Abstract
Purpose
KX2-391 is an oral non–ATP-competitive inhibitor of Src kinase and tubulin polymerization. In phase 1 trials, prostate-specific antigen (PSA) declines were seen in patients with advanced prostate cancer. We conducted a single-arm phase 2 study evaluating KX2-391 in men with chemotherapy-naïve bone-metastatic castration-resistant prostate cancer (CRPC).
Methods
We treated 31 patients with oral KX2-391 (40mg twice-daily) until disease progression or unacceptable toxicity. The primary endpoint was 24-week progression-free survival (PFS); a 50% success rate was predefined as clinically significant. Secondary endpoints included PSA progression-free survival (PPFS) and PSA response rates. Exploratory outcomes included pharmacokinetic studies, circulating tumor cell (CTC) enumeration, and analysis of markers of bone resorption (urinary N-telopeptide [uNTx]; C-telopeptide [CTx]) and formation (bone alkaline phosphatase [BAP]; osteocalcin).
Results
The trial closed early after accrual of 31 patients, due to a prespecified futility rule. PFS at 24 weeks was 8%, and median PFS was 18.6 weeks. The PSA response rate (≥30% decline) was 10%, and median PPFS was 5.0 weeks. Additionally, 18% of men with unfavorable (≥5) CTCs at baseline converted to favorable (<5) CTCs with treatment. The proportion of men with declines in bone turnover markers was 32% for uNTx, 21% for CTx, 10% for BAP, and 25% for osteocalcin. In pharmacokinetic studies, median Cmax was 61 (range 16–129) ng/mL, and median AUC was 156 (35–348) ng*hr/mL. Common toxicities included hepatic derangements, myelosuppression, fatigue, nausea and constipation.
Conclusion
KX2-391 dosed at 40mg twice-daily lacks antitumor activity in men with CRPC, but has modest effects on bone turnover markers. Because a Cmax of ≥142 ng/mL is required for tubulin polymerization inhibition (defined from preclinical studies), higher once-daily dosing will be used in future trials.
Keywords: KX2-391, prostate cancer, Src inhibitor, tubulin polymerization
INTRODUCTION
Prostate cancer is the most prevalent non-cutaneous malignancy in men worldwide, and accounts for the second largest number of cancer deaths [1]. Although androgen suppression is an effective initial therapy for men with advanced prostate cancer, most patients will eventually progress to a state of castration-resistant prostate cancer (CRPC), which is invariably fatal. In the past few years, several new agents have been introduced for the treatment of advanced prostate cancer [2]. However, because none of these therapies are curative, and survival for men with metastatic CRPC remains short, novel biological targets continue to be explored [3] in order to expand treatment options for these patients.
One such therapeutic target is Src, a family of intracellular protein tyrosine kinases that transduce signals from a range of upstream proteins, including receptors for epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and vascular-endothelial growth factor (VEGF) [4]. Preclinical studies have suggested a role for Src-family kinases in prostate cancer oncogenesis [5], and Src is active or overexpressed during prostate tumor growth and metastasis [6]. In addition, Src signaling is required during osteoclast maturation and activation, and plays a role in the formation and maintenance of prostate cancer bone metastases [7]. In a study evaluating metastatic tumor biopsies from patients with CRPC, Src was overexpressed in 28% of tumor samples and was associated with shorter overall survival [8]. The suggestion that Src may serve as a valid therapeutic target has been strengthened by several clinical trials investigating the oral Src inhibitor, dasatinib, which has demonstrated encouraging antitumor activity as well as favorable effects on markers of bone resorption and formation [9,10].
KX2-391 (Kinex Pharmaceuticals, Buffalo, NY) is an orally-bioavailable highly-selective small molecule Src-family kinase inhibitor [11] that uniquely targets the peptide substrate-binding domain and not the ATP-binding site like all other Src inhibitors. In addition, this agent inhibits tubulin polymerization (via a novel α,β-tubulin heterodimer binding site), and therefore possesses a dual mechanism of action. In preclinical studies, KX2-391 demonstrated potent antineoplastic activity across a wide spectrum of tumor cell lines (including several prostate cancer cell lines) and inhibited primary tumor growth as well as metastasis in an orthotopic prostate cancer mouse model [12,13]. A phase 1 trial of KX2-391 in advanced solid tumor patients defined the maximal tolerated dose as 40 mg orally twice-daily [14]. In that trial, twice-daily dosing was selected due to the short half-life of the drug (4 hours), and once-daily dosing was not evaluated. Dose-limiting toxicities included elevated liver transaminases, neutropenia and fatigue, all of which improved within 7 days of drug discontinuation. Encouraging clinical activity was seen in several patients, including two men with metastatic CRPC who demonstrated declines in prostate-specific antigen (PSA) levels.
Following from these preclinical and clinical findings, we designed a single-arm phase 2 study to evaluate the efficacy and safety of KX2-391 (at an oral dose of 40 mg twice-daily) in patients with metastatic CRPC who had not received prior chemotherapy. Because Src is overexpressed in prostate cancer bone metastases and in osteoclasts, we decided to focus our study on patients with documented bone metastases, and to also examine the effect of KX2-391 on bone metabolism.
METHODS
Patients
Our target population was men with bone-metastatic CRPC who had not received chemotherapy. Patients were required to have histologically-confirmed prostate adenocarcinoma, progressive disease despite “castrate levels” of serum testosterone (<50 ng/dL), and radiographically-visible bone metastases on technetium-99 bone scan (additional soft tissue metastases visualized on CT scan were permitted but not required). Patients had to have ≥3 rising serum PSA values taken two weeks apart with the last value being ≥2.0 ng/mL, in accordance with Prostate Cancer Working Group (PCWG2) guidelines [15]. Other eligibility criteria included age >18, Eastern Cooperative Oncology Group (ECOG) score ≤2, life expectancy >6 months, and adequate kidney/liver/bone marrow function.
Patients were excluded if they had received an oral antiandrogen or a 5α-reductase inhibitor in the last 6 weeks, if they had ever received chemotherapy for metastatic CRPC, if they were receiving other concurrent endocrine therapy, if they had received investigational drugs in the last 4 weeks, if they had received extensive (≥50% of bone marrow) radiation therapy or bone-targeting radiopharmaceuticals in the last 4 weeks, if they had a malabsorption syndrome, if they took drugs metabolized by CYP3A4, if they had a prior malignancy (except non-melanoma skin cancers and superficial bladder cancers) within 3 years, if they had a history of pathologic bone fracture, if they had undergone major surgery in the last 4 weeks, if they had persistent ≥grade 2 treatment-related toxicities from prior anticancer therapy, if they had major infectious/pulmonary/gastrointestinal/metabolic illnesses, if they had symptomatic congestive heart failure or other major cardiovascular disease, or if they had a corrected-QT interval >450 milliseconds on electrocardiography. Prior ketoconazole treatment was allowed as long as it had been discontinued 4 weeks prior to enrolment. Use of bisphosphonates was permitted if the patient was on a stable dose for at least 4 weeks.
The institutional review boards at all centers approved the study, which was conducted according to good clinical practice guidelines. All patients provided written informed consent.
Study design
This was a single-arm open-label phase 2 study conducted at five institutions of the Prostate Cancer Clinical Trials Consortium [16]. Patients were treated on a continuous basis with oral KX2-391 at a dose of 40 mg twice daily. KX2-391 (Kinex Pharmaceuticals, Buffalo, NY) was supplied as an oral solution dispensed in single-use unit dose bottles containing 40 mg of the investigational product. Patients that were unable to tolerate 40 mg of the study drug twice daily could have their dose reduced to 40 mg once daily. Patients were required to fast for 2 hours before and 1 hour after taking the study drug. Treatment continued either until unmanageable drug-related toxicity, or until clinical or radiographic progression. Importantly, treatment was not discontinued for PSA elevations alone [15].
Assessments
Clinical evaluations included physical examination, vital sign measurements, assessment of ECOG performance status, review of concomitant medications, laboratory evaluations (chemical and hematologic studies), and review of adverse events; and were performed every 4 weeks. Efficacy assessments included serum PSA measurements every 4 weeks, as well as CT (chest/abdomen/pelvis) and whole-body technetium-99 bone scan evaluations every 12 weeks.
Outcome measures
The primary endpoint was freedom from progression (progression-free survival; PFS) at 24 weeks after randomization. Progression was defined [15] as clinical progression (worsening disease-related symptoms or new cancer-related complications), or radiographic progression (on CT scan: ≥20% enlargement in the sum short-axis diameter of soft-tissue target lesions [RECIST 1.1 criteria [17]]; on bone scan: ≥2 new bone lesions that do not represent tumor flare), or death, whichever occurred first. To define radiographic progression, a confirmatory scan (≥6 weeks later for CT scan; ≥12 weeks later for bone scan) was required, consistent with PCWG2 criteria. A key secondary endpoint was freedom from PSA progression (PSA progression-free survival; PPFS) at 24 weeks. PSA progression was defined as a ≥25% increase in PSA from nadir (and by ≥2 ng/mL), requiring confirmation ≥4 weeks later (PCWG2 criteria) [15].
Other secondary endpoints included median PFS, median PPFS, PSA response rate (≥30% PSA decline from baseline), best PSA response (maximal percentage PSA decrease from baseline), and objective response rate in measurable soft-tissue lesions (partial response: ≥30% decrease in the sum short-axis diameter of target lesions; progressive disease: ≥20% increase in the sum diameter of target lesions or ≥1 new lesion; stable disease: changes in the sum diameter of target lesions that do not meet the above parameters; RECIST criteria [17]). A final secondary endpoint was safety; adverse events were graded using the National Cancer Institute Common Toxicity Criteria for Adverse Events, v4.0.
Circulating tumor cell (CTC) analysis
Blood samples (7.5 mL) for CTC enumeration were collected at baseline and after 4, 12 and 24 weeks on study (although some patients had fewer samples collected, if they came off study earlier). CTCs were analyzed using the CellSearch system (Veridex, Raritan, NJ) as previously described [18]. Results were expressed as numbers of CTCs per 7.5mL of blood.
Pharmacokinetics
Pharmacokinetic (PK) analysis involved measurement of plasma concentrations of KX2-391 using a validated liquid-chromatography/tandem mass-spectrometry assay (XenoBiotic Laboratories, Plainsboro, NJ), as previously described [14]. PKs were performed in order to evaluate whether plasma drug parameters might be influenced by the castrated state, as previously reported with other agents [19]. PK sampling was performed at baseline (30 minutes pre-dose) and at 1, 2, and 4 hours after initial study drug administration. Subsequently, PK samples were collected 1 hour post-dose at 4, 12, and 24 weeks after study entry. PK parameters of interest included minimum and maximum plasma concentrations (Cmin, Cmax) of KX2-391, time to maximum plasma concentration (Tmax), area under the concentration–time curve (AUC), systemic clearance (CL), volume of distribution at steady state (Vss), and plasma half-life (t1/2).
Bone turnover markers
Blood and urine samples for evaluation of bone metabolism were collected at baseline and after 4, 12 and 24 weeks on study. To assess markers of bone resorption, urinary N-telopeptide (uNTx) and serum C-telopeptide (CTx) levels were measured at each time point. To evaluate markers of bone formation, serum bone-specific alkaline phosphatase (BAP) and serum osteocalcin levels were measured. These bone turnover markers were analyzed and quantified as previously described [20]. Results are reported as best percentage change in each biomarker from baseline levels.
Statistical analysis
Based on prior studies [21], we estimated that no more than 30% of patients with metastatic chemotherapy-naïve CRPC would be free of disease progression (as defined above) after 24 weeks. We hypothesize that KX2-391 would prevent progression at 24 weeks in approximately 50% of such men. Therefore, a 50% 24-week PFS rate in KX2-391–treated patients was predefined as constituting a success. Because KX2-391 has not previously been studied in men with prostate cancer, patients were assessed for treatment futility by incorporating a Simon minimax two-stage design [22]. Using this design, 42 total patients granted 91% power to detect an improvement in the primary endpoint from 30% (historic controls) to 50% (active treatment), with a type-I error (α) of 10%. The trial would be closed prematurely if ≤5 of the first 20 enrolled and evaluable patients failed to achieve the primary endpoint. If the trial accrued to completion, the study would be deemed successful if ≥17 patients (out of 42) achieved the primary endpoint. As these decision rules were contingent upon enrolling certain numbers of patients (both at the interim and at the final analysis), additional patients were enrolled as a precaution against having too few evaluable patients at each stage. In order that the expected sample size be maintained for the interim and final analyses, the protocol prespecified that 31 patients should be enrolled prior to halting for the interim analysis, and that 65 patients should be enrolled in total.
Efficacy endpoints were analyzed using descriptive statistics and 95% confidence intervals. The Kaplan-Meier product-limit method was used to estimate time-to-event endpoints (e.g. median PFS, median PPFS) and their 95% confidence intervals. Waterfall plots were used to depict changes in PSA levels and bone biomarker levels from baseline in each patient. Pharmacokinetic parameters were analyzed using non-compartmental models; descriptive statistics were generated for Cmin, Cmax, AUC, and t1/2. CTC enumeration results were reported as the percentage of patients who converted from pre-treatment unfavorable counts (≥5 CTCs/7.5mL blood) to post-treatment favorable counts (<5 CTCs/7.5mL).
RESULTS
Patients
A total of 31 patients were enrolled between March 2010 and April 2011. The study was halted early for futility at the time of the interim analysis, because the minimum number of responses was not achieved. Median treatment duration was 17.6 weeks. Baseline patient characteristics are shown in Table 1. Mean age was 68.9 years, and 25.8% of participants were non-white. The majority of patients had pathological Gleason scores of ≥7; the mean number of prior hormonal therapies was 4.0; and the mean number of distant metastases was 5.4 (about two-thirds of patients had soft-tissue metastases in addition to bone metastases).
Table 1.
Baseline demographic and clinical characteristics.
| Baseline Characteristic | N | % |
|---|---|---|
| Age, years | ||
| mean (range) | 68.9 (50–90) | |
| Race | ||
| white | 23/31 | 74.2 |
| non-white | 8/31 | 25.8 |
| Gleason score | ||
| ≤6 | 4/31 | 12.9 |
| 7 | 8/31 | 25.8 |
| 8–10 | 19/31 | 61.3 |
| Baseline PSA, ng/mL | ||
| median (range) | 35.0 (7.3–997.8) | |
| ECOG performance status | ||
| 0 | 16/31 | 51.6 |
| 1 or 2 | 15/31 | 48.4 |
| Metastatic sites | ||
| bone only | 11/31 | 35.5 |
| bone and soft tissue | 20/31 | 64.5 |
| Number of metastases | ||
| mean (range) | 5.4 (1–18) | |
| Number of prior hormonal therapies | ||
| mean (range) | 4.0 (2–6) | |
| Prior bisphosphonates | ||
| yes | 11/31 | 35.5 |
| no | 20/31 | 64.5 |
| Baseline hemoglobin, g/dL | ||
| median (range) | 13.0 (9.6–15.1) | |
| Baseline albumin, g/dL | ||
| median (range) | 4.0 (3.2–4.7) | |
| Baseline alkaline phosphatase, U/L | ||
| median (range) | 90 (54–594) |
Primary endpoint
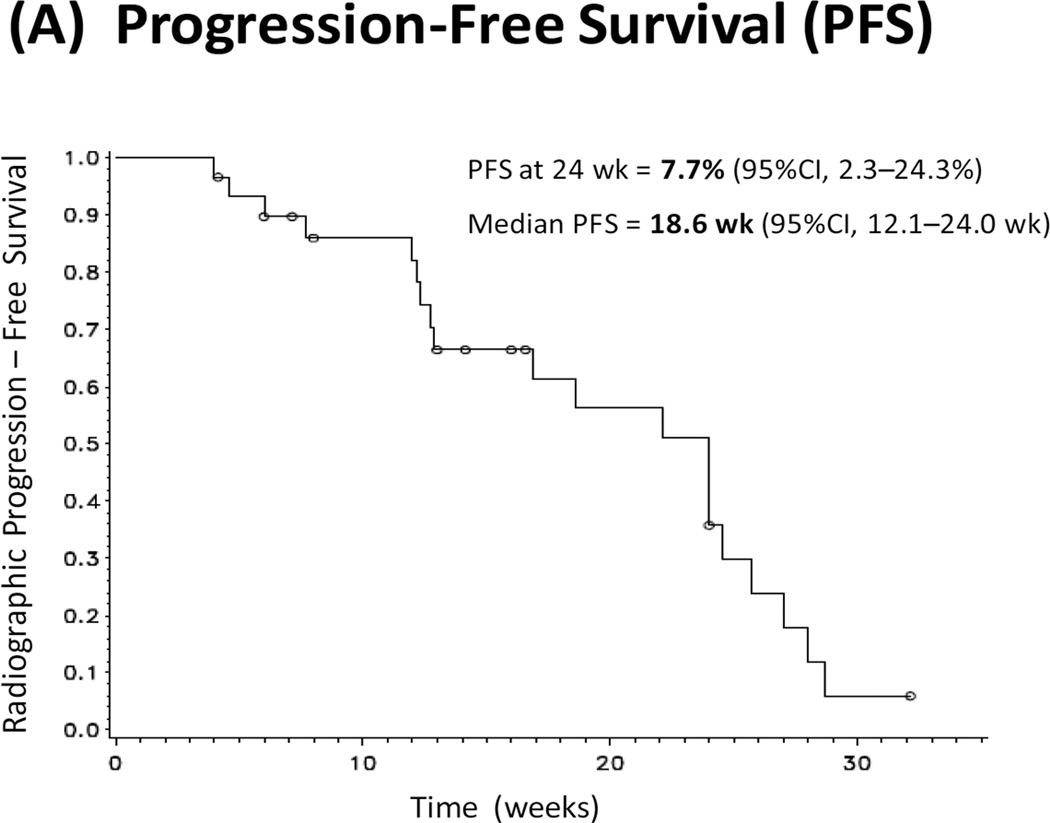
Of the 31 enrolled patients, 26 were evaluable for the primary endpoint (5 men came off study before 24 weeks due to toxicity). The PFS rate at 24 weeks was 7.7% (95%CI, 2.3–24.3%) (Fig. 1A), failing to achieve the desired 24-week PFS rate of 50% as initially hypothesized. Median PFS was 18.6 weeks (95%CI, 12.1–24.0 weeks) (Fig. 1A).
Figure 1. Clinical Outcomes.
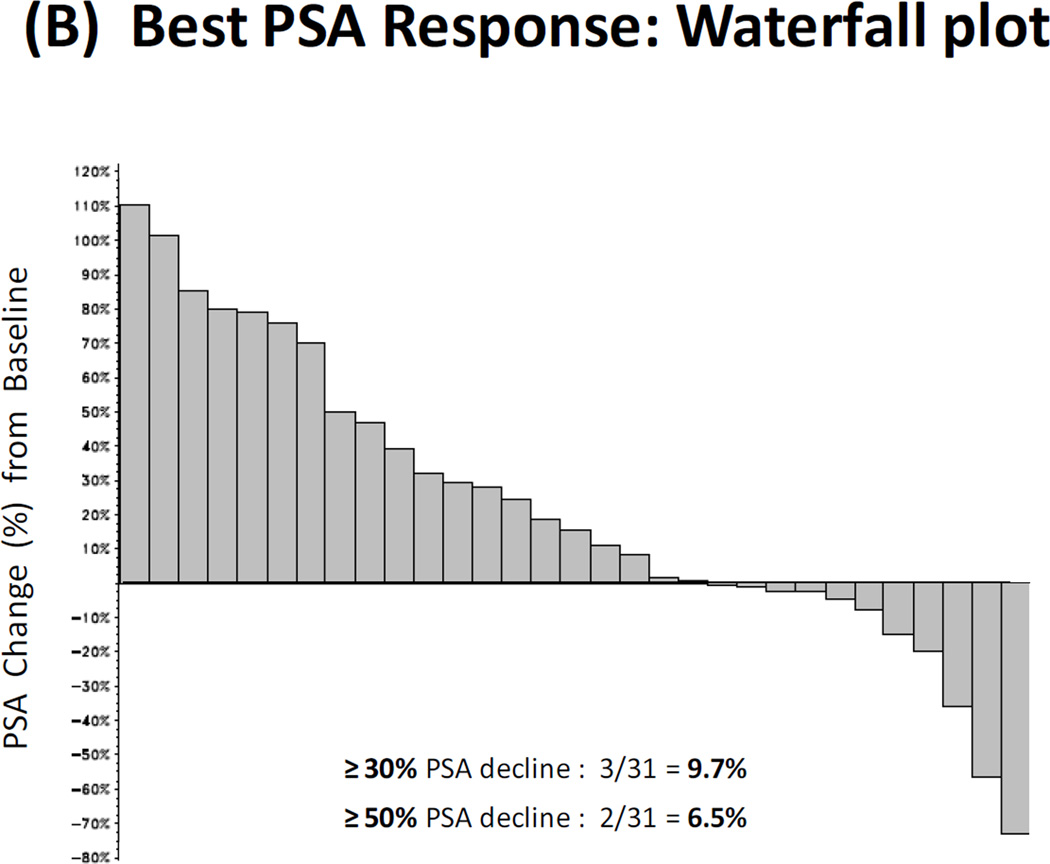
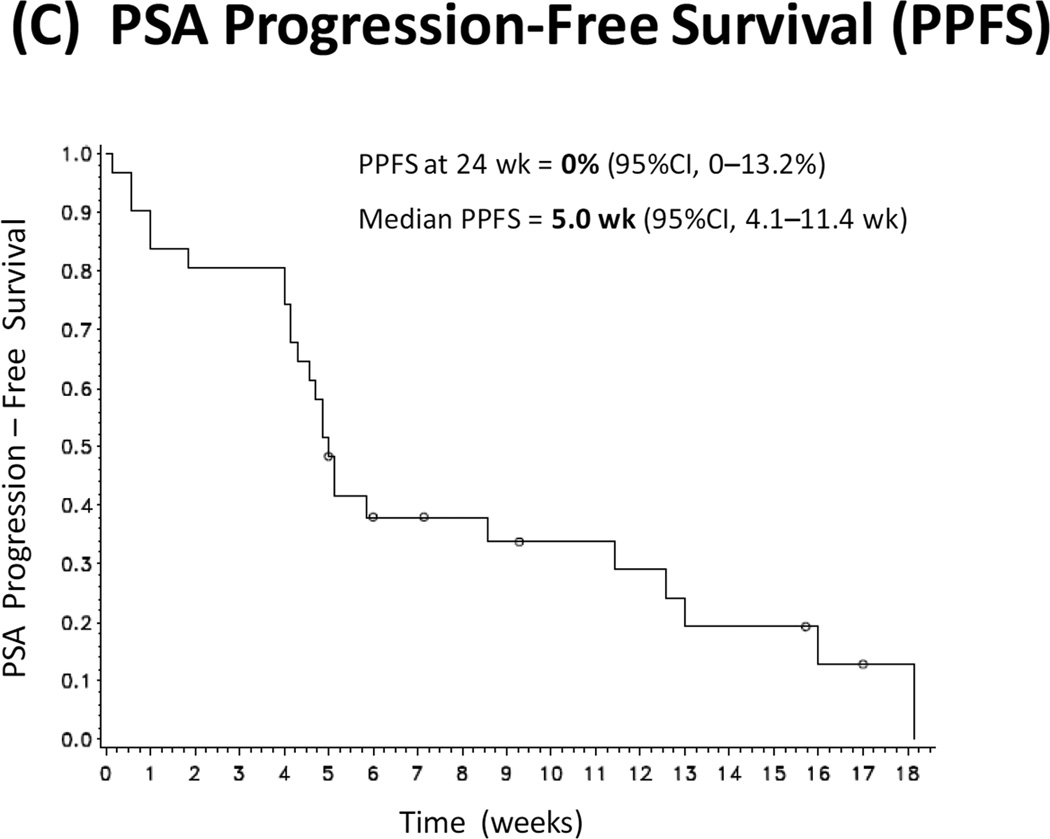
(A) Kaplan-Meier curve depicting radiographic progression-free survival (PFS). Progression is defined according to the PCWG2 criteria. (B) Waterfall plot showing best PSA response. Each bar represents the percentage change in PSA from baseline in an individual patient. (C) Kaplan-Meier curve depicting PSA progression-free survival (PPFS). PSA progression is defined as a confirmed ≥25% increase in PSA from nadir.
Secondary endpoints
The PSA response rate (≥30% PSA decline) was 9.7% (95%CI, 3.5–25.0%), while a larger number of patients showed lesser degrees of PSA reductions (Fig. 1B). The 24-week PPFS rate was 0% (95%CI, 0–13.2%), meaning that all patients experienced PSA progression before 24 weeks. Median PPFS was 5.0 weeks (95%CI, 4.1–11.4) (Fig. 1C). Among those with measurable disease at baseline (n=20), no patient achieved an objective tumor response (data not shown).
Safety
Adverse events were generally mild to moderate; those occurring in ≥5% of patients are summarized in Table 2. Grade 3–4 toxicities included neutropenia (9.7%), thrombocytopenia(6.5%), leucopenia (6.5%), anemia (6.5%), and liver function test abnormalities (6.5%) including elevations in bilirubin and transaminases. All grade 3–4 adverse events improved to grade 1–2 within 2 weeks of study drug discontinuation or dose reduction (3/31 patients required dose modifications to 40 mg once daily). Additional low-grade toxicities included fatigue (51.6%), nausea (38.7%), anorexia (25.8%), constipation (19.4%) and diarrhea (16.1%). The percentage of patients who came off study due to adverse events was 16.1%; all of these patients came off study prior to the 24 week evaluation.
Table 2.
Adverse events, regardless of their relationship to the study drug.
| Adverse Event | All Grades | % | Grade 3–4 | % |
|---|---|---|---|---|
| Fatigue | 16 | 51.6% | ||
| LFT elevations | 15 | 48.4% | 2 | 6.5% |
| Nausea | 12 | 38.7% | ||
| Anorexia | 8 | 25.8% | ||
| Constipation | 6 | 19.4% | ||
| Diarrhea | 5 | 16.1% | ||
| Alopecia | 4 | 12.9% | ||
| Dehydration | 4 | 12.9% | 2 | 6.5% |
| Leukopenia | 3 | 9.7% | 2 | 6.5% |
| Neutropenia | 3 | 9.7% | 3 | 9.7% |
| Thrombocytopenia | 3 | 9.7% | 2 | 6.5% |
| Creatinine elevation | 3 | 9.7% | ||
| Headache | 3 | 9.7% | ||
| Bone pain | 3 | 9.7% | 2 | 6.5% |
| Anemia | 2 | 6.5% | 2 | 6.5% |
CTC enumeration
Thirty patients had paired baseline and post-treatment blood samples collected for CTC enumeration studies (Table 3). Nineteen men had favorable baseline CTC counts (<5 CTCs/7.5mL blood); 94.7% of them (n=18) retained favorable CTC counts for at least 12 weeks. Eleven men had unfavorable baseline CTC counts (≥5 CTCs/7.5mL); 18.2% of them (n=2) converted to favorable CTC counts post-treatment. Neither of these 2 patients were receiving concurrent bisphosphonates while on study. One patient had a post-treatment reduction in uNTx levels, while the other patient had a post-treatment reduction in serum osteocalcin levels. Neither patient achieved a PSA response.
Table 3.
Circulating tumor cell (CTC) changes with treatment.
| Baseline CTCs <5 (per 7.5 mL) | N = 19 | |
|---|---|---|
| Post-treatment CTCs <5 | 18/19 | 94.7% |
| Post-treatment CTCs ≥5 | 1/19 | 5.3% |
| Baseline CTCs ≥5 (per 7.5 mL) | N = 11 | |
| Post-treatment CTCs <5 | 2/11 | 18.2% |
| Post-treatment CTCs ≥5 | 9/11 | 81.8% |
Bone metabolism markers
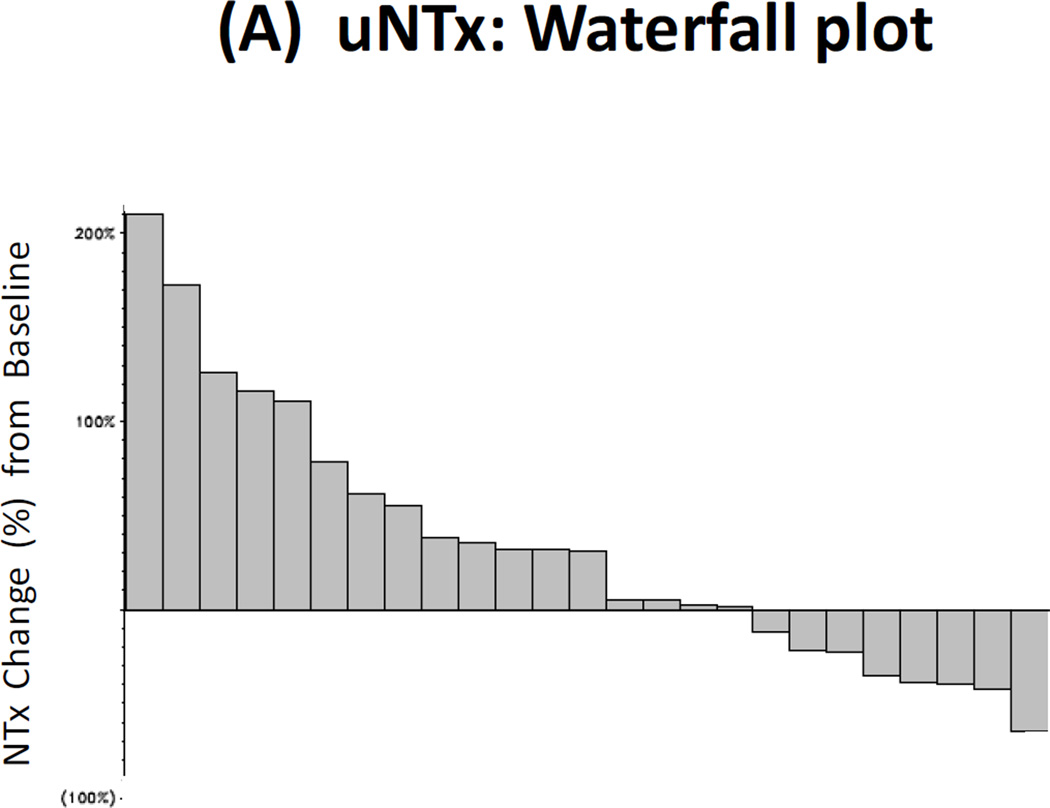
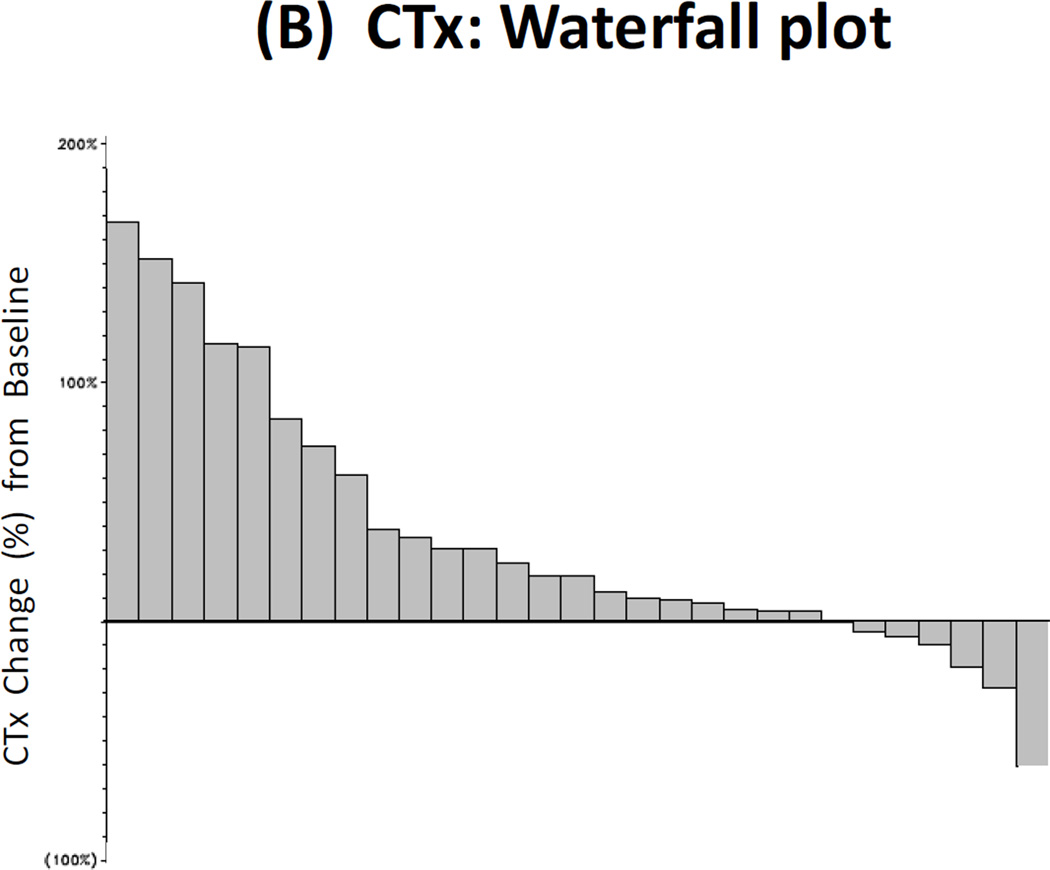
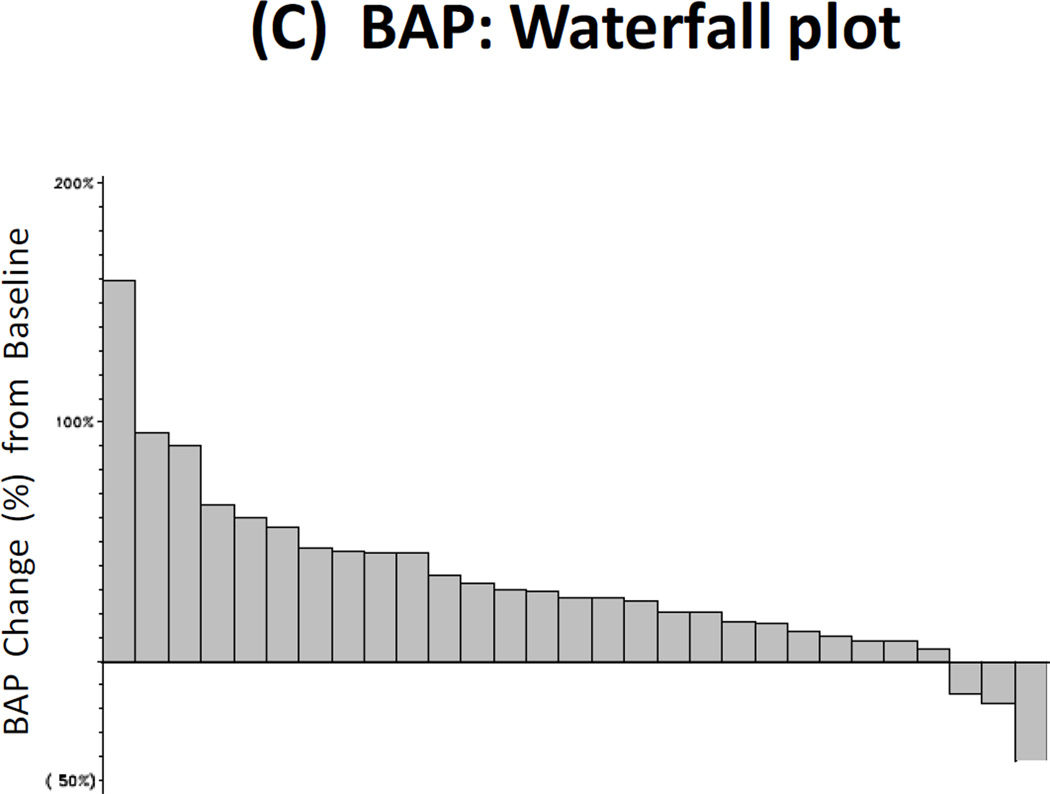
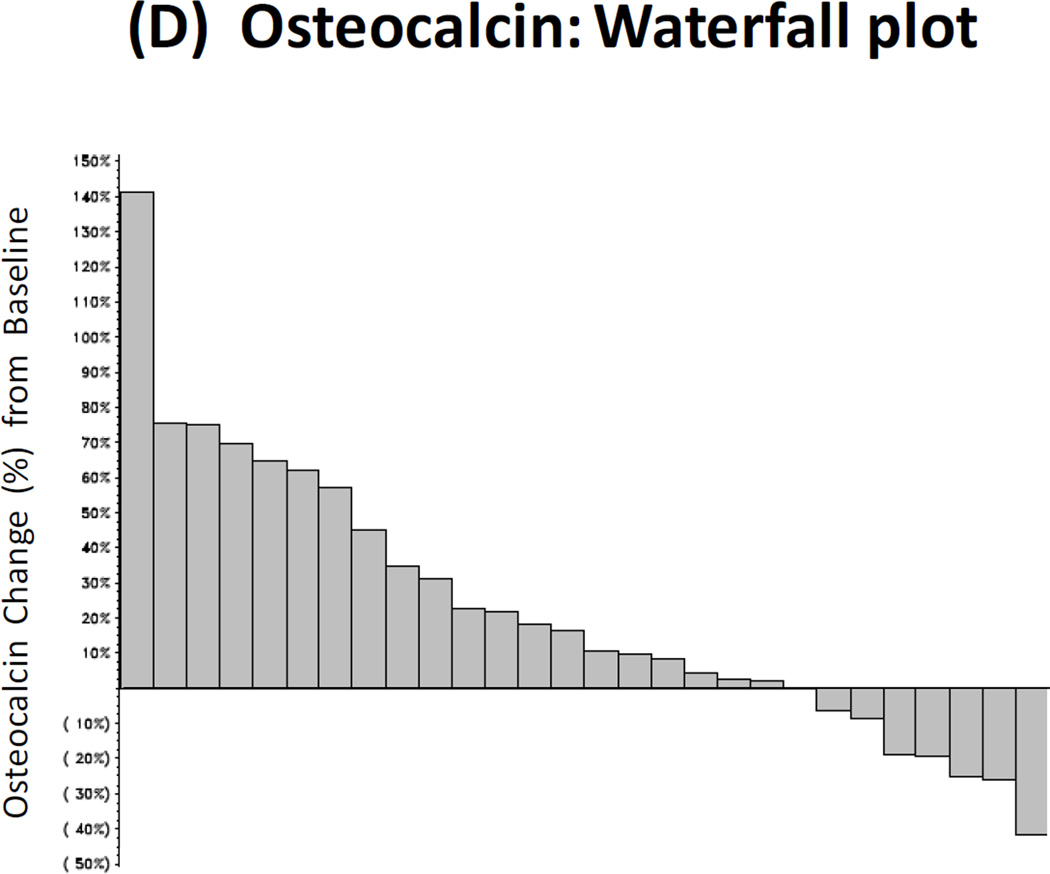
Twenty-nine patients had paired baseline and post-treatment serum samples collected for bone marker analysis, and 25 men had paired urine samples collected for this purpose. Reductions in uNTx were observed in 32% of patients (≥40% declines were seen in 16%), reductions in CTx were observed in 21% of patients (≥40% declines in 4%), reductions in BAP were observed in 10% of patients (≥40% declines in 4%), and reductions in osteocalcin were observed in 25% of patients (≥40% declines in 4%). These results are summarized in Fig. 2A–2D.
Figure 2. Bone Biomarker Outcomes.
(A) Waterfall plot showing best treatment response in urinary N-telopeptide (uNTx), a marker of bone resorption. (B) Waterfall plot showing best treatment response in serum C-telopeptide (CTx), a marker of bone resorption. (C) Waterfall plot showing best treatment response in serum bone alkaline phosphatase (BAP), a marker of bone formation. (D) Waterfall plot showing best treatment response in serum osteocalcin, a marker of bone formation.
Pharmacokinetics
Plasma pharmacokinetic analyses were performed on all 31 subjects, and linear PKs were observed. Following an initial 40 mg oral dose of KX2-391, median Cmax was 60.8 ng/mL (range, 16–129 ng/mL), median Tmax was 1 hour (range, 1–2 hours), and median AUC was 156 ng*hr/mL (range, 35–348 ng*hr/mL). With continued oral administration of KX2-391 at a dose of 40 mg twice daily, median peak (Cmax) and trough (Cmin) levels of KX2-391 were similar across all subsequent cycles of therapy. Of note, although the observed Cmax levels appear to exceed those required for Src kinase inhibition (minimum threshold 28 ng/mL), the Cmax levels are substantially lower than those needed for inhibition of tubulin polymerization (minimum threshold 142 ng/mL; see Appendix for derivation).
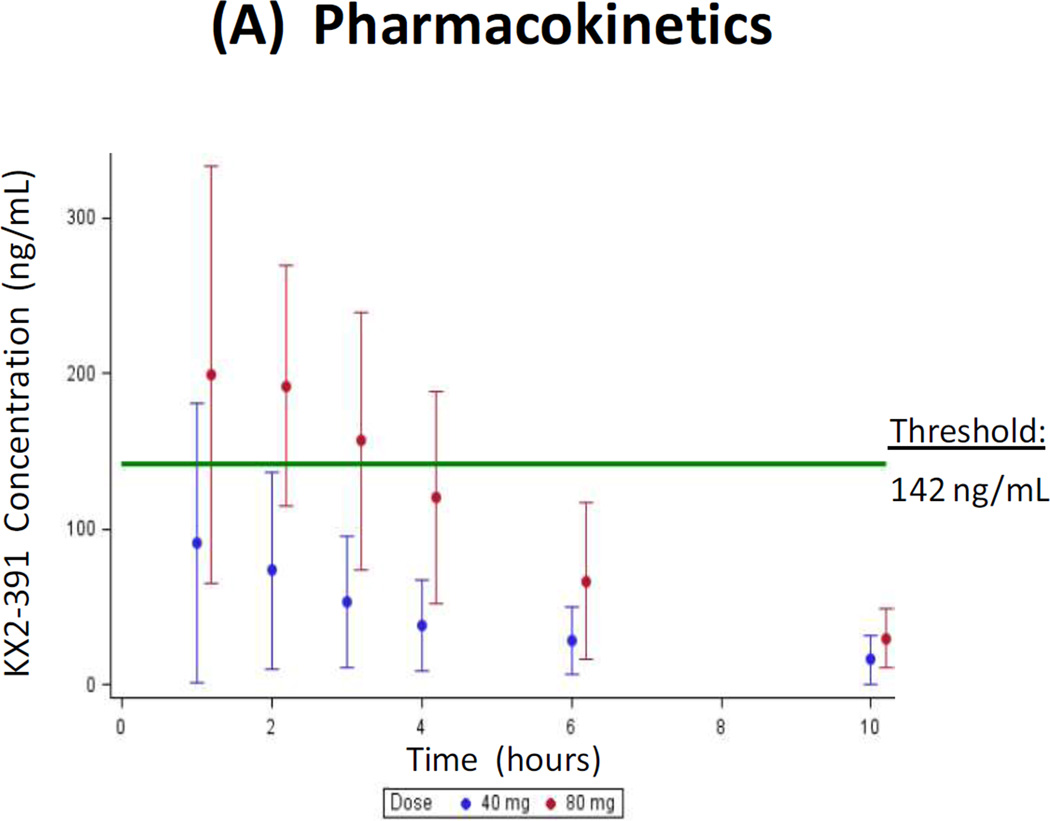
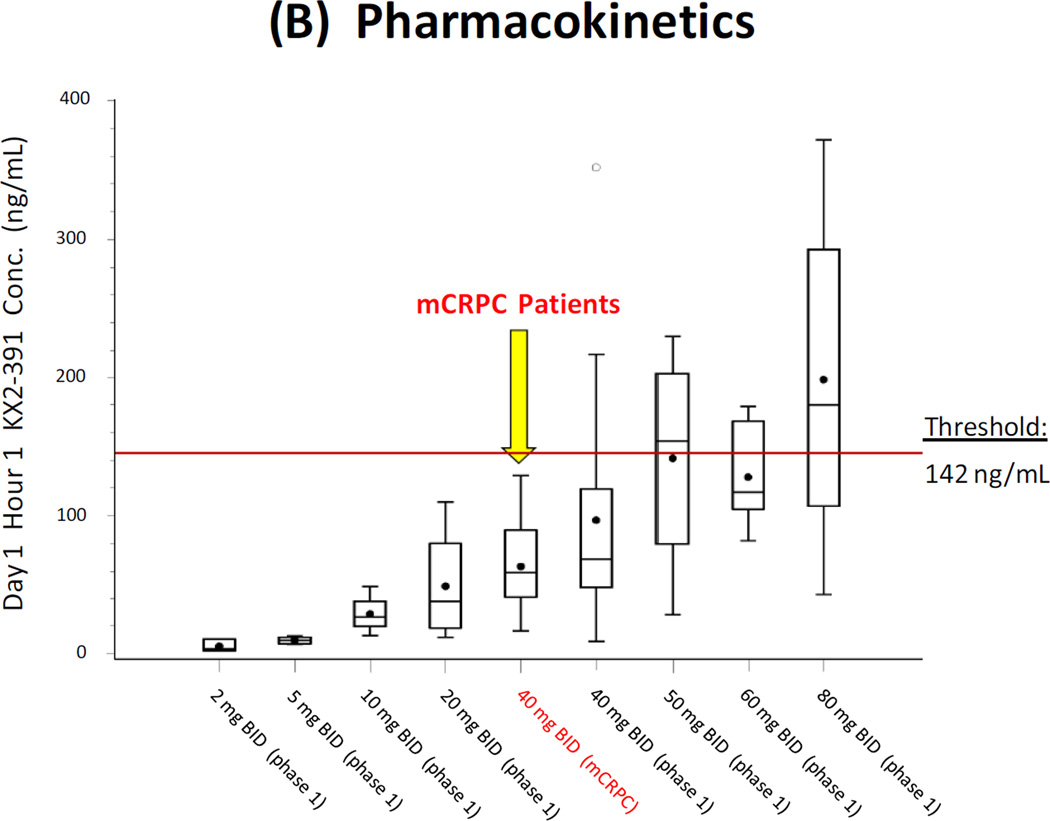
Preclinical studies predict that the minimum Cmax level required for tubulin polymerization inhibition by KX2-391 is 142 ng/mL (see Appendix). Data from ongoing trials (NCT01397799) suggest that this minimum threshold is achieved only when KX2-391 is administered at oral doses of ≥80 mg given all at one time, and not at a dose of 40 mg at one time (Fig. 3A). A summary of PK data from the prior phase 1 trial [14] and the current phase 2 trial suggests that Cmax levels achieved in men with metastatic CRPC are somewhat lower than those achieved in patients with other advanced solid malignancies (Fig. 3B).
Figure 3. Pharmacokinetic Outcomes.
(A) Pharmacokinetic results following the morning administration of KX2-391 at doses of 40 mg (current phase 2 study) and 80 mg (prior phase 1 study [14]; NCT00646139). Circles represent median values, and bars represent the range of values. The minimum plasma threshold thought to be required for tubulin polymerization inhibition is also depicted. (B) Summary of pharmacokinetic data from the current phase 2 trial and the prior phase 1 trial [14] (NCT00646139). Results are shown as box-and-whisker plots: the height of the box is the interquartile range (IQR), the horizontal line inside the box is the median, the circle inside the box is the mean, and the whiskers extend beyond the upper and lower quartiles by 1.5× IQR.
DISCUSSION
This is the first trial to assess KX2-391, a dual Src kinase and tubulin polymerization inhibitor, in men with advanced prostate cancer, and the first phase 2 study of this agent in any cancer. Although the trial did not meet its primary efficacy endpoint, modest benefits were observed with respect to CTC modulation and effects on bone metabolism. The inclusion of basic PK analyses was critical in aiding to potentially explain why the drug did not achieve its desired clinical effects, and lessons learned from these PK studies are being applied to future trials. KX2-391 was generally well-tolerated, with common adverse effects being liver function test abnormalities, myelosuppression, fatigue, nausea and constipation.
The use of Src inhibitors in men with advanced prostate cancer has met with some early evidence of success. Dasatinib, an oral inhibitor of multiple protein kinases including Src, showed encouraging preliminary activity in a phase 2 study where it was used as a single agent in men with metastatic CRPC [9]. In that trial, although PSA reductions and objective tumor responses were infrequent, positive effects on bone metabolism were observed in many patients (more than half of subjects had ≥40% declines in urinary N-telopeptide levels, and 60% had reductions in bone alkaline phosphatase levels). In a subsequent phase 1/2 study combining dasatinib with docetaxel in a similar patient population, PSA responses were observed in 57% of participants, objective tumor responses were seen in 60% of men, and 30% of patients with osseous metastases showed improvements in bone scans [10]. Finally, in another phase 2 trial using a different oral Src inhibitor (saracatinib) in men with advanced CRPC, 18% of patients had transient PSA reductions although bone biomarkers were not assessed [23]. The lack of a pharmacodynamic evaluation of drug target inhibition in the present trial was a clear limitation of this study. In addition, because we did not assess pain responses in our patients, we were unable to seek a potential association between bone biomarker changes and pain responses.
In the present trial, a significant minority of patients achieved modest improvements in markers of bone resorption (urinary N-telopeptide, serum C-telopeptide) and formation (bone alkaline phosphatase, osteocalcin). Previous studies have shown that treatment-related changes in these bone turnover markers in patients with bone metastases can impact on survival. For example, a ≥40% reduction in urinary N-telopeptide in CRPC patients receiving zoledronic acid was associated with a 17% reduction in the risk of death [24]. In the current trial, 16% of patients achieved uNTx declines of ≥40%, while in the dasatinib trial discussed above 51% of men achieved this effect. Although the relationship between other bone biomarkers (CTx, BAP, osteocalcin) and survival is less clear, reductions in these markers were also seen in some patients receiving KX2-391. However, bone biomarker effects with KX2-391 were less pronounced than those observed with dasatinib as well as other osteoclast-directed therapies (e.g. zoledronic acid and denosumab) [20].
The inclusion of PK data gave us the ability to hypothesize why KX2-391 did not achieve its desired therapeutic effects in the current clinical trial. Preclinical in vitro and in vivo studies conducted in parallel with this trial have informed us that the antitumor activity of KX2-391 appears to be driven primarily by Cmax levels and not by AUC levels. Furthermore, different plasma concentration thresholds are required to engage the two distinct mechanisms of action of this drug. To this end, while the minimum threshold for effective Src kinase inhibition is 28 ng/mL, the threshold for tubulin polymerization inhibition is 142 ng/mL (see Appendix for derivation). Because the antitumor activity of KX2-391 appears to rely on both mechanisms of action, it is hypothesized that plasma Cmax levels in KX2-391–treated patients should exceed 142 ng/mL.
In the current study, Cmax levels achieved with 40 mg doses of the study drug were below this threshold for all patients (median 61 ng/mL, range 16–129 ng/mL). Examination of Fig. 3A reveals that Cmax levels above this threshold are only achieved with oral KX2-391 doses of ≥80 mg, a fact that was not fully appreciated at the time when the current study was being designed. Examination of Fig. 3B confirms this notion, and also reveals that Cmax levels at the 40 mg dose are lower in patients with CRPC than in patients with other solid tumors. Although this finding was somewhat unexpected, similar discrepancies in plasma levels of docetaxel have been described in castrate (compared to non-castrate) prostate cancer patients [19], suggesting that the castrate state itself may affect metabolism of certain anticancer drugs.
Taken together, these data suggest that patients in the present study may have been under-dosed and did not achieve adequate plasma drug levels to engage the second mechanism of action of KX2-391: tubulin polymerization inhibition. With this in mind, ongoing phase 1b studies of KX2-391 in other tumor types (NCT01397799) are investigating higher once-daily doses of the drug (i.e. 80 mg, 120 mg, and even higher), with the hope that these doses will remain safe. Indeed, preliminary data suggests that this is the case. To this end, a study using dasatinib at a dose of 100 mg once daily in men with metastatic CRPC suggested that this dose was better tolerated and equally effective compared to prior studies using dasatinib 70–100 mg twice daily in this patient population [25].
In conclusion, KX2-391 given at a dose of 40 mg twice daily lacks significant antitumor activity in men with metastatic CRPC, but does have modest effects on CTC counts and markers of bone metabolism. Ongoing studies are currently evaluating higher (≥80 mg) once-daily doses of KX2-391 in other malignancies, with the hope that the drug will be safe and tolerable at these doses, although additional trials in prostate cancer patients have not been planned. This study illustrates the importance of including PK analyses in early phase 2 trials, which may inform the design of subsequent studies.
ACKGNOWLEDGEMENT
FUNDING
Supported by Kinex Pharmaceuticals LLC; a Conquer Cancer Foundation 2009 Young Investigator Award; an NIH/NCI training grant (T32 CA009071); the Department of Defense Prostate Cancer Research Program (PC051382); and the Prostate Cancer Foundation.
We thank the patients who volunteered to participate in this study and their families, as well as the staff members who cared for them at each site.
APPENDIX
Derivation of 142 ng/mL as the plasma threshold for KX2-391 activity
KX2-391 is 83% protein-bound in the serum. This correlates with an approximate 4-fold reduction in potency for both mechanisms of action (Src inhibition, and tubulin polymerization inhibition) measured in whole cells in the presence of human plasma. The IC50 for Src inhibition in human tumor cells is about 25 nM in the absence of human plasma and about 100 nM in the presence of human plasma. However, the IC50 for tubulin polymerization inhibition in human tumor cells is about 125 nM in the absence of plasma and about 500 nM in the presence of plasma. 500 nM of KX2-391 corresponds to a plasma concentration of 216 ng/mL. In mouse HT29 xenograft studies, it was determined that KX2-391 partitions into tumor tissue vs plasma at a ratio of 1.52. This reduces the required plasma concentration by 1.52-fold (i.e. 216/1.52 = 142 ng/mL). Based upon these assumptions, both mechanisms of action of KX2-391 will be engaged in patients when plasma levels reach approximately 142 ng/mL or higher (which should result in tumor tissue levels of at least 216 ng/mL). In addition, the drug level needs to remain above 142 ng/mL for long enough to cause significant apoptosis in tumor cells. An 80 mg dose of KX2-391 produces a Cmax of 242 ng/mL, and plasma levels stay above the 142 ng/mL threshold for about 3 hours [1]. This suggests that significant efficacy may be obtained with KX2-391 when it is dosed at or above 80 mg in patients. Preclinical toxicity and efficacy studies in mice indicated that once-daily dosing of KX2-391 was less toxic, allowing at least 3-fold higher drug doses to be administered, and appeared more efficacious than twice-daily dosing. Once-daily KX2-391 dosing in ongoing trials (NCT01397799) suggests that the maximum tolerated dose will be above 80 mg.
Reference
1. Adjei AA, Cohen RB, Kurzrock R et al (2009) Results of a phase I trial of KX2-391, a novel non-ATP competitive substrate-pocket directed Src inhibitor, in patients with advanced malignancies. J Clin Oncol 27(Suppl): abstract 3511
Footnotes
Conflicts of Interest: ESA, EIH, EMP, MRH, JYB, EYY, SYC, and GJF have no relevant conflicts of interest. GEW is a paid consultant/advisor for Kinex Pharmaceuticals. DHG is the Chief Scientific Officer of Kinex Pharmaceuticals, and has stock ownership in Kinex Pharmaceuticals. MRK is the Chief Medical Officer of Kinex Pharmaceuticals, and has stock ownership in Kinex Pharmaceuticals. LMD is the Vice President of Operations of Kinex Pharmaceuticals, and has stock ownership in Kinex Pharmaceuticals. MAC received research funding from Kinex Pharmaceuticals.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012 CA. Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Antonarakis ES, Eisenberger MA. Expanding treatment options for metastatic prostate cancer. N Engl J Med. 2011;364:2055–2058. doi: 10.1056/NEJMe1102758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Chang YM, Kung HJ, Evans CP. Non-receptor tyrosine kinases in prostate cancer. Neoplasia. 2007;9:90–100. doi: 10.1593/neo.06694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Posadas EM, Al-Ahmadie H, Robinson VL, et al. FYN is overexpressed in human prostate cancer. BJU Int. 2009;103:171–177. doi: 10.1111/j.1464-410X.2008.08009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fizazi K. The role of Src in prostate cancer. Annals of Oncology. 2007;18:1765–1773. doi: 10.1093/annonc/mdm086. [DOI] [PubMed] [Google Scholar]
- 7.Araujo JC, Logothetis CJ. Targeting Src signaling in metastatic bone disease. Int J Cancer. 2009;124:1–6. doi: 10.1002/ijc.23998. [DOI] [PubMed] [Google Scholar]
- 8.Tatarov O, Mitchell TJ, Seywright M, et al. Src family kinase activity is upregulated in hormone-refractory prostate cancer. Clin Cancer Res. 2009;15:3540–3549. doi: 10.1158/1078-0432.CCR-08-1857. [DOI] [PubMed] [Google Scholar]
- 9.Yu EY, Wilding G, Posadas E, et al. Phase II study of dasatinib in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res. 2009;15:7421–7428. doi: 10.1158/1078-0432.CCR-09-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Araujo JC, Mathew P, Armstrong AJ, et al. Dasatinib combined with docetaxel for castration-resistant prostate cancer: results from a phase 1-2 study. Cancer. 2012;118:63–71. doi: 10.1002/cncr.26204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fallah-Tafti A, Foroumadi A, Tiwari R, et al. Thiazolyl N-benzyl-substituted acetamide derivatives: synthesis, Src kinase inhibitory and anticancer activities. Eur J Med Chem. 2011;46:4853–4858. doi: 10.1016/j.ejmech.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 12.Hangauer D, Gelman I, Dyster L, et al. Potent and selective in vitro and in vivo inhibition of tumor proliferation by KXO1, a novel non-ATP competitive Src inhibitor. Proc Am Assoc Cancer Res. 2007 2007: abstract 3245. [Google Scholar]
- 13.Bu Y, Gao L, Smolinski M, et al. KX01 (KX2-391), a Src-family kinase inhibitor targeting the peptide-binding domain, suppresses oncogenic proliferation in vitro and in vivo . Proc Am Assoc Cancer Res. 2008 2008: abstract 4983. [Google Scholar]
- 14.Adjei AA, Cohen RB, Kurzrock R, et al. Results of a phase I trial of KX2-391, a novel non-ATP competitive substrate-pocket directed Src inhibitor, in patients with advanced malignancies. J Clin Oncol. 2009;27(Suppl) doi: 10.1007/s10637-013-9929-8. abstract 3511. [DOI] [PubMed] [Google Scholar]
- 15.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris MJ, Basch EM, Wilding G, et al. Department of Defense prostate cancer clinical trials consortium: a new instrument for prostate cancer clinical research. Clin Genitourin Cancer. 2009;7:51–57. doi: 10.3816/CGC.2009.n.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.Shaffer DR, Leversha MA, Danila DC, et al. Circulating tumor cell analysis in patients with progressive castration-resistant prostate cancer. Clin Cancer Res. 2007;13:2023–2029. doi: 10.1158/1078-0432.CCR-06-2701. [DOI] [PubMed] [Google Scholar]
- 19.Franke RM, Carducci MA, Rudek MA, et al. Castration-dependent pharmacokinetics of docetaxel in patients with prostate cancer. J Clin Oncol. 2010;28:4562–4567. doi: 10.1200/JCO.2010.30.7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fizazi K, Lipton A, Mariette X, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27:1564–1571. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 21.Carducci MA, Saad F, Abrahamsson PA, et al. A phase 3 randomized controlled trial of the efficacy and safety of atrasentan in men with metastatic hormone-refractory prostate cancer. Cancer. 2007;110:1959–1966. doi: 10.1002/cncr.22996. [DOI] [PubMed] [Google Scholar]
- 22.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 23.Lara PN, Jr, Longmate J, Evans CP, et al. A phase II trial of the Src-kinase inhibitor AZD0530 in patients with advanced castration-resistant prostate cancer: a California Cancer Consortium study. Anticancer Drugs. 2009;20:179–184. doi: 10.1097/CAD.0b013e328325a867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipton A, Cook R, Saad F, et al. Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acid. Cancer. 2008;113:193–201. doi: 10.1002/cncr.23529. [DOI] [PubMed] [Google Scholar]
- 25.Yu EY, Massard C, Gross ME, et al. Once-daily dasatinib: expansion of phase II study evaluating safety and efficacy of dasatinib in patients with metastatic castration-resistant prostate cancer. Urology. 2011;77:1166–1171. doi: 10.1016/j.urology.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]