Abstract
Background
We investigated the role of calcium-activated potassium (KCa) channel activation in myogenic tone in human peripheral microvasculature after heart surgery.
Methods
Human skeletal muscle arterioles (90–180 µm diameter) were dissected from tissue harvested pre- and post-cardiopulmonary bypass (CPB) during cardiac surgery. Myogenic reactivity in response to stepwise increases in intraluminal pressure was studied between pressure steps. Microvessel tone was determined pre-CPB, post-CPB, and after blockade of KCa channels. Expression and localization of large conductance (BK) KCa channels in the coronary microvasculature was assessed by immunoblot and immunofluorescence photomicroscopy.
Results
Myogenic tone of skeletal muscle arterioles was significantly reduced post-CPB compared with pre-CPB. Decrease in myogenic tone after CPB was reflected by the increase in microvessel internal diameter. Myogenic tone of post-CPB microvessels was significantly increased after treatment with BKCa-blocker iberiotoxin, but unchanged in the combined presence of the blockers of intermediate (IKCa) and small conductance (SKCa) KCa channels, TRAM34/apamin. The increases in myogenic tone after iberiotoxin treatment were demonstrated as a decrease in microvessel internal diameter. No significant differences in BKCa protein levels were noted comparing pre- and post-CPB conditions judged by immunoblot and by immunofluorescence staining of skeletal muscle microvessels. Prominent staining for BKCa-α and BKCa-β1 subunits localized to the microvascularsmoothmuscle.
Conclusion
CPB-associated decrease in peripheral myogenic reactivity is likely due to activation of BKCa, but not IKCa or SKCa. CPB may increase BKCa activity without increasing BK polypeptide level.
Keywords: calcium-activated potassium channels, cardiopulmonary bypass, myogenic tone, vasomotor dysfunction, microcirculation, human, skeletal muscle
INTRODUCTION
Myogenic tone is an intrinsic property of vascular smooth muscle cells and crucial component of autoregulation of the coronary and peripheral microcirculations. Myogenic tone is manifested by an increase in wall tension and a decrease in vessel diameter in response to increases in vascular transmural pressure [1, 2]. Reduced myogenic tone has been found consistently in the coronary and peripheral microcirculations following cardiopulmonary bypass (CPB) in animal and humans [1–5]. Reduced myogenic tone in peripheral microvasculature may present clinically as post-CPB systemic hypotension, resulting in poor organ perfusion [5, 7]. Decreases in activation or phosphorylation of protein kinase C (PKC), mitogen-activated protein kinases (MAPK), and extracellular signal regulated kinases 1/2 (ERK1/2), have been implicated as mediators for the CPB-related vasomotor dysfunction [2, 6, 7].
More recently, we have found that in human coronary and peripheral microvasculature, calcium-activated potassium (KCa) channels may play an important role in CPB-related endothelium dysfunction [8, 9]. However, the role of KCa channel activation in CPB-related vasomotor dysfunction in human peripheral resistance arterioles and the regulatory properties of KCa channels in this vascular bed have been little investigated. This study was designed to examine the role of KCa channels in CPB-related changes in myogenic tone of human skeletal muscle microvessels, and to relate these responses to possible alterations in expression and localization of KCa polypeptides in human skeletal muscle tissue.
MATERIALS AND METHODS
Human Subjects and Tissue Harvesting
Samples of skeletal muscle from the left internal mammary artery bed were harvested before and after CPB. The pre-CPB specimen was taken after cannulation, and the post-CPB specimen was collected from a different location in the left internal mammary artery bed after removal of the aortic cross-clamp and weaning from CPB. Tissue samples for immunoblot analysis assay were immediately frozen in liquid nitrogen. Tissue for immunofluorescent staining was fixed in 10% formalin buffered solution for 24 h followed by paraffin mounting and sectioning into 5-µm slices. Tissue for microvascular studies was placed in cold (5 to 10 °C) Krebs buffer solution. All procedures were approved by the Institutional Review Board of Beth Israel Deaconess Medical Center, Harvard Medical School, and informed consent was obtained from all enrolled patients as required by the Institutional Review Board.
Microvessel Reactivity
Microvessel studies were performed by in vitro organ bath videomicroscopy. Myogenic tone of skeletal muscle arterioles was indirectly determined by measurement of the microvessel internal diameter at different intraluminal pressures of 10 to 100 mm Hg as described in detail previously [5–7]. At each pressure, the vessel was allowed to reach a steady diameter for 3 min and the steady-state diameter was measured. The internal diameter of each vessel was normalized to the microvessel diameter at a pressure of 40 mm Hg after application of papaverine. Microvessels were analyzed from pre-CPB (n = 8) and post-CPB (n = 8) skeletal muscle tissue. In several vessels, post-CPB microvessels were pretreated with the BKCa channel inhibitor, iberiotoxin (10−7 M) (n = 8), or with a mixture of the IKCa/SKCa inhibitors TRAM34/apamin (10−7 M/10−6 M), respectively, n = 8). At the end of each experiment, microvessels were washed with KHB-1% albumin buffer solution; then exposed to potassium chloride (75 mmol/L) was applied to verify retention of microvessel viability and responsiveness.
Immunoblot
Small arteries from six patients were dissected and cleaned of connective tissues, and solubilized in SDS-PAGE buffer. Total protein (40 µg) was fractionated on an 8–16% SDS-PAGE, then transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore Corp., Bedford, MA) as previously described [3]. Membranes were incubated for 1 h at room temperature with 1:200 dilutions of individual rabbit polyclonal primary antibodies to BKCa-α (Sigma-Aldrich, St. Louis, MO). The membranes were then incubated for 1 h with horseradish peroxidase-conjugated secondary anti-Ig washed 3 times in Tris saline buffer (TBS), and processed for chemiluminescent detection (Pierce, Rockford, IL) on X-ray film (Kodak, Rochester, NY). Band intensity was measured by densitometric analysis of autoradiograph films using NIH Image J 1.33.
Confocal Immunofluorescence Photomicroscopy
Skeletal tissue sections from five patients were deparaffinized in xylene, rehydrated in graded ethanol, and phosphate-buffered saline solution (PBS), and antigen-unmasked with sodium citrate (10 mmol/L, pH = 6.0), followed by PBS wash and blocking with 2% bovine serum albumin in PBS at room temperature for 2 h. After PBS wash, overnight incubation with anti-BKCa-α (Sigma-Aldrich) and BKCa-β1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, each at 1:200 dilution) were performed at 4 °C. Anti-mouse, α-smooth muscle actin (1:1000 dilution; Sigma-Aldrich) was used to detect microvascular smooth muscle. Sections were then washed in PBS, incubated with the appropriate Alexa Fluor-conjugated secondary antibody, then mounted in fluorescent mounting medium (Vector Labs, Burlingame, CA). Tissue was visualized using a Zeiss LSM510 confocal microscope system (Carl Zeiss MicroImaging, Inc., Thornwood, NY). Tissue labeling with secondary antibody alone or with normal rabbit IgG or serum in place of primary antibody served as negative controls.
Chemicals
Iberiotoxin, TRAM34, and apamin were obtained from Sigma- Aldrich. Stock solutions of iberiotoxin and TRAM34 were prepared in dimethylsulfoxide. Stock solutions of apamin were prepared in acetic acid. All stock solutions were stored at −20°C. All dilutions were prepared fresh daily.
Data Analysis
Data are presented as the mean and standard error of the mean (SEM). Repeated-measures ANOVA and Student’s t-test were used to compare variables among vessels. The treatment effects were statistically examined by paired or independent two-tailed Student’s t-test. Statistical significance was taken at P < 0.05.
RESULTS
Patient Characteristics
Tissue samples from 19 patients were studied. Nineteen patients (mean age 65 ± 6 y) underwent coronary artery bypass graft (CABG) with duration of cardioplegic arrest of 61 ± 5 min andCPBtime of 71.0 ± 14 min. Fourteen patients were male and five were female. Fifteen out of 19 patients carried a preoperative diagnosis of hypertension. All patients with preoperative hypertension were on medication (β-blocker, aspirin, calcium channel blocker, or angiotensin-converting enzyme inhibitor), and received perioperative β-blockade. Diabetes mellitus (type I or type II) was present in 6 of 19 patients.
Myogenic Tone
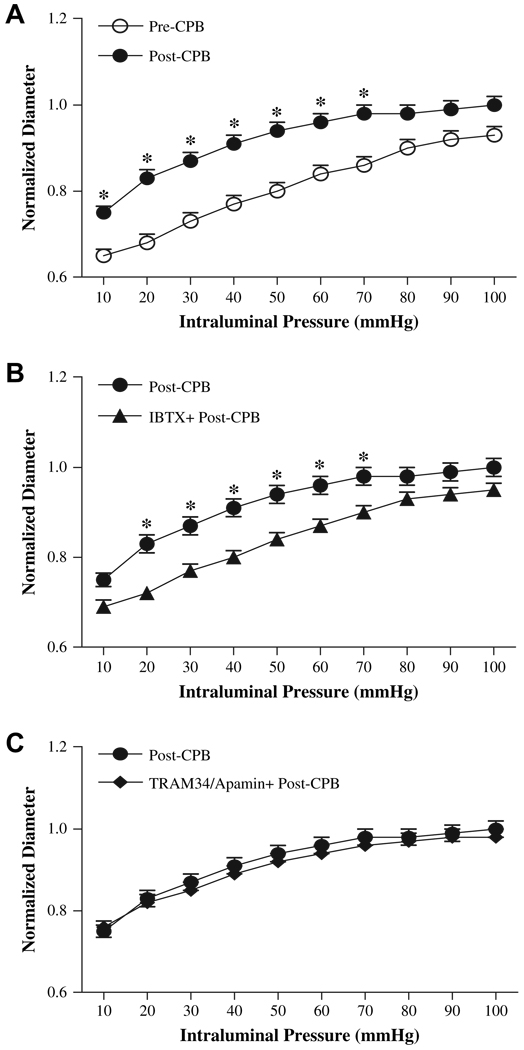
Skeletal muscle arteriole myogenic tone at transmural pressures of 10 to 100 mm Hg was demonstrated in pre-CPB microvessels. Myogenic tone of peripheral arterioles was significantly reduced post-CPB compared with pre-CPB (P < 0.05, post-C/CPB versus pre-CPB, n = 8). The decrease in myogenic tone post-CPB was indicated an increase in microvessel internal diameter and a significant upward shift of the pressure-diameter curve (Fig. 1A). Treatment of post-CPB microvessels with iberiotoxin (n = 8) to block BKCa channel resulted in increased myogenic tone (P < 0.05 versus post-CPB). The increased myogenic tone after iberiotoxin treatment was demonstrated as the decreases in microvessel internal diameter and significant downward shifts of the pressure-diameter curves (Fig. 1B). In contrast, combined treatment of post-CPB microvessels with TRAM34 and apamin (n = 8) to block IKCa/SKCa channels failed to affect the myogenic tone of skeletal muscle (Fig. 1C).
FIG. 1.
Effects of intraluminal pressure on normalized intraluminal diameter. (A) pre-CPB vessels versus post-CPB; (B) post-CP vessels treated with absence or presence of Iberiotoxin (IBTX), a selective BKCa blocker; (C) post-CP vessels treated with absence or presence of the IKCa blocker TRAM34 and the SKCa blocker apamin (n = 8/group). *P < 0.05 versus pre-CPB or post-CPB, respectively.
Effect of CPB on Levels of BKCa Polypeptides
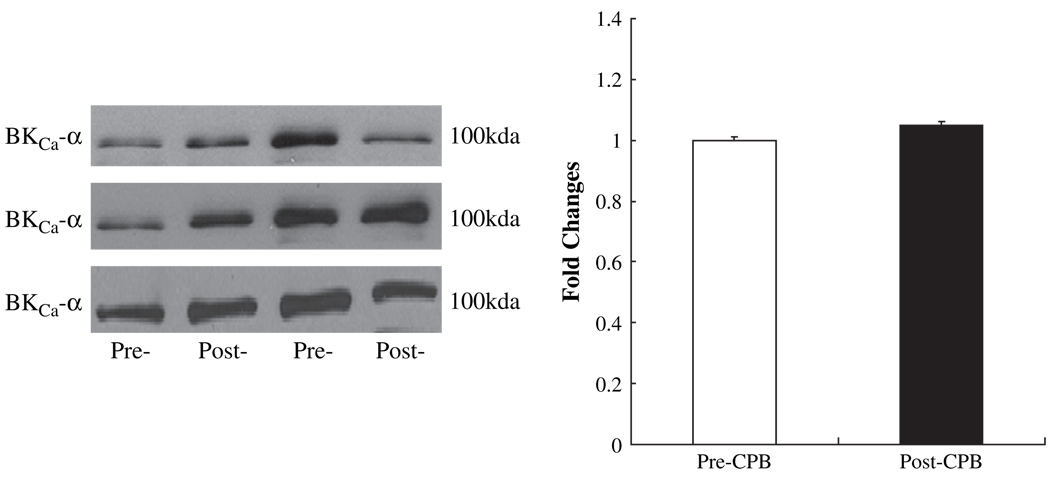
Pre-CPB skeletal muscle artery levels of the BKCa-α channel polypeptide were unchanged in the post-CPB period as detected by immunoblot (Fig. 2).
FIG. 2.
Left: Representative immunoblots of human skeletal muscle microvessels (40 µg loaded protein) developed with antibodies to BKCa-α; right: densitometric evaluation of immunoblot band intensity normalized to pre-CPB values shows unaltered levels of BKCa-α polypeptides after CPB (n = 6).
Effect of CPB on Microvessel Distribution of BKCa Polypeptides
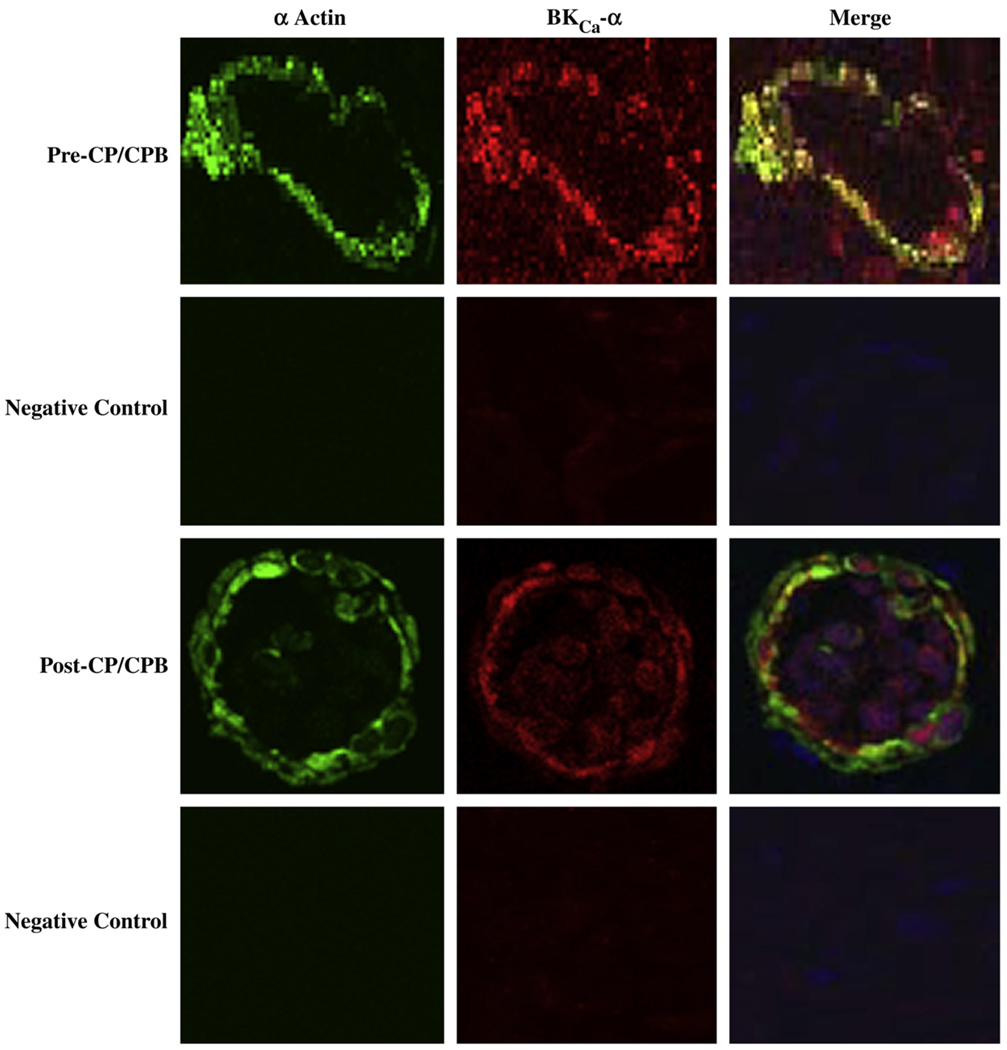
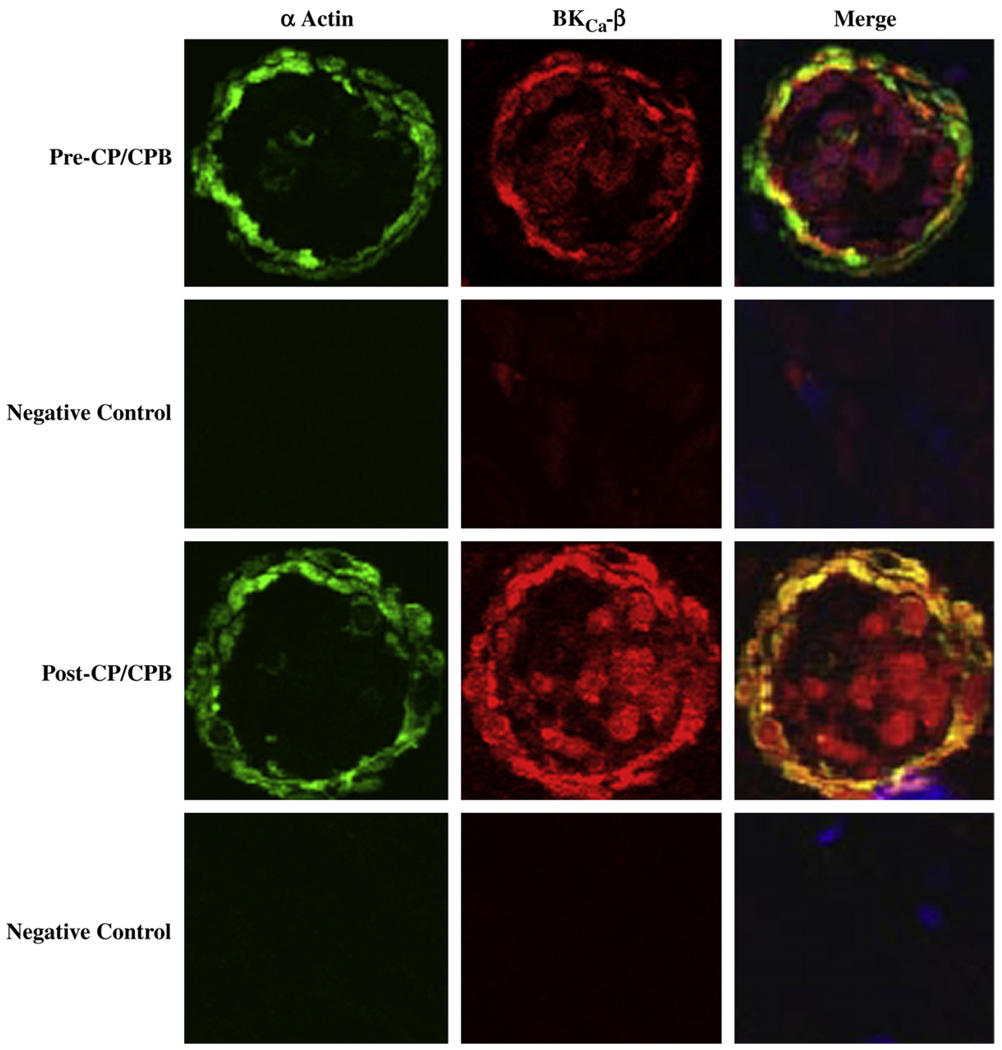
Immunofluorescent staining of skeletal microvessels displayed a strong signal for BKCa-α (Fig. 3) and BKCa-β1 (Fig. 4) subunits localized to the microvascular smooth muscle cells. Negative controls stained only with α-actin documented low level background fluorescence without BKCa-α (Fig. 3), or BKCa-β1 signals, respectively (Fig.4).
FIG. 3.
Immunolocalization of BKCa-α channel polypeptides inhumanskeletal microvessels. Vessels were co-stained for smooth muscle α-actin and BKCa-α. Matched negative controls are displayed below each row of primary antibody staining as indicated. Merged images are at right. (Color version of figure is available online.)
FIG. 4.
Immunolocalization of BKCa-β1 channel polypeptides in human skeletal microvessels. Vessels were co-stained for smooth muscle α-actin and BKCa-β1. Matched negative controls are displayed below each row of primary antibody staining as indicated. Merged images are at right. (Color version of figure is available online.)
DISCUSSION
The effect of CPB on myogenic tone in pigs and humans is believed to be a systemic phenomenon [2–7]. Systemic loss of myogenic tone may contribute to hypotension due to vasomotor dysfunction and decreased systemic vascular resistance. Hypotension secondary to impaired systemic myogenic tone may potentially reduce organ or tissue perfusion, including that of skeletal muscle [2–7]. This mechanism of vasomotor deficiency leading to hypotension is consistent with the findings of increased myogenic tone in skeletal muscle arterioles from spontaneously hypertensive rats [1]. In our porcine model of CPB, the reduction in myogenic tone was significantly less with blood cardioplegia compared with crystalloid cardioplegia [10]. The present study demonstrates significant reduction in myogenic tone of skeletal muscle arterioles after CPB.
The peripheral microvessels in this study lacked myogenic contraction, the intrinsic contractile response of vascular smooth muscle cells that decreases vessel diameter in response to increases in intraluminal pressure. The observation of myogenic tone without myogenic contraction may reflect the pre-operative chronic use of calcium channel blockers. CPB-induced activation of complement and neutrophils, release of pro-inflammatory cytokines and free radicals, and excessive adrenergic stimulation may each contribute to increased vascular permeability and smooth muscle myocyte swelling, resulting in peripheral microvascular vasomotor dysfunction [1, 2, 11].
The mechanism of myogenic regulation in the microvasculature is not completely understood. Studies of the arterioles denuded of endothelium provided evidence to suggest that myogenic tone is not dependent on the presence or integrity of vascular endothelium [12]. Several protein kinases, such as PKC, MAPK, and ERK1/2, have been implicated in myogenic tone regulation of the human coronary and peripheral microcirculations [2, 6, 7]. Voltage-gated calcium channels have also been demonstrated to mediate myogenic tone [2, 7]. The present study demonstrated that post-CPB peripheral microvascular myogenic tone is partially dependent on activation of BKCa channels. This finding may have significant implications regarding post-operative regulation of peripheral organ perfusion. BKCa channels may serve as a n end-effector of the protein kinase activation in vascular smooth muscles in response to CPB. Indeed, recent studies have demonstrated that phosphorylation/activation of several protein and tyrosine kinases may phosphorylate BKCa channels or their regulatory proteins, resulting in BKCa channel activation [13, 14].
Vascular KCa channels are of three physiological classes: BKCa, IKCa and SKCa [8, 9, 15, 16]. We recently found that the responses to the BKCa agonist NS1619 were unchanged or even slightly increased in the peripheral microvasculature after cardioplegia and CPB [8, 9]. In the present study, blockade of BKCa channels with iberiotoxin significantly increased the myogenic tone of post-CPB microvessels, suggesting BKCa may play a role in regulating CPB-related microvascular myogenic tone. In contrast, blockade of IKCa/SKCa with TRAM34/apamin had no affect on the reduced microvascular myogenic tone following CPB. More recently, we have found that IKCa and SKCa are expressed predominately in endothelial cells and less in smooth muscle cells, suggesting that inactivation of IKCa and/or SKCa may contribute to CP/CPB-related endothelial dysfunction [8, 9]. These previous findings are consistent with the present results, indicating little or no role for IKCa and SKCa in regulation of microvascular myogenic tone. The present study confirmed expression of BKCa polypeptides in human skeletal muscle microvessels, consistent with our previous findings in human atrial vessels [8]. Several studies have found that BKCa proteins can be upregulated in shock, and ischemia [17, 18]. However, CPB in the present study did not alter total BKCa channels polypeptide abundance in skeletal muscle vasculature, suggesting that brief hypothermic CPB modifies channel activity without change in channel polypeptide abundance.
In clinic, frequent use of high dose of norepinephrine or vasopressin to increase post-CPB blood pressure causes several side effects and complications, such as tissue edema, tissue damage and poor organ perfusion. The present study provides a potential, new therapeutic strategy for maintaining post-CPB myogenic tone. Future development of BKCa channel blockers of greater specificity and lower toxicity may prove useful to counteract post-CPB systemic hypotension.
In conclusion, this study has demonstrated that BKCa channels are expressed in human skeletal muscle microvasculature. Activation of BKCa channels may contribute to CPB-related microvascular vasomotor dysfunction of human peripheral tissue. Continued investigation of the molecular mechanisms of vasomotor dysfunction may further development of methods to decrease the morbidity associated with cardiac surgery.
ACKNOWLEDGMENTS
This research project was supported in part by the National Heart, Lung, and Blood Institute grants HL-69024-05, HL085647, and HL-46716-15 (FWS) and HL-077765 (SLA). YL, NRS, and RMO were supported by a cardiovascular research training grant (T32) from the National Institute of Health (HL076130-02).
Footnotes
Presented at the Fourth Annual Academic Surgical Congress, February 3–6, 2009, Fort Myers, Florida.
REFERENCES
- 1.Sellke FW, Boyle EM, Jr, Verrier ED. Endothelial cell injury in cardiovascular surgery: The pathophysiology of vasomotor dysfunction. Ann Thorac Surg. 1996;62:1222. doi: 10.1016/0003-4975(96)00538-3. [DOI] [PubMed] [Google Scholar]
- 2.Ruel M, Khan TA, Voisine P, et al. Vasomotor dysfunction after cardiac surgery. Eur J Cardiothorac Surg. 2004;26:1002. doi: 10.1016/j.ejcts.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 3.Stamler A, Wang SY, Aguirre DE, et al. Cardiopulmonary bypass alters vasomotor regulation of the skeletal muscle microcirculation. Ann Thorac Surg. 1997;64:460. doi: 10.1016/S0003-4975(97)00539-0. [DOI] [PubMed] [Google Scholar]
- 4.Wang SY, Stamler A, Li J, et al. Decreased myogenic reactivity in skeletal muscle arterioles after hypothermic cardiopulmonary bypass. J Surg Res. 1997;69:40. doi: 10.1006/jsre.1997.5020. [DOI] [PubMed] [Google Scholar]
- 5.Wang SY, Friedman M, Franklin A, et al. Myogenic reactivity of coronary resistance arteries after cardiopulmonary bypass and hyperkalemic cardioplegia. Circulation. 1995;92:1590. doi: 10.1161/01.cir.92.6.1590. [DOI] [PubMed] [Google Scholar]
- 6.Khan TA, Bianchi C, Araujo EG, et al. Cardiopulmonary bypass reduces peripheral microvascular contractile function by inhibition of mitogen-activated protein kinase activity. Surgery. 2003;134:247. doi: 10.1067/msy.2003.229. [DOI] [PubMed] [Google Scholar]
- 7.Khan TA, Bianchi F, Ruel M, et al. Mitogen-activated protein kinase inhibition and cardioplegia-cardiopulmonary bypass reduce coronary myogenic tone. Circulation. 2003;108(Suppl 1):II348. doi: 10.1161/01.cir.0000087652.93751.0e. [DOI] [PubMed] [Google Scholar]
- 8.Feng J, Liu YH, Clements RT, et al. Calcium-activated potassium channels contribute to human coronary microvascular dysfunction after cardioplegia arrest. Circulation Surg Suppl. 2008;118(Suppl I):S46. doi: 10.1161/CIRCULATIONAHA.107.755827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu YH, Sellke EW, Feng J, et al. Calcium-activated potassium channels contribute to human skeletal muscle microvascular endothelial dysfunction related to cardiopulmonary bypass. Surgery. 2008;144:239. doi: 10.1016/j.surg.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang SY, Stamler A, Tofukuji M, et al. Effects of blood and crystalloid cardioplegia on adrenergic and myogenic vascular mechanisms. Ann Thorac Surg. 1997;63:41. doi: 10.1016/s0003-4975(96)00644-3. [DOI] [PubMed] [Google Scholar]
- 11.Edmunds LH., Jr Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 1998;66:S12. doi: 10.1016/s0003-4975(98)00967-9. [DOI] [PubMed] [Google Scholar]
- 12.Kuo L, Chilian WM, Davis MJ. Coronary arteriolar myogenic response is independent of endothelium. Circ Res. 1990;66:860. doi: 10.1161/01.res.66.3.860. [DOI] [PubMed] [Google Scholar]
- 13.Schubert R, Nelson MT. Protein kinases: Tuners of the BKCa channel in smooth muscle. Trends in Pharmacol Sci. 2001;22:505. doi: 10.1016/s0165-6147(00)01775-2. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Terata K, Chai Q, et al. Peroxynitrite inhibits Ca2+-activated K+ channel activity in smooth muscle of human coronary arterioles. Circ Res. 2002;9:1070. doi: 10.1161/01.res.0000046003.14031.98. [DOI] [PubMed] [Google Scholar]
- 15.Ledoux J, Werner ME, Brayden JE, et al. Calcium-activated potassium channels and the regulation of vascular tone. Physiology. 2006;21:69. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- 16.Wei AD, Gutman GA, Aldrich R, et al. International Union of Pharmacology. LII. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol Rev. 2005;5:463. doi: 10.1124/pr.57.4.9. [DOI] [PubMed] [Google Scholar]
- 17.Zhao GL, Zhao Y, Pan BX, et al. Hypersensitivity of BKCa to Ca2+ sparks underlies hyporeactivity of arterial smooth muscle in shock. Circ Res. 2007;101:493. doi: 10.1161/CIRCRESAHA.107.157271. [DOI] [PubMed] [Google Scholar]
- 18.Shintani Y, Node K, Asanuma H, et al. Opening of Ca2+-activated K+ channels is involved in ischemic preconditioning in canine hearts. J Mol Cell Cardiol. 2004;37:1213. doi: 10.1016/j.yjmcc.2004.09.012. [DOI] [PubMed] [Google Scholar]