Abstract
Phosphine, which is released continuously from sediment, can affect the eco-physiological strategies and molecular responses of phytoplankton. To examine the effects of phosphine on phosphorus uptake and utilization in Thalassiosira pseudonana, we examined the transcriptional level of the phosphate transporter gene (TpPHO) and the activity of alkaline phosphatase (AKP) in relation to supplement of various concentrations of phosphine. TpPHO expression was markedly promoted by phosphine in both the phosphate-deficient and phosphate-4 µM culture. However, high phosphine concentrations can inhibit TpPHO transcription in the declining growth phase. AKP activity was also higher in the phosphine treatment groups than that of the control. It increased with increasing phosphine concentration in the range of 0 to 0.056 µM but was inhibited by higher levels of phosphine. These responses revealed that phosphine can affect phosphate uptake and utilization in T. pseudonana. This result was consistent with the effect of phosphine on algal growth, while TpPHO expression and AKP were even more sensitive to phosphine than algal growth. This work provides a basic understanding for further research about how phosphine affects phytoplankton.
Introduction
Phosphorus (P) is essential for the growth of planktonic algae and plays a significant role in cell physiology and biochemistry in the marine environment. Increasing evidence indicates that P is frequently a limiting nutrient for phytoplankton, and its absence can inhibit primary productivity [1]–[3]. Previously, P was commonly assumed existing in the environment as fully oxidized phosphate. Along with the development of analytical technology, reduced inorganic forms of P, including phosphite, hypophosphite, phosphide and phosphine (PH3), have been frequently detected in the natural environment [4]. Therefore, it is likely that organisms with the ability to utilize reduced P as alternative sources of P may be at an ecological advantage under P limiting conditions. Recent biochemical evidence suggests that some organisms can utilize phosphite and hypophosphite as alternative sources of P [4]. However, whether phosphine can serve as an alternative source of P under P limiting conditions is still not known.
Phosphine, even as a trace component, is a ubiquitous constituent of the hydrosphere [5]–[10]. It exists in two forms: free gaseous phosphine and matrix-bound phosphine (MBP) [10]–[14]. MBP, normally presented in soils, sediments, sludge, and other condensed media, can hydrolyze in biological aquatic media to form free phosphine gas. Phosphine is a reduced compound that can be easily oxidized to other forms of P. Therefore, phosphine should not be neglected in the marine environment considering its potential ecological roles to marine organisms. Besides, phosphine is commonly used as a fumigant to control insects that affect stored products throughout the world [15]–[19]. It is toxic to animals such as mites [15], insects [15]–[17], and mammals [18]. The effect of phosphine on animals has been examined in various studies [18], [19], but only a few focused primarily on the effects of phosphine on phytoplankton (e.g. Microcystis aeruginosa [20]). Niu et al. [20] reported that phosphine stimulated the growth of M. aeruginosa and that the release of phosphine may play an important role in outbreaks of algal blooms in eutrophic waters. However, little is known about what the fate of phosphine is and how it affects marine phytoplankton.
Changes in expression of phosphate transporter genes and activity of alkaline phosphatase (AKP) can be useful for investigating the effects of phosphine on microalgae and how it is utilized by microalgae. Dong et al. [21] reported a strong relationship between phosphate uptake and the expression of phosphate transporter genes. Other researchers have studied phosphorus uptake by cells [22], and the first step is believed to be transport of phosphorus across the cell membrane by a membrane-associated transporter. Willsky et al. [23] proposed that phosphorus uptake is maintained by two distinct systems: the Pit (phosphate inorganic transport) system and the Pst (phosphate specific transport) system. Pit is a low-affinity, high-velocity system and Pst is a high-affinity, low-velocity system. A high-affinity phosphate transporter gene encoding a previously described Pst system was identified in several plankton species, such as Anabaena sp. PCC7120 [24] and Tetraselmis chui [25]. Furthermore, its expression was regulated by the phosphate concentration in the medium. Low phosphate concentrations also can induce AKP activity. This enzyme hydrolyzes various forms of organic P and releases P, making it available for direct uptake [26]. Like phosphate transporter genes, AKP is another bioreporter of P metabolism [24]. Vrba et al. [27] reported that algae might adapt to phosphorus depletion by increasing AKP activity.
Thalassiosira pseudonana was used to investigate the mRNA expression of a high-affinity phosphate transporter gene and the activity of AKP in response to phosphine. This species is a common red tide algae and its full genome has been sequenced making this species an attractive model organism in genetic studies [28]–[30]. The nucleotide sequence of high-affinity phosphate transporter gene in T. pseudonana, TpPHO, has been deposited in the GenBank database under accession number XM002292288.1. In this study, we investigated the effects of phosphine on P uptake and utilization by T. pseudonana by assessing the responses of the phosphate transporter gene and AKP to various levels of phosphine. Our results provides basic understanding of how phosphine affects phytoplankton and will benefit further research to explore the fate of phosphine in the oceanic biogeochemical cycle of phosphorus.
Materials and Methods
Algae and Culture Conditions
T. pseudonana was provided by Xiamen University, Fujian, China. The unialgal stock and experimental cultures were maintained at 20±1°C under a light:dark cycle of 12∶12 h, with 80 µmol photon m−2s−1 provided by four cool-white fluorescent light bulbs. The stock culture was grown in L1-enriched seawater medium [31] and maintained in the exponential growth phase by inoculating cells into fresh L1 medium every 5 d. T. pseudonana was cultured at low P concentration (4 µM) for at least five generations before the experiments were started. Glassware was washed with ∼10% v/v HCl to remove possible contaminants, rinsed with distilled water, and then autoclaved at 121 kPa for 30 min.
Cultures at mid-exponential phase were inoculated into 2.5 L of sterilized seawater in 3L sealable culture flasks. The initial cell density for all experiments was on average 5.45×105 cells/ml, and they were grown under identical conditions as the stock culture. Nutrients, except phosphate (PO4 3−), were added to the cultures. All experimental cultures were inoculated for 24 h to ensure that the original algal cells exhausted the P in the medium before the addition of P to the medium, in between each flask was agitated gently every 12 h.
Experimental Design
To test the effects of phosphine on the transcriptional level of TpPHO, nine treatments with different initial P concentrations were prepared (Table 1). According to the concentration of phosphine in the marine environment [8] and the preliminary experiment, we chose 0.022 and 0.22 µmol/l (represent low-PH3 and high-PH3 respectively) for the experiment. Samples were collected at different growth stages (1, 2, 4, and 6 d) for analysis of TpPHO transcription. The timing of phosphine introduction also is described in Table 1.
Table 1. Control and P conditions for TpPHO gene expression experiment.
| Treatment | Description |
| Control (no-PH3 treatment in P-deficient culture) | The initial added concentrations of phosphate and phosphine were both 0 µM |
| 0.022 µM PH3 (low-PH3 treatment in P-deficient culture)a | The initial added concentration of PO4 3− was 0 µM, and 0.022 µM PH3 was introduced into the medium every day |
| 0.022 µM PO4 3− (low- PO4 3- treatment in P-deficient culture)a | The initial added concentration of PO4 3− was 0 µM, and 0.022 µM PO4 3− was introduced into the medium every day |
| 0.22 µM PH3 (high-PH3 treatment in P-deficient culture)a | The initial added concentration of PO4 3− was 0 µM, and 0.22 µM PH3 was introduced into the medium every day |
| 0.22 µM PO4 3− (high-PO4 3- treatment in P-deficient culture)a | The initial added concentration of PO4 3− was 0 µM, and 0.22 µM PO4 3− was introduced into the medium every day |
| 2 µM PO4 3− +0.022 µM PH3 (low-PH3 treatment in phosphate-2 µMculture)b | The initial added concentration of PO4 3− was 2 µM, and 0.022 µM PH3 was introduced into the medium every day |
| 4 µM PO4 3− +0.022 µM PH3 (low-PH3 treatment in phosphate-4 µMculture)b | The initial added concentration of PO4 3− was 4 µM, and 0.022 µM PH3 was introduced into the medium every day |
| 4 µM PO4 3− +0 µM PH3 (no-PH3 treatment in phosphate-4 µM culture) b | The initial concentration of PO4 3− was 4 µM, and no PH3 was introduced |
| 4 µM PO4 3− +0.22 µM PH3 (high-PH3 treatment in phosphate-4 µMculture)b | The initial concentration of PO4 3− was 4 µM, and 0.22 µM PH3 was introduced into the medium every day |
The concentrations of phosphine or phosphate were added for the first time at 24 h after inoculation and then added after sampling each morning.
Phosphate was added at 24 h after inoculation, and phosphine was introduced first at the same time. Thereafter, phosphine was introduced after sampling each morning.
To examine the effects of phosphine on AKP activity, initial phosphine concentrations were 0 µM, 0.022 µM, 0.056 µM, and 0.22 µM in cultures, respectively. No phosphate (0 µM) was added to the treatments. Nitrate, silicate and other nutrients were the same as in L1 medium. Phosphine was introduced into the phosphate-depleted culture at 24 h after inoculation. Cultures were sampled at 3, 6, 24 and 48 h after the addition of phosphine.
All treatments were carried out in triplicate.
Growth Parameters
During the course of the TpPHO experiments, cell densities of the algae were determined every day. After illumination for 2 h each day, ∼10 ml of algal suspension were sampled from the culture and fixed with Lugol’s solution for determination of cell densities. Cell densities were determined using a light microscope (Nikon Eclipse 50i, Tokyo, Japan) at a magnification of ×400 and were used to calculate the specific growth rate (µ) of the algae with the equation: µ = (lnN2− lnN1)/(t2− t1), where N1 and N2 represent cell numbers at t1 and t2, respectively, and t is the incubation time.
Extraction of Total RNA and First-strand cDNA Synthesis
The samples from the TpPHO experimental cultures at 1, 2, 4, and 6 d were centrifuged at 3500 rev/min (2512×g) for 5 min at 20°C. Approximately 108 cells were harvested and stored in liquid nitrogen. Cells were disrupted by grinding in lysis buffer containing β-mercaptoethanol. The total RNA was extracted using the RNAprep pure Plant Kit (TIANGEN Biotech, Beijing, China) following the manufacturer’s instructions. The isolated RNA was resuspended in 30 µl RNase-free water. RNA concentration was determined using a spectrophotometer (Thermo Scientific, NanoDrop 1000, Waltham, MA, USA) and the purity was estimated by the 260/280 nm absorption ratio (all ratios ranged from 1.8 to 2.1). RNA integrity was verified by agarose gel electrophoresis. RNA of appropriate quality was obtained for all samples.
DNase I-treated total RNA (1 µg) from each of the different samples was reverse transcribed into cDNA using an oligo(dT)18 primer (Sangon Biotech, Shanghai, China) and PrimeScript reverse transcriptase (TaKaRa, Kyoto, Japan) according to the manufacturer’s instructions.
Real-time Quantitative RT-PCR
First-strand cDNA was synthesized as described above. Real-time quantitative RT-PCR, based on the SYBR Green method was performed using SYBR Premix Ex Taq™ (TaKaRa) with a real-time thermal cycler system (Eppendorf, Hamburg, Germany). The PCR cycle program included an initial denaturation at 95°C for 30 s for 1 cycle, followed by 40 cycles at 95°C for 5 s, 60°C for 20 s, and 72°C for 30 s. A melting curve analysis was conducted to verify formation of a single unique product and the absence of potential primer dimerization. Triplicate qRT-PCR assays were performed for each sample [32].
To standardize the amount of total RNA present in each reaction, the housekeeping gene (internal reference gene) 18S rRNA was co-amplified and used as the standard. Primers were chosen using the Primer Premier 5 program to have a melting temperature of 58–60.4°C. Primers (Table 2) were synthesized at Sangon Biotech. PCR products were sequenced (Sangon Biotech) to verify the validity of these primers.
Table 2. List of primers and probes used for real-time RT-PCR.
| Gene name | Sequence (5′→3′) | Tm(°C) | Amplicon length |
| Tp18SrRNAf | TCTTAGTTGGTGGAGTGATTTGTC | 58.0 | 140 bp |
| Tp18SrRNAr | CGCCATCTTCCTTCATCTTGTA | 59.6 | |
| TpPHOf | TCTTCTATGCGGATGCCTGAG | 60.4 | 157 bp |
| TpPHOr | GCCGTGGCACATTGTTCTG | 60.2 |
Amplification efficiency of all RT-PCR reactions was analyzed through serial dilutions of cDNA originating from the control culture. Transcriptional levels of the target genes were calculated using the 2–ΔΔC T method [33] according to the equation ΔΔCT = (CT, Target-CT, 18s rRNA)sample-(CT, Target-CT, 18s rRNA)control. The CT values of three runs of the same sample were averaged before carrying out the ΔΔCT calculation. The relative abundance of TpPHO transcripts was 2–ΔΔC T fold relative to the control (fold is the unit for TpPHO transcriptional level).
AKP Activity
Samples (taken from cultures for each treatment at 3, 6, 24, and 48 h) used to measure cellular AKP activity were collected onto 25 mm diameter Whatman GF/F glass fiber filter membranes (pre-combusted at 450°C for 5 h) and stored at –20°C. Blank filters were prepared similarly. Cells were disrupted by grinding in Tris-HCl buffer (pH = 7.8) in an external ice-bath, and AKP activity then was determined using an AKP assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer’s instructions.
Statistical Analysis
The software SPSS 17.0 for Windows was used for statistical analysis. One-way analysis of variance (ANOVA) with Tukey’s multiple comparison test was applied to test differences in cell densities, specific growth rates, TpPHO transcription, and AKP activity of different phosphine treatments. A significance level of 0.05 was used in the analysis.
Results
Algal Growth Under Different P-conditions
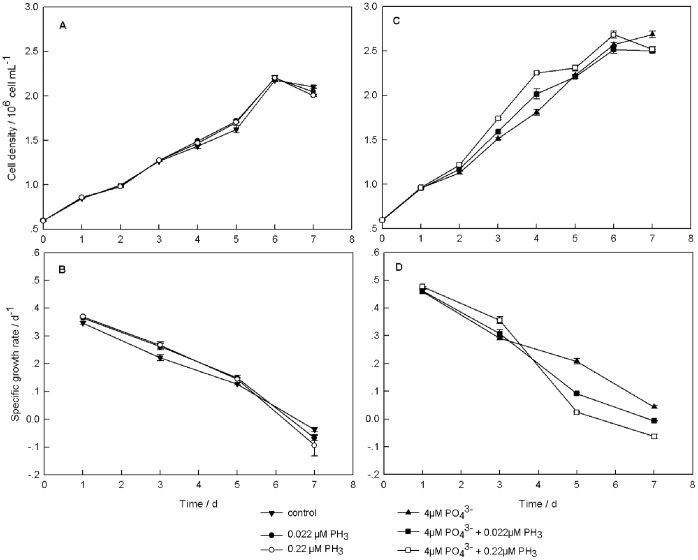
When T. pseudonana was cultured in phosphate-deficient medium (PO4 3− = 0 µM), the cell density increased during the first 6 days of the experiment. On day 6, the cell density of control (0 µM PH3), low-PH3 treatment (0.022 µM PH3), and high-PH3 treatment (0.22 µM PH3) reached 2.17×106, 2.20×106, 2.20×106 cells mL−1, respectively, but the culture reached the stationary stage on day 7. In this condition, different concentrations of phosphine had no significant effect on cell density (P>0.05) (Figure 1A). In the phosphate-deficient medium, specific growth rate (μ) in the high-PH3 treatment was higher than in the low-PH3 treatment, and both of the phosphine treatments had a higher µ than the control during the early culture period (day 1 and day 3, Figure 1B). The highest μ was 0.370 d−1 in the high-PH3 treatment on day 1.When incubation time was prolonged, specific growth rates of all treatments decreased. On day 5, there were no significant differences among the three phosphate-deficient treatments with different concentrations of phosphine (P>0.05). The specific growth rates of the three phosphate-deficient treatments were lower than 0 d−1 on day 7. The cell mortality rate in the high-PH3 treatment was higher than that in the low-PH3 treatment, and both of the phosphine treatments had a higher mortality rate than the control. The mortality rate was 0.094 d−1 for the high-PH3 treatment, 0.068 d−1 for the low-PH3 treatment, and 0.038 d−1 for the control.
Figure 1. Algal growth parameters of T. pseudonana with different phosphine levels.
(A) Cell density under the phosphate-deficient condition. (B) Growth rate under the phosphate-deficient condition. (C) Cell density under the phosphate-4 µM condition. (D) Growth rate under the phosphate-4 µM condition. The error bars represent standard deviations about the mean. For data points without an error bar, the error bar is smaller than the symbol.
In the phosphate-4 µM medium (PO4 3− = 4 µM), T. pseudonana also grew during the first 6 d and reached the stationary stage on day 7. The maximum cell density was 2.69×106 cells mL−1 in the high-PH3 treatment on day 6 and the no-PH3 treatment on day 7. Different cell densities were found in different treatments beginning on day 3, and the most pronounced difference occurred on day 4. The cell density of the high-PH3 treatment was higher than that of the low-PH3 treatment and no-PH3 treatment from days 2 to 6. The low-PH3 treatment had higher cell density than the no-PH3 treatment (Figure 1C). Figure 1D shows specific growth rates of the phosphine treatments in the phosphate-4 µM culture. The variation among the three different phosphine treatments was similar to the variation observed in the phosphate-deficient medium on days 1 and 3. The highest µ was 0.477 d−1 in the high-PH3 treatment on day 1. On day 5, µ of the no-PH3 treatment was higher than that of the low-PH3 treatment; and µ of the high-PH3 treatment was lowest among these treatments. On day 7, µ of the no-PH3 treatment was higher than 0 d−1. However, the mortality rate was 0.063 d−1 in the high-PH3 treatment and 0.006 d−1 in the low-PH3 treatment. Compared to the phosphate-deficient cultures, the phosphate-4 µM cultures had higher specific growth rates.
Amplification Efficiencies of TpPHO and 18SrRNA
To validate the 2–ΔΔC T method, all reactions were ensured to have the same amplification efficiencies. To assess the homogeneity of amplification efficiencies of the target and reference genes, variation of ΔCT in serial dilutions of template cDNA was evaluated. Figure 2 shows the standard curves of log cDNA diluted over a 200-fold range. The threshold cycles (CT) of TpPHO and 18SrRNA were both linearly correlated with log cDNA dilution. Slopes of the standard curves were –3.25 for TpPHO (Figure 2A) and –3.26 for 18SrRNA (Figure 2B). Both were close to the theoretical value of –3.32. A plot of ΔC T (C T, TpPHO – C T, 18S) versus log cDNA dilution (Figure 2C) revealed that the value of the slope was 0.01, which is close to zero. Therefore, the amplification efficiencies of the two genes were equal, and the 2–ΔΔC T method could be used to analyze the data [33].
Figure 2. Amplification efficiencies of TpPHO and 18SrRNA.
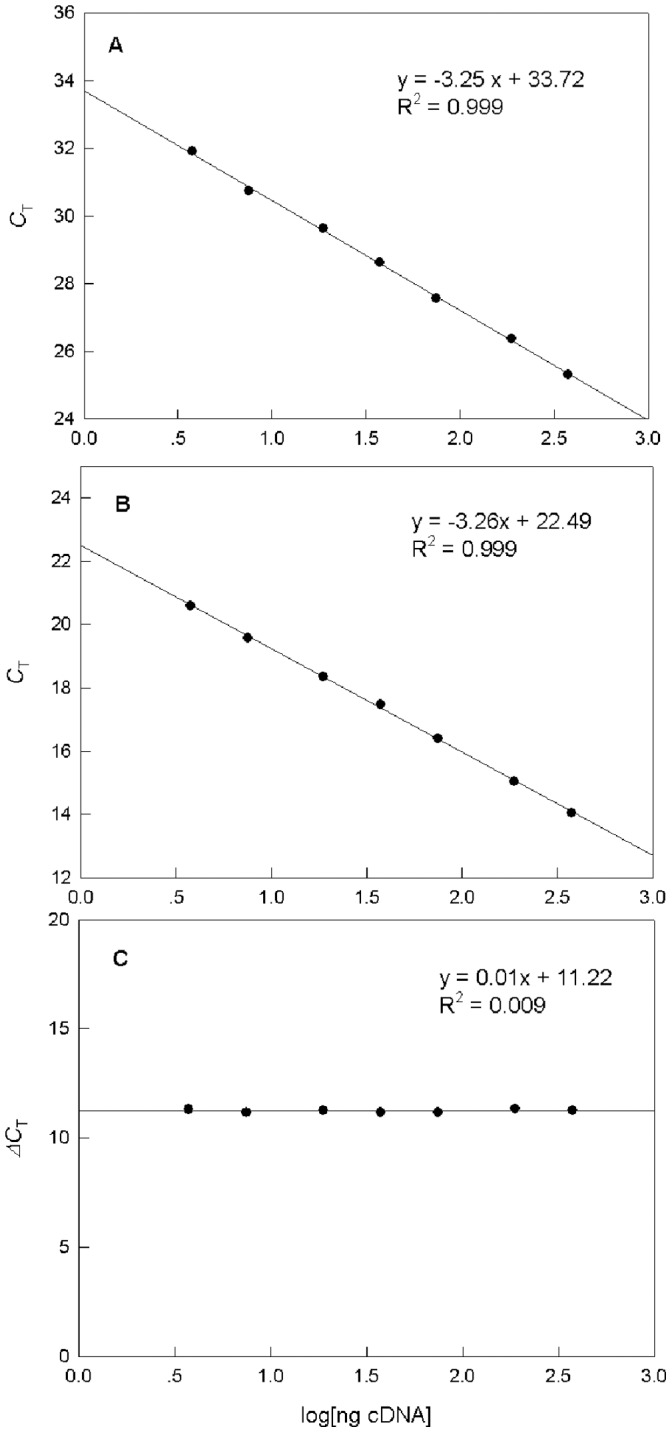
Real-time quantitative RT-PCR standard curves of (A) TpPHO and (B) 18S rRNA. (C) ΔC T. Curves were generated by plotting the logarithm of various cDNA concentrations (3 to 400 ng) versus C T or ΔC T values.
Effect of Phosphine on Transcriptional Level of TpPHO
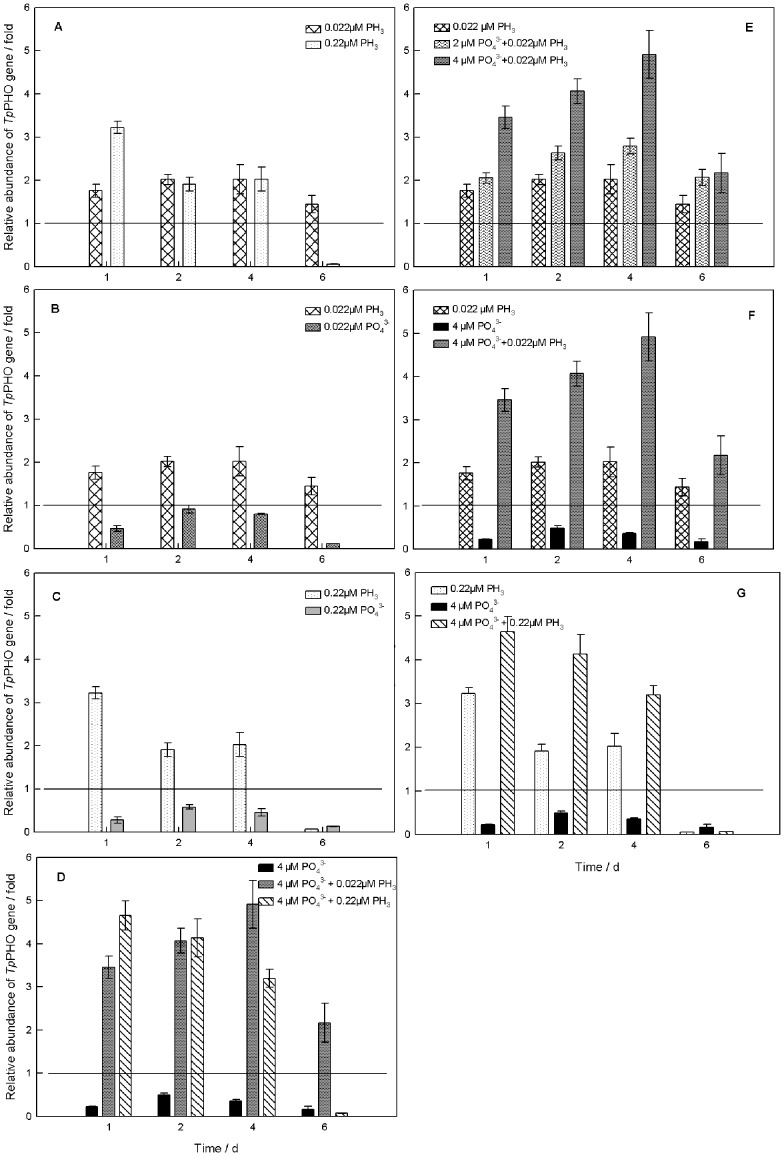
In the phosphate-deficient culture, the TpPHO transcriptional level increased with increased concentration of phosphine on the first day. The TpPHO transcriptional level in the low-PH3 (0.022 µM PH3) treatment was 1.760 fold relative to the control and the transcriptional level in the high-PH3 (0.22 µM PH3) treatment was 3.226 fold. The relative abundance of TpPHO transcripts in the low-PH3 treatment increased on days 2 and 4 and decreased on day 6, but it still was higher than the abundance in the control (>1 fold). In contrast, the TpPHO transcriptional level decreased with culture age in the high-PH3 treatment. The lowest level of TpPHO transcription among all samples tested was observed in this treatment on day 6 (0.064 fold) (Figure 3A).
Figure 3. Effects of phosphine on transcriptional level of TpPHO in T. pseudonana.
The horizontal lines show that the relative abundance of TpPHO in the control (reference group) was 1 fold. (A) Under the phosphate-deficient condition, different concentrations of phosphine were introduced. (B) Under the phosphate-deficient condition, the same low concentrations (0.022 µM) of phosphine and phosphate were added. (C) The same high concentrations (0.22 µM) of phosphine and phosphate were added, respectively, under the phosphate-deficient condition. (D) Different concentrations of phosphine were added under the phosphate-4 µM condition. (E) The low concentration (0.022 µM) of phosphine was introduced into cultures with different initial concentrations of phosphate. (F) and (G) Phosphine and phosphate have a synergistic effect. For all treatments, the first sampling occurred at 24 h (day 1) after the first addition of phosphate and/or phosphine, which was 48 h after inoculation. The error bars represent standard deviations.
Two treatments of phosphate in the same concentrations as phosphine (0.022 µM, 0.22 µM), also were tested under phosphate-deficient conditions. The TpPHO transcriptional level of these two PO4 3− treatments was lower than that of the control (<1 fold), and much lower than those of the phosphine treatments (Figure 3B and 3C).
The temporal change in TpPHO transcriptional level in the phosphate-4 µM cultures was similar to that observed in the phosphate-deficient culture (Figure 3D). After 1 day, the TpPHO transcriptional level increased with the concentration of phosphine. In the phosphate-4µM culture, the TpPHO transcriptional level in the low-PH3 (4 µM PO4 3− +0.022 µM PH3) treatment was 3.455 fold. It was lower than the level in the high-PH3 (4 µM PO4 3− +0.22 µM PH3) treatment (4.652 fold) but much higher than the level in the no-PH3 (4 µM PO4 3− +0 µM PH3) treatment (0.228 fold). After 2 days, the TpPHO transcriptional level in both the low-PH3 and no-PH3 treatments increased slightly, and the low-PH3 treatment reached the highest level (4.913 fold) on day 4. As in the phosphate-deficient culture, the TpPHO transcriptional level in the low-PH3 treatment (2.17 fold) was higher than that in the control. In contrast, the level in the high-PH3 treatment was significantly lower than that in the control after day 6. The transcriptional level in the no-PH3 treatment was always lower than that in the phosphine treatments and in the control.
Figure 3E shows the effect of phosphine on the TpPHO transcriptional level in the treatments with different initial concentrations of phosphate. For the low-PH3 treatments with different concentrations of phosphate (0 µM PO4 3− +0.022 µM PH3, 2 µM PO4 3− +0.022 µM PH3, 4 µM PO4 3− +0.022 µM PH3), the TpPHO transcriptional level increased with increased phosphate concentration. The TpPHO levels of all three treatments increased over the first 4 days of the experiment and then decreased by day 6. The differences in transcript levels among the three treatments on day 6 were not significant (P>0.05).
Figure 3F illustrates a synergistic effect between phosphine and phosphate on transcription of TpPHO. On day 1, the relative abundances of TpPHO in the low-PH3 treatment (0 µM PO4 3− +0.022 µM PH3) and phosphate-4 µM treatment (4 µM PO4 3− +0 µM PH3) were 1.760 fold and 0.228 fold, respectively. However, the TpPHO transcriptional level of the low-PH3 treatment in the phosphate-4 µM culture (4 µM PO4 3− +0.022 µM PH3) dramatically increased to 4.070 fold. Moreover, throughout the experiment, the TpPHO gene expression of the 4 µM PO4 3− +0.022 µM PH3 treatment was higher than the sum of the other two treatments. The synergistic effect could be illustrated by the high- PH3 treatment (0 µM PO4 3− +0.22 µM PH3), phosphate-4 µM treatment (4 µM PO4 3− +0 µM PH3), and phosphate-4 µM culture (4 µM PO4 3− +0.22 µM PH3) (Figure 3G).
Effect of Phosphine on AKP Activity
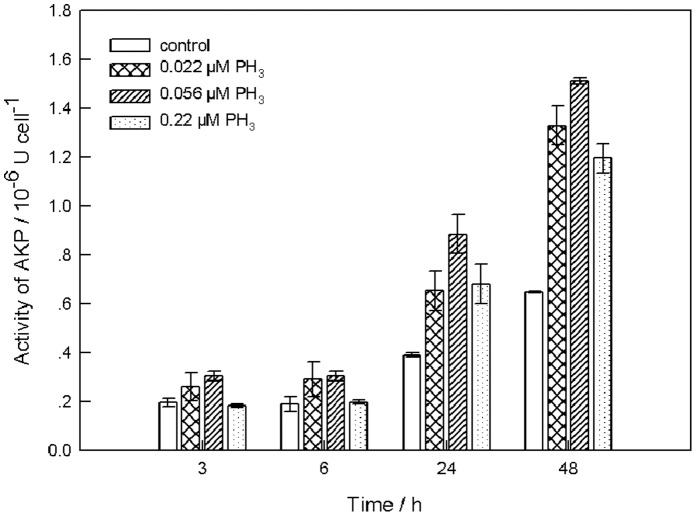
Results of the previous experiment revealed that TpPHO gene expression varied with phosphine concentration; in other words, phosphine may affect the P metabolism of phosphate. Phosphine concentration markedly affected gene expression on the first day of the experiment. To further confirm that phosphine affects P metabolism, a short-term experiment to measure the effect of phosphine on AKP activity was conducted with different phosphine concentrations and with the influence of growth stage eliminated. Figure 4 shows the results of the experiment. AKP activity increased with culture age in all treatments, and it was generally higher in the three phosphine treatments than in the control. The highest level, 1.511×10−6 U cell−1, was observed in the treatment with the middle concentration of phosphine (0.056 µM PH3) after 48 h. The variation in AKP activity did not completely coincide with the variation in the TpPHO transcriptional level. However, low phosphine concentration (0.022 µM PH3) had a greater effect (i.e., promotion) on both parameters than high concentration (0.22 µM PH3).
Figure 4. Effects of phosphine on AKP activity in T. pseudonana.
Enzymatic activity, 1 U Cell−1 is expressed as 1µg P-nitrophenol liberated by AKP per algal cell for 1.5 h. The error bars represent standard deviations from three replicate treatments.
Discussion
In this study, effects of phosphine on the cell density and specific growth rates in T. pseudonana were investigated. The phosphine treatments had higher specific growth rates during the early culture period; however, with continuous introduction of phosphine and prolonged stress time, specific growth rates of the phosphine treatments became lower than that of controls. During the decline phase, the mortality rate of the high-PH3 treatment was higher than that of the low-PH3 treatment; and the mortality rate of the low-PH3 treatment was higher than that of the control. These results suggest that phosphine has a dual effect on algae: phosphine can stimulate cells to reproduce at a low concentration, while has inhibitory effect on cell vitality at a high concentration. Niu et al. [20] reported a similar result for Microcysis aeruginosa. Before the microalgae enter a low growth phase and even die, a transient higher growth rate of phosphine treatment during the first few days may reflect a kind of "stress response" of the organisms to phosphine. Chefurka et al. [18] found that phosphine can affect the electron transport chain, and Nocter and Foyer [34] suggested that disturbances of the photosynthetic electron transport chain can result in oxidative stress in plants. Phosphine could influence the anti-oxidative defense system. Bolter and Chefurka [17] reported that phosphine induced an increase of superoxide dismutase activity, and the anti-oxidation mechanisms could protect the cell against oxygen-derived radicals. Low phosphine concentrations may induce the production of reactive oxygen species (ROS), which could stimulate anti-oxidative mechanisms. The activation of the anti-oxidative system could help enhance the cell’s tolerance to radicals, meaning that phosphine at low concentrations is beneficial to cell growth. In contrast, high concentrations of phosphine would induce too much ROS for the anti-oxidative system, resulting in lipid peroxidation and reduce algal growth subsequently [17], [35]. The effect of phosphine is likely consistent with the effect of ROS. Pelicano et al. [36] demonstrated that ROS have a dual effect on cell growth: low levels of ROS induced cell proliferation, whereas excess ROS led to cell death.
The result of the phosphine experiments clearly indicated that TpPHO expression was markedly promoted by phosphine in the phosphate-deficient medium. On the other hand, high phosphine concentration had inhibitory effects on TpPHO transcription during the decline phase (Figure 3A). Comparison of the phosphine treatment with the phosphate treatment of the same concentration revealed that the TpPHO transcriptional level of the former was much higher than that of the latter (Figure 3B and 3C). Therefore, phosphine appears to influence the algae in a manner other than by being oxidized to phosphate. A similar result was obtained in the 4 µM phosphate culture: TpPHO exhibited much higher expression in the phosphine treatments than in the control (Figure 3D). Previous studies have found a strong relationship between phosphate uptake and the expression of phosphate transporter genes [21]. For example, the expression of PHO84 and PHO89 in Saccharomyces cerevisiae was controlled by the phosphate concentration [37]. In Tetraselmis chui, TcPHO expression responded to the external concentration of phosphate [25]. Thus, under the same concentration of phosphate and the same experimental conditions, the observed effects of phosphine on TpPHO transcriptional level means that phosphine influenced phosphate uptake. These results suggest that phosphine triggers a P-deficiency signal to stimulate transcription of phosphate transporter genes, but the origin and nature of this signal is unknown. It could be considered a survival strategy to overcome the pressure from phosphine. Growing evidence supports the role of ROS as secondary messengers in signal pathways to regulate cell division [36], [38], [39]. This might be a mechanism that can explain the effect of phosphine on gene expression.
The response of TpPHO transcription to phosphine increased with the concentration of phosphate in culture (Figure 3E). The highest level of TpPHO transcription was observed in the low-PH3 treatment in the phosphate-4 µM culture(4 µM PO4 3− +0.022 µM PH3), which means that phosphine gave a bigger boost to TpPHO transcription as the phosphate increased in the culture. TpPHO expression is linked to the level of ambient phosphate. Nutritional conditions can impact the physiological characteristics of plants, such as signal transduction, enzymatic activity, and gene expression [40]. Algal cell vitality was strengthened at higher phosphate concentrations, thereby making proteins efficient in transmitting or responding to signals. Consequently, with higher phosphate concentration, the TpPHO transcriptional level is increased and the capacity for phosphate transport is improved subsequently.
As the culture aged, the sum of the added concentration of phosphine in the culture increased to a high level. Hsu et al. [35] reported that phosphine can induce DNA damage in rats. Meanwhile, algal cell vitality weakened in the later stage of the growth cycle. Therefore, gene expression was inhibited by phosphine. The observation that a low phosphine concentration boosted TpPHO gene expression was consistent with the variation of growth rate. Moreover, the effect of phosphine on phosphate uptake would further influence algal growth. This deduction was in agreement with published studies about the effect of phosphate on algal growth [20]. Furthermore, the response of TpPHO expression was more sensitive than that of algal growth.
The data revealed that changes of AKP activity and the TpPHO transcriptional level caused by phosphine were similar. Values of the two parameters in the phosphine treatments were higher than those in the control. A considerable amount of research has shown that AKP activity is negatively related to phosphate concentration [41], [42]. In the present study, if all of the phosphine was oxidized to phosphate, the concentration of phosphate in the medium should have increased as the added concentration of phosphine increased. Thus, AKP activity should have decreased with the increasing concentration of phosphine. In the experiment, AKP activity increased with the increase of phosphine in the range of 0 to 0.056 µM. This increasing AKP activity suggests that phosphine has other ways of influencing algae in addition to being oxidized to phosphate for uptake. These results demonstrate that phosphine influences the P metabolism of phosphate. The toxicity of phosphine is the reason why AKP activity in the high phosphine treatment was lower than that in the low phosphine treatment.
AKP hydrolyzes a variety of forms of organic P and releases P, making it available for uptake [26]. Meanwhile, the phosphate transporter is supposed to be the first step in phosphate transport [25]. However, there is not a direct relationship between AKP and the phosphate transporter. The only connection is that they are closely related to P metabolism and improved by phosphate deficiency. The similar variations in these parameters in response to varying levels of phosphine indicate that phosphine triggers a P-deficiency signal.
Conclusions and Perspectives
Our experimental data demonstrate that phosphine influences algae in a way other than by oxidation to release P for uptake. Both TpPHO transcriptional level and AKP activity of T. pseudonana increased at low phosphine concentration. These response mechanisms suggest that phosphine improves phosphate uptake and utilization, probably by triggering a P-deficiency signal. On the other hand, the toxicity of phosphine can induce damage to DNA and the plasma membrane system, thus gene expression and enzyme activity were inhibited at high phosphine concentrations. These variations indicate the dual effect of phosphine on T. pseudonana. However, different phytoplankton groups have different ecophysiological strategies and molecular level response mechanisms, resulting in different responses to phosphine. Whether other phytoplankton species have the same response to phosphine remains to be determined. In addition, a better understanding of the effect of phosphine on phosphorus metabolism will help explain the mechanism by which phosphine influences phytoplankton. Studies of the effect of phosphine on the electron transport chain and anti-oxidative system will be helpful to explain this mechanism.
Acknowledgments
We greatly thank Prof. Chaolun Li, Dr. Minxiao Wang, Hongjian Lv for their assistance and generosity in providing experimental instruments. We also thank Prof. Caiwen Li for language modification.
Funding Statement
This work was financially supported by the National Natural Science Foundation of China (30970522), the Funds for Creative Research Groups of China (41121064), and the State Key Development Program for Basic Research of China (973 program) (2010CB428706). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1. Krom MD, Kress N, Brenner S, Gordon LI (1991) Phosphorus limitation of primary productivity in the Eastern Mediterranean-Sea. Limnol Oceanogr 36: 424–432. [Google Scholar]
- 2. Li H, Veldhuis MJW, Post AF (1998) Alkaline phosphatase activities among planktonic communities in the northern Red Sea. Mar Ecol Prog Ser 173: 107–115. [Google Scholar]
- 3. Wu JF, Sunda W, Boyle EA, Karl DM (2000) Phosphate depletion in the western North Atlantic Ocean. Science 289: 759–762. [DOI] [PubMed] [Google Scholar]
- 4. Hanrahan G, Salmassi TM, Khachikian CS, Foster KL (2005) Reduced inorganic phosphorus in the natural environment: significance, speciation and determination. Talanta 66: 435–444. [DOI] [PubMed] [Google Scholar]
- 5. Devai I, Felfoldy L, Wittner I, Plosz S (1988) Detection of phosphine - new aspects of the phosphorus cycle in the hydrosphere. Nature 333: 343–345. [Google Scholar]
- 6. Glindemann D, Edwards M, Liu J, Kuschk P (2005) Phosphine in soils, sludges, biogases and atmospheric implications - a review. Ecol Eng 24: 457–463. [Google Scholar]
- 7. Devai I, Delaune RD (1995) Evidence for phosphine production and emission from Louisiana and Florida Marsh Soils. Org Geochem 23: 277–279. [Google Scholar]
- 8. Gassmann G (1994) Phosphine in the fluvial and marine hydrosphere. Mar Chem 45: 197–205. [Google Scholar]
- 9. Geng JJ, Niu XJ, Wang XR, Edwards M, Glindemann D (2010) The presence of trace phosphine in Lake Taihu water. Int J Environ Anal Chem 90: 737–746. [Google Scholar]
- 10. Niu XJ, Geng JJ, Wang XR, Wang CH, Gu XH, et al. (2004) Temporal and spatial distributions of phosphine in Taihu Lake, China. Sci Total Environ 323: 169–178. [DOI] [PubMed] [Google Scholar]
- 11. Feng ZH, Song XX, Yu ZM (2008) Distribution characteristics of matrix-bound phosphine along the coast of China and possible environmental controls. Chemosphere 73: 519–525. [DOI] [PubMed] [Google Scholar]
- 12. Yu ZM, Song XX (2003) Matrix-bound phosphine: A new form of phosphorus found in sediment of Jiaozhou Bay. Chin Sci Bull 48: 31–35. [Google Scholar]
- 13. Song XX, Morrison RJ, Feng ZH, Liu DY, Harrison JJ, et al. (2011) Matrix-bound phosphine in sediments from Lake Illawarra, New South Wales, Australia. Mar Pollut Bull 62: 1744–1750. [DOI] [PubMed] [Google Scholar]
- 14. Feng ZH, Song XX, Yu ZM (2008) Seasonal and spatial distribution of matrix-bound phosphine and its relationship with the environment in the Changjiang River Estuary, China. Mar Pollut Bull 56: 1630–1636. [DOI] [PubMed] [Google Scholar]
- 15. Sinha RN, Berck B, Wallace HAH (1967) Effect of phosphine on mites, insects, and microorganisms. J Econ Entomol 60: 125–132. [Google Scholar]
- 16. Bond EJ, Robinson JR, Buckland CT (1969) Toxic action of phosphine - absorption and symptoms of poisoning in insects. J Stored Prod Res 5: 289–298. [Google Scholar]
- 17. Bolter CJ, Chefurka W (1990) The effect of phosphine treatment on superoxide-dismutase, catalase, and peroxidase in the granary weevil, Sitophilus granarius . Pestic Biochem and Physiol 36: 52–60. [Google Scholar]
- 18. Chefurka W, Kashi KP, Bond EJ (1976) Effect of phosphine on electron-transport in mitochondria. Pestic Biochem and Physiol 6: 65–84. [Google Scholar]
- 19. Price NR (1984) Active exclusion of phosphine as a mechanism of resistance in Rhyzopertha dominica (F.) (Coleoptera: Bostrychidae). J Stored Prod Res 20: 163–168. [Google Scholar]
- 20. Niu XJ, Zhang JF, Shi XL, Wang XR, Gao G, et al. (2003) Studies on Microcystis aeruginosa affected by phosphine and its oxidation dynamically released from eutrophic lakes. J Lake Sci 15: 263–268 (In Chinese).. [Google Scholar]
- 21. Dong B, Ryan PR, Rengel Z, Delhaize E (1999) Phosphate uptake in Arabidopsis thaliana: dependence of uptake on the expression of transporter genes and internal phosphate concentrations. Plant Cell and Environ 22: 1455–1461. [Google Scholar]
- 22. Jansson M (1988) Phosphate uptake and utilization by bacteria and algae. Hydrobiologia 170: 177–189. [Google Scholar]
- 23. Willsky GR, Bennett RL, Malamy MH (1973) Inorganic-phosphate transport in Escherichia coli: involvement of 2 genes which play a role in alkaline-phosphatase regulation. J Bacteriol 113: 529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Muñoz-Martín MÁ, Mateo P, Leganés F, Fernández-Piñas F (2011) Novel cyanobacterial bioreporters of phosphorus bioavailability based on alkaline phosphatase and phosphate transporter genes of Anabaena sp. PCC 7120. Anal Bioanal Chem 400: 3573–3584. [DOI] [PubMed] [Google Scholar]
- 25. Chung CC, Hwang SPL, Chang J (2003) Identification of a high-affinity phosphate transporter gene in a prasinophyte alga, Tetraselmis chui, and its expression under nutrient limitation. Appl Environ Microbiol 69: 754–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ivancic I, Fuks D, Radic T, Lyons DM, Silovic T, et al. (2010) Phytoplankton and bacterial alkaline phosphatase activity in the northern Adriatic Sea. Mar Environ Res 69: 85–94. [DOI] [PubMed] [Google Scholar]
- 27. Vrba J, Komarkova J, Vyhnalek V (1993) Enhanced activity of alkaline phosphatases - phytoplankton response to epilimnetic phosphorus depletion. Water Sci Technol 28: 15–24. [Google Scholar]
- 28. Armbrust EV, Berges JA, Bowler C, Green BR, Martinez D, et al. (2004) The genome of the diatom Thalassiosira pseudonana: Ecology, evolution, and metabolism. Science 306: 79–86. [DOI] [PubMed] [Google Scholar]
- 29. Huang AY, He LW, Wang GC (2011) Identification and characterization of microRNAs from Phaeodactylum tricornutum by high-throughput sequencing and bioinformatics analysis. BMC Genomics 12: 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brunner E, Richthammer P, Ehrlich H, Paasch S, Simon P, et al. (2009) Chitin-based organic networks: an integral part of cell wall biosilica in the diatom Thalassiosira pseudonana . Angew Chem-Int Edit 48: 9724–9727. [DOI] [PubMed] [Google Scholar]
- 31. Guillard RRL, Hargraves PE (1993) Stichochrysis immobilis is a diatom, not a chyrsophyte. Phycologia 32: 234–236. [Google Scholar]
- 32. Zhang BY, Yang F, Wang GC, Peng G (2010) Cloning and quantitative analysis of the Carbonic Anhydrase gene from Porphyra Yezoensis . J Phycol 46: 290–296. [Google Scholar]
- 33. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔC T method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 34. Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49: 249–279. [DOI] [PubMed] [Google Scholar]
- 35. Hsu C-H, Han B-C, Liu M-Y, Yeh C-Y, Casida JE (2000) Phosphine-induced oxidative damage in rats: attenuation by melatonin. Free Radic Biol Med 28: 636–642. [DOI] [PubMed] [Google Scholar]
- 36. Pelicano H, Carney D, Huang P (2004) ROS stress in cancer cells and therapeutic implications. Drug Resist Updat 7: 97–110. [DOI] [PubMed] [Google Scholar]
- 37. Martinez P, Persson BL (1998) Identification, cloning and characterization of a derepressible Na+-coupled phosphate transporter in Saccharomyces cerevisiae . Mol Gen Genet 258: 628–638. [DOI] [PubMed] [Google Scholar]
- 38. Lu M, Gong XG, Lu YW, Guo JJ, Wang CH, et al. (2006) Molecular cloning and functional characterization of a cell-permeable superoxide dismutase targeted to lung adenocarcinoma cells. J Biol Chem 281: 13620–13627. [DOI] [PubMed] [Google Scholar]
- 39. Thannickal VJ, Fanburg BL (2000) Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 279: L1005–L1028. [DOI] [PubMed] [Google Scholar]
- 40. Franco-Zorrilla JM, Gonzalez E, Bustos R, Linhares F, Leyva A, et al. (2004) The transcriptional control of plant responses to phosphate limitation. J Exp Bot 55: 285–293. [DOI] [PubMed] [Google Scholar]
- 41. Pettersson K (1980) Alkaline phosphatase activity and algal surplus phosphorus as phosphorus-deficiency indicators in Lake Erken. Arch Hydrobiol 89: 54–87. [Google Scholar]
- 42. Gonzalez-Gil S, Keafer BA, Jovine RVM, Aguilera A, Lu SH, et al. (1998) Detection and quantification of alkaline phosphatase in single cells of phosphorus-starved marine phytoplankton. Mar Ecol Prog Ser 164: 21–35. [Google Scholar]