Abstract
Polymodal sensory neurons inform organisms about the nature of the physical world around them. The activity of these cells guide behaviors including the withdrawal from nocifensive stimuli such as intense heat or harsh force to feeling the comforting weight of a warm blanket. Molecular and genetic analysis of the channel proteins required for these divers behavioral responses have revealed an elaborate and disparate collection of channel proteins within the polymodal sensory neuron. Recent data supports that the biophysical traits of the channel proteins combined with the collection of channels activated during stimulation is sufficient to describe the nature of the stimulus. It is currently unclear what the functional arrangement of channel proteins are during perception. Specifically, are channel proteins arranged in parallel and function independently during perception, or are these channel proteins arranged in functional sensory networks. We propose a hierarchal functional arrangement of channels within polymodal sensory neurons that incorporates aspects of both parallel and serial arrangements of channel proteins.
Keywords: ASIC channel, Degenerin, Trp channel, mechanosensation, sensory transduction
A Molecular Sensory Network
The polymodal sensory neuron must transform disparate stimuli such as heat and touch into informative neural activity that relays the physical description of the stimulus to the nervous system. Like mammalian sensory neurons, polymodal md sensory neurons from Drosophila express multiple channel types including members of the Deg/ENaC, Trp and Piezo channel families, that are required for md neurons to respond to a broad range of noxious stimuli including chemical, mechanical, or thermal.1-8 Existing data about these channel proteins are consistent with them possessing unique biophysical properties. Thus, they are able to generate currents with unique and discrete properties. Whether the biophysical properties of the individual channels are sufficient to inform the nervous system about the nature of stimuli remains unclear. Considering the data demonstrating the genetic separation of sensory modalities in invertebrates, a simple model is that the channels sensing these modalities function in parallel and that perception is the result of a unique current signature produced from the specific combination of channels responding to a distinct stimuli (Fig. 1A).
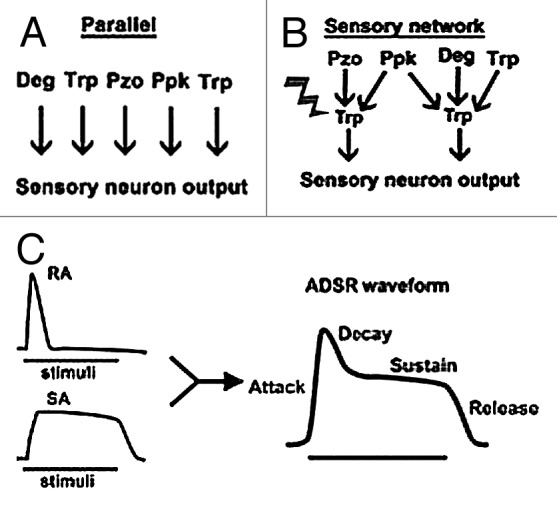
Figure 1. Sensory transduction in md neurons. (A) Parallel organization of channel receptors. Channel protein function independently. (B) Organization of channel proteins into a network. In this scenario, Trp channels play an important role in modifying and filtering the current generated by the upstream incidence receptors. (C) The ADSR waveform as a result of the combination of RA and SA currents.
Interestingly, some Trp channels, such as the Painless or TrpA1 channels, are required in the md neuron for the appropriate response to more than one sensory modality.4-7 In addition, genetic epistasis analysis of channel protein mutants in flies finds that these channels are arranged in genetic pathways. For example, in Drosophila md sensory neurons the mechanosensitive Piezo channel, the Trp family member Painless and the Deg/ENaC family member Ppk1 are all required for mechanical nociception.1-3 Genetic epistasis finds that Piezo and ppk1 function independently during mechanical nociception but that Piezo and painless function in the same genetic pathway (Fig. 1B).2 Combined with the observation that Painless is required for multiple modalities in md neurons, these data suggest an organization where Trp channels function downstream of Deg and Pzo channels in a sensory network (Fig. 1B). This organization could allow for the encoding of a broader range of stimuli and places Trp channels as critical modifiers of incidence detection.
Feel the Music
The collection of ion channel currents found in sensory neurons can be characterized by their nonstationary response to continued stimulation: currents that rapidly adapt (RA) resulting in transient responses, slowly adapting (SA) currents resulting in a persistent response and currents that are intermediate to RA and SA in adaptation (IA) to stimuli.1,9-13 Furthermore, sensory neurons can contain multiple adapting currents for the same modality.1 During stimulation it is predicted that a large number of channels, conducting both RA and SA currents, are recruited contributing to a cell-wide current signature that is representative of the stimuli. Currently there is little evidence that the current signatures generated in md neurons during stimulation encode sensory information.
We find that Drosophila md neurons harbor molecularly distinct RA and SA acid-sensitive currents. Specific activation of the RA current generated by the Ppk1 channel is sufficient to generate a burst of action potentials that precisely reflects the kinetics of the adaptation of this Ppk1-dependent current.1 Furthermore, a gain of function mutation in Ppk1 that alterschannel gating generates a sustained burst of action potentials reflective of the more sustained adaptation of the mutant channel. Importantly, larvae harboring this mutant Ppk1 channel have impaired nociception, providing evidence that the adaptation kinetics of the Ppk1 channel is important for encoding information about the nature of the stimuli. Also consider Drosophila larvae that have the same behavioral response to harsh touch and high heat, even though these modalities utilize distinct collections of channel proteins.2,4 If the current signature of the md neuron encodes information about the nature of the stimuli, than we predict that the current signatures generated by harsh touch and high heat should be very similar. Although data from heterologous cells support that the channels supporting this behavior have similar adaptation kinetics, it will be important to extend these analyses into sensory neurons to evaluate the bursting patterns generated during nociceptive stimuli.
Because our stimulation of Ppk1 is heterotypic, it remains to be seen what the in vivo bursting pattern of md neurons during stimulation is. One possibility is that these currents combine to create a distinct current waveform with a shape that describes the stimulus. This waveform could resemble the attack-decay-sustain-release (ADSR) waveform used by moog synthesizers to generate sounds as diverse as a cymbal crash to a low hum (Fig. 1C). The utility of the ADSR waveform for information encoding is demonstrated by the broad range of unique waveforms that can be generated as well as the ability of this waveform to report changes in the quality of stimuli over time.14 Thus, perception by md sensory neurons could consist of the activation of unique sets of currents (RA and SA) that generate a cellular current signature resulting in a unique bursting event that sufficiently describes the stimuli. Further comparisons of sensory neuron bursting, channel currents and the resulting behavior will be required to determine how the biophysical properties of channels can guide behavior.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/23469
References
- 1.Boiko N, Kucher V, Stockand JD, Eaton BA. Pickpocket1 is an ionotropic molecular sensory transducer. J Biol Chem. 2012;287:39878–86. doi: 10.1074/jbc.M112.411736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim SE, Coste B, Chadha A, Cook B, Patapoutian A. The role of Drosophila Piezo in mechanical nociception. Nature. 2012;483:209–12. doi: 10.1038/nature10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhong L, Hwang RY, Tracey WD. Pickpocket is a DEG/ENaC protein required for mechanical nociception in Drosophila larvae. Curr Biol. 2010;20:429–34. doi: 10.1016/j.cub.2009.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhong L, Bellemer A, Yan H, Ken H, Jessica R, Hwang RY, et al. Thermosensory and nonthermosensory isoforms of Drosophila melanogaster TRPA1 reveal heat-sensor domains of a thermoTRP Channel. Cell Rep. 2012;1:43–55. doi: 10.1016/j.celrep.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang K, Panzano VC, Chang EC, Ni L, Dainis AM, Jenkins AM, et al. Modulation of TRPA1 thermal sensitivity enables sensory discrimination in Drosophila. Nature. 2012;481:76–80. doi: 10.1038/nature10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Anzi B, Tracey WD, Jr., Benzer S. Response of Drosophila to wasabi is mediated by painless, the fly homolog of mammalian TRPA1/ANKTM1. Curr Biol. 2006;16:1034–40. doi: 10.1016/j.cub.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Tracey WD, Jr., Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–73. doi: 10.1016/S0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 8.Ainsley JA, Pettus JM, Bosenko D, Gerstein CE, Zinkevich N, Anderson MG, et al. Enhanced locomotion caused by loss of the Drosophila DEG/ENaC protein Pickpocket1. Curr Biol. 2003;13:1557–63. doi: 10.1016/S0960-9822(03)00596-7. [DOI] [PubMed] [Google Scholar]
- 9.Hao J, Delmas P. Multiple desensitization mechanisms of mechanotransducer channels shape firing of mechanosensory neurons. J Neurosci. 2010;30:13384–95. doi: 10.1523/JNEUROSCI.2926-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu J, Lewin GR. Mechanosensitive currents in the neurites of cultured mouse sensory neurones. J Physiol. 2006;577:815–28. doi: 10.1113/jphysiol.2006.117648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Hagan R, Chalfie M, Goodman MB. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat Neurosci. 2005;8:43–50. doi: 10.1038/nn1362. [DOI] [PubMed] [Google Scholar]
- 12.Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, et al. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci USA. 2002;99:2338–43. doi: 10.1073/pnas.032678399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krishtal OA, Pidoplichko VI. Receptor for protons in the membrane of sensory neurons. Brain Res. 1981;214:150–4. doi: 10.1016/0006-8993(81)90446-7. [DOI] [PubMed] [Google Scholar]
- 14.Pinch T. Analog days the invention and impact of the Moog synthesizer. Cambridge, MA, Harvard University Press, 2002. [Google Scholar]


