann Neurol. 1982 Apr;11(4):353–358. doi: 10.1002/ana.410110406. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Groth D. F., Bolton D. C., Kent S. B., Hood L. E. Purification and structural studies of a major scrapie prion protein. Cell. 1984 Aug;38(1):127–134. doi: 10.1016/0092-8674(84)90533-6. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Groth D. F., Cochran S. P., Masiarz F. R., McKinley M. P., Martinez H. M. Molecular properties, partial purification, and assay by incubation period measurements of the hamster scrapie agent. Biochemistry. 1980 Oct 14;19(21):4883–4891. doi: 10.1021/bi00562a028. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., McKinley M. P., Bowman K. A., Bolton D. C., Bendheim P. E., Groth D. F., Glenner G. G. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell. 1983 Dec;35(2 Pt 1):349–358. doi: 10.1016/0092-8674(83)90168-x. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Prions and neurodegenerative diseases. N Engl J Med. 1987 Dec 17;317(25):1571–1581. doi: 10.1056/NEJM198712173172505. [DOI] [PubMed] [Google Scholar]
- Roberts G. W., Lofthouse R., Brown R., Crow T. J., Barry R. A., Prusiner S. B. Prion-protein immunoreactivity in human transmissible dementias. N Engl J Med. 1986 Nov 6;315(19):1231–1233. doi: 10.1056/NEJM198611063151919. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Scott M., Foster D., Mirenda C., Serban D., Coufal F., Wälchli M., Torchia M., Groth D., Carlson G., DeArmond S. J. Transgenic mice expressing hamster prion protein produce species-specific scrapie infectivity and amyloid plaques. Cell. 1989 Dec 1;59(5):847–857. doi: 10.1016/0092-8674(89)90608-9. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Hughes S. H., Kung H. J., Majors J. E., Quintrell N., Guntaka R. V., Bishop J. M., Varmus H. E. Mapping unintegrated avian sarcoma virus DNA: termini of linear DNA bear 300 nucleotides present once or twice in two species of circular DNA. Cell. 1978 Dec;15(4):1383–1395. doi: 10.1016/0092-8674(78)90063-6. [DOI] [PubMed] [Google Scholar]
- Singh L., Jones K. W. The use of heparin as a simple cost-effective means of controlling background in nucleic acid hybridization procedures. Nucleic Acids Res. 1984 Jul 25;12(14):5627–5638. doi: 10.1093/nar/12.14.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sparkes R. S., Simon M., Cohn V. H., Fournier R. E., Lem J., Klisak I., Heinzmann C., Blatt C., Lucero M., Mohandas T. Assignment of the human and mouse prion protein genes to homologous chromosomes. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7358–7362. doi: 10.1073/pnas.83.19.7358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindall K. R., Kunkel T. A. Fidelity of DNA synthesis by the Thermus aquaticus DNA polymerase. Biochemistry. 1988 Aug 9;27(16):6008–6013. doi: 10.1021/bi00416a027. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westaway D., Carlson G. A., Prusiner S. B. Unraveling prion diseases through molecular genetics. Trends Neurosci. 1989 Jun;12(6):221–227. doi: 10.1016/0166-2236(89)90126-4. [DOI] [PubMed] [Google Scholar]
- Westaway D., Goodman P. A., Mirenda C. A., McKinley M. P., Carlson G. A., Prusiner S. B. Distinct prion proteins in short and long scrapie incubation period mice. Cell. 1987 Nov 20;51(4):651–662. doi: 10.1016/0092-8674(87)90134-6. [DOI] [PubMed] [Google Scholar]
- Westaway D., Prusiner S. B. Conservation of the cellular gene encoding the scrapie prion protein. Nucleic Acids Res. 1986 Mar 11;14(5):2035–2044. doi: 10.1093/nar/14.5.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. B. Host specificity of DNA produced by Escherichia coli: bacterial mutations affecting the restriction and modification of DNA. J Mol Biol. 1966 Mar;16(1):118–133. doi: 10.1016/s0022-2836(66)80267-x. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- ZLOTNIK I., RENNIE J. C. EXPERIMENTAL TRANSMISSION OF MOUSE PASSAGED SCRAPIE TO GOATS, SHEEP, RATS AND HAMSTERS. J Comp Pathol. 1965 Apr;75:147–157. doi: 10.1016/0021-9975(65)90005-8. [DOI] [PubMed] [Google Scholar]