Abstract
Objective
High resolution optical imaging is an imaging modality which allows visualization of structural changes in epithelial tissue in real time. Our prior studies using contrast-enhanced microendoscopy to image squamous cell carcinoma in the head and neck demonstrated that the contrast agent, proflavine, has high affinity for keratinized tissue. Thus, high-resolution microendoscopy with proflavine provides a potential mechanism to identify ectopic keratin production, such as that associated with cholesteatoma formation and distinguish between uninvolved mucosa and residual keratin at the time of surgery.
Study Design
Ex vivo imaging of histopathologically-confirmed samples of cholesteatoma and uninvolved middle-ear epithelium.
Methods
Seven separate specimens collected from patients who underwent surgical treatment for cholesteatoma were imaged ex vivo with the fiberoptic endoscope after surface staining with proflavine. Following imaging, the specimens were submitted for hematoxylin &eosin staining to allow histopathological correlation.
Results
Cholesteatoma and surrounding middle ear epithelium have distinct imaging characteristics. Keratin-bearing areas of cholesteatoma lack nuclei and appear as confluent hyperfluorescence, while nuclei are easily visualized in specimens containing normal middle ear epithelium. Hyperfluorescence and loss of cellular detail is the imaging hallmark of keratin allowing for discrimination of cholesteatoma from normal middle ear epithelium.
Conclusions
This study demonstrates the feasibility of high-resolution optical imaging to discriminate cholesteatoma from uninvolved middle ear mucosa, based on the unique staining properties of keratin. Use of real-time imaging may facilitate more complete extirpation of cholesteatoma by identifying areas of residual disease.
Keywords: cholesteatoma, keratin, optical imaging, otology, high-resolution microendoscopy
Introduction
Cholesteatomas are sacs of keratinizing squamous epithelium that can occur in the middle ear and mastoid. Cholesteatomas can enlarge, become infected, and cause local destruction through bony erosion. The only available treatment for this disease is complete surgical resection via tympnomastoidectomy1. However, surgical treatment is not always curative because re-growth of disease (“recidivism”) is relatively common even in experienced hands, with rates ranging from 5% to 50% in published series.2-4 Revision surgery is mandatory in these cases, but this subjects the patient to a second operation and does not fully assure against further recurrences. Cholesteatoma recidivism occurs by two different mechanisms: “recurrence” is the re-formation of an epithelial sac because eustachian tube dysfunction allows a new retraction pocket to develop; “residual” cholesteatoma is disease left behind by the surgeon that persists and later re-grows. The rate of residual disease has been reported as high as 30% in some published series.4 Thus it is critical to completely remove all microscopic disease at the initial surgery. To date, there is no well-established intraoperative imaging device or technique that can prevent residual disease.
Histologically, cholesteatoma is distinct from the simple cuboidal or ciliated columnar epithelium lining the middle ear and mastoid air cells. Cholesteatoma is comprised of keratinizing squamous epithelium and has four layers, the same as in skin epidermis, which include the basal, squamous, granulosum, and stratum corneum, and which is supported underneath by loose connective tissue.5-6 These lesions, like skin, shed keratin debris and stain positive for cytokeratins.7-9
Optical imaging technologies provide non-invasive visualization of tissue epithelium in real time.10-12 A novel endoscopic technique using high-resolution microendoscopy (HRME) allows for the spatial resolution of individual nuclei and cellular structures.13 The potential applications of this approach are broad, including the detection of neoplastic changes in the mucosa of the head and neck and esophagus.14-15 Our previous studies using this device to image squamous cell carcinomas in the head and neck have demonstrated that keratinized tissue has a high affinity for the contrast agent proflavine, thus providing a mechanism to potentially identify and differentiate cholesteatoma from the surrounding middle ear epithelium.15-16
In this paper, we describe the use of contrast-enhanced HRME for the detection of cholesteatoma in the middle ear. The flexible fiberoptic microscope provides a mechanism to obtain real-time intraoperative images of tissue epithelium and is a promising approach to discriminate between cholesteatoma and surrounding normal mucosa at the point of care.
Methods
Imaging System
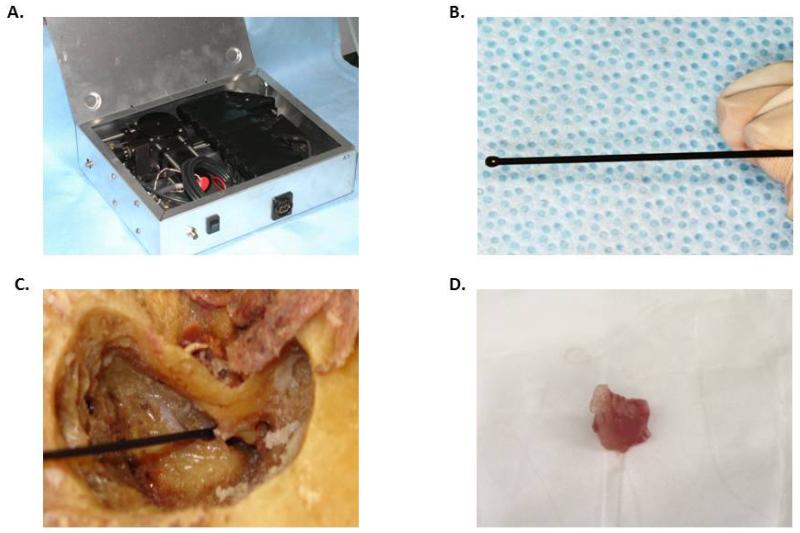
The HRME has been previously described.13 Briefly, the system consists of a fiberoptic probe, an LED light source, and a CCD camera connected to a laptop computer for image capture and storage (Figure 1a-b). The fiberoptic probe tip is small and flexible allowing for access to the middle ear space (Figure 1c). This imaging platform is designed for use with proflavine (Sigma-Aldrich, St. Louis, MO), a fluorescent contrast agent from the acriflavine family that reversibly binds to DNA and stains cell nuclei.17 Proflavine is buffered with saline to 0.01% solution and a small amount applied topically to tissue epithelium with a cotton-tipped applicator. Proflavine has been used in in vivo studies of the gastrointestinal tract in Europe and Australia without any reported adverse events, and is a component of the dye used to prevent infection of the umbilical stump of newborns.18-20 Proflavine has never been applied to the middle ear, thus the ototoxicity of this agent is currently unknown. Future clinical studies are therefore required to determine the safety profile for use in the middle ear.
Figure 1.
A. Internal configuration of the HRME device; B. Fiberoptic probe tip; C. Fiberoptic probe tip delivered to cadaveric temporal bone after mastoidectiomy; D. Representative cholesteatoma specimen.
Specimen acquisition and imaging
Through a study protocol approved by the Mount Sinai School of Medicine Institutional Review Board (GCO# 11-1262), we obtained 7 anonymized surgical specimens of cholesteatoma which had been stored at −80°C for 1-2 years prior to this study. Specimens were thawed to room temperature prior to application of proflavine solution (0.01% in saline) to the specimen with a cotton-tipped applicator and subsequent imaging with the fiberoptic probe (Figure 1d). The specimens were then sent for histological processing and preparation of hematoxylin and eosin (H&E) slides correlating with the area imaged.
Images of the gingiva and head and neck squamous cell carcinoma specimens were obtained through a study protocol approved by the Mount Sinai School of Medicine Institutional Review Board (GCO 09-0954). Grossly normal and malignant areas of the fresh tumor specimen were stained immediately with proflavine following standard-of-care surgical resection. After topical application of the dye, the fiberoptic probe was used to view these areas and capture images or movie clips of 3 seconds duration. Movie clips were converted to still images using Windows Movie Maker (Microsoft, Redmond, WA). A 3-mm punch biopsy of the imaged site was analyzed by conventional H&E histopathology by a board-certified pathologist.
Results
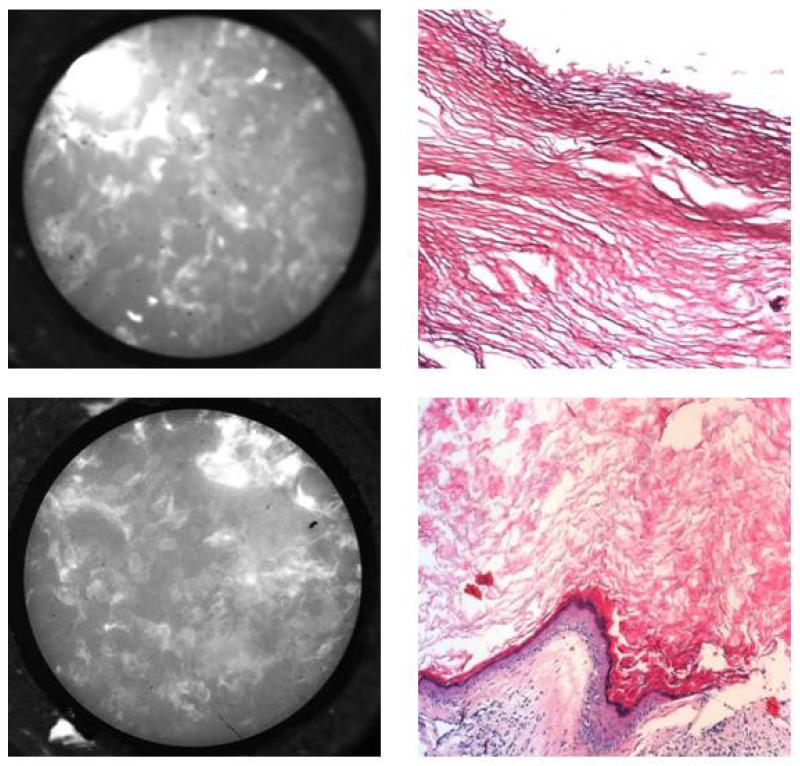
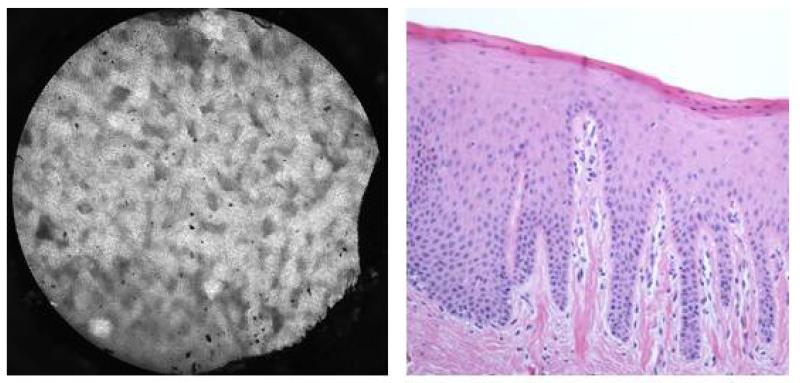
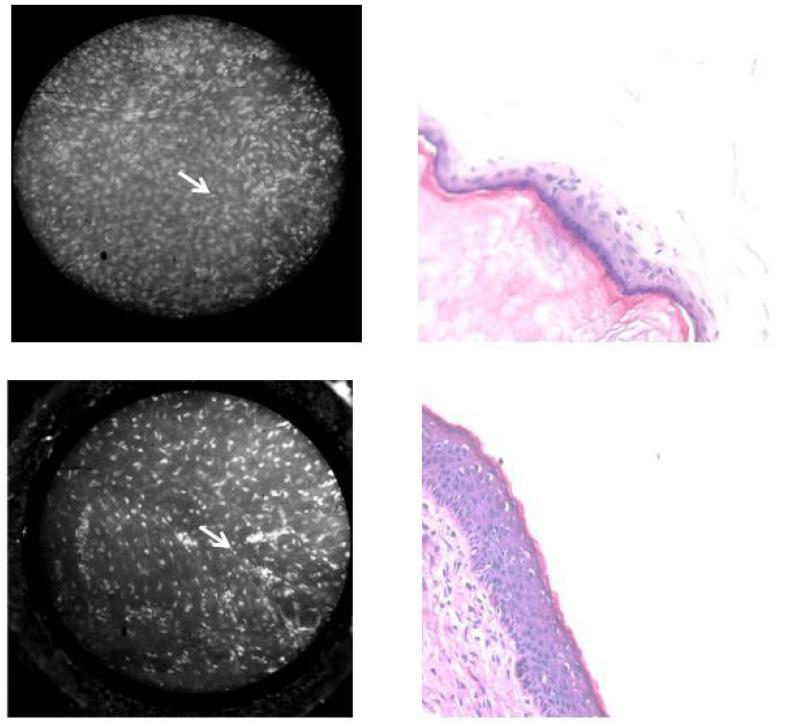
We could successfully image cholesteatoma and uninvolved mucosa from all 7 specimens. Contrast-enhanced images of cholesteatoma specimens appeared as areas of confluent hyperfluorescence that correlated to histologically confirmed keratin (Figure 2). The high-resolution images of cholesteatoma, composed of mostly keratin, appeared similar to images from areas of keratinized epithelium in the upper aerodigestive tract (Figure 3). Images of middle ear epithelium surrounding the cholesteatoma displayed obvious normal-appearing nuclei, which correlated with corresponding histology (Figure 4). Therefore, hyperfluorescence and loss of cellular architecture are the microendoscopic imaging hallmarks of cholesteatomas due to high keratin composition. The striking affinity of proflavine for keratin allows straightforward differentiation from surrounding middle ear epithelium in which nuclei are readily visualized.
Figure 2.
HRME images (left) of cholesteatoma specimens with corresponding H&E appearance at 20× (right). Notice that keratin appears as disorganized hyperflourescence without visible nuclei.
Figure 3.
Appearance of keratin imaged with contrast-enhanced HRME. A. HRME image (left) of histologically (H& E original magnification 100X) confirmed (right) keratin from the gingiva of a composite resection of the oral cavity. Notice that the image shows hyperflourescence without discrete visualization of nuclei.
Figure 4.
HRME images (left) of middle ear epithelium specimens with corresponding H&E at 20× (right). Nuclei (arrow) are readily apparent.
Discussion
Here we describe the first report of an optical imaging device applied to otologic surgery. The differences between cholesteatoma and middle ear epithelium are readily apparent in the proflavine-enhanced images providing a mechanism to ensure elimination of all diseased tissue. These results support future in vivo intraoperative application of HRME, as the fiberoptic endoscope can readily be applied during mastoid procedures and provide real-time imaging that can assist the otologic surgeon.
The treatment of cholesteatoma is thwarted by the tendency of the disease to regrow, even after adequate surgical removal in the most experienced hands. Cholesteatoma regrowth can be the result of disease recurrence (the formation of a new sac by invasion of skin from the external canal into the middle ear), or residual cholesteatoma (disease unknowingly left behind by the surgeon).21,22 Cholesteatoma recurrences are associated with increased morbidity, including the need for additional surgery, further loss of hearing, recurrent infection, and potential complications including labyrinthitis and intracranial spread.23 Therefore, a mechanism that could identify residual disease intraoperatively would be a worthwhile advance in the treatment of this aggressive disease in order to reduce the chance of residual disease.
In our prior studies, utilizing HRME for imaging head and neck squamous cell carcinomas, keratinizing epithelium appeared hyperfluorescent, which obstructed visualization of nuclei and was a limitation in distinguishing benign from cancerous mucosa. Keratin absorbs proflavine, thus these contrast-enhanced images appear as hyperflourescent. Proflavine, a member of the acridine family, is positively-charged, and thus intercalates between negatively-charged double-stranded DNA in a non-covalent manner.24 Although the precise mechanism of proflavine’s affinity for keratin is not understood, it may in part be a result of the overall negative charge of keratin molecules.25-26 Although hyperkeratosis presents an obstacle to detecting squamous cell carcinoma of the upper aerodigestive tract, for cholesteatoma, keratin provides the key diagnostic imaging hallmark in HRME images.
This is the first report utilizing the HRME in combination with proflavine on samples that were frozen for an extended period of time. In our prior head and neck studies, ex vivo imaging was performed on fresh specimens immediately following surgical resection.13-14,16 Although we have not done a controlled comparison, in general, we observe that in vivo images show higher contrast than ex vivo images of fresh or frozen/thawed tissue. We speculate that the reduction in contrast is due to partial autolysis that occurs in the in ex vivo specimens, resulting in additional cytoplasmic staining. Nonetheless, the image features otherwise appear quite similar in the ex vivo and in vivo images despite the temperature differences. This is true across a wide variety of organ sites including oral cavity, breast, uterine cervix, and esophagus, for which our group has performed both published and ongoing in vivo studies.
In addition to the obvious application of the HRME to direct in vivo imaging of cholesteatomas to aid in surgical removal, the highly keratinized cholesteatoma specimens can serve as a platform for further investigations of the unique imaging properties of keratin including its interactions with proflavine and, potentially, other contrast agents. A better understanding of the imaging properties of keratin and its associated hyperfluorescence and obstruction of nuclear detail would improve the utility of HRME for cancer detection in the upper aerodigestive tract, and potentially allow application of contrast-enhanced HRME to other highly keratinized organ systems, such as skin.
Conclusion
This ex vivo study demonstrates the feasibility of proflavine-enhanced HRME to distinguish cholesteatoma from uninvolved middle ear mucosa. Further in vivo studies are required to test the hypothesis that intra-operative real-time imaging can enhance surgical removal by identifying residual disease, and thereby improve patient prognosis and surgical outcome.
Acknowledgments
Financial Disclosure: This work was supported by the NCI Biomedical Research Partnership (BRP) Grant R01 CA103830-06, the National Institute of Biomedical Imaging and Bioengineering Grant R01 EB007594, as well as a grant from the Doris Duke Charitable Foundation to Mount Sinai School of Medicine to fund clinical research fellow Lauren L. Levy. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Biomedical Imaging And Bioengineering or the National Institutes of Health. Lauren Levy, Lauren Levy,Dr. Nancy Jiang, Dr. Eric Smouha and Dr. Andrew Sikora have no conflicts of interests. Dr. Rebecca Richards-Kortum holds patents related to the optical imaging modality used in this study in addition to minority ownership stake in the company to which these technologies have been licensed.
Footnotes
Work was performed at Mount Sinai School of Medicine in the Department of Otolaryngology
References
- 1.Smouha EE, Bojrab DI. General considerations in cholesteatoma. In: Smouha EE, Borjab DI, editors. Cholesteatoma. Thieme; New York, NY: 2011. pp. 1–8. [Google Scholar]
- 2.Vartiainen E. Factors associated with recurrence of cholesteatoma. J Laryngol Otol. 1995;109:590–2. doi: 10.1017/s0022215100130804. [DOI] [PubMed] [Google Scholar]
- 3.Edelstein DR, Parisier SC. Surgical techniques and recidivism in cholesteatoma. Otolaryngol Clin North Am. 1989;22:1029–40. [PubMed] [Google Scholar]
- 4.Sheehy JL, Brackmann DE, Graham MD. Cholesteatoma surgery: residual and recurrent disease. A review of 1,024 cases. Ann Otol Rhinol Laryngol. 1977;86:451–62. doi: 10.1177/000348947708600405. [DOI] [PubMed] [Google Scholar]
- 5.Olszewska E, Wagner M, Bernal-Sprekelsen M, et al. Etiopathogenesis of cholesteatoma. Eur Arch Otorhinolaryngol. 2004;261:6–24. doi: 10.1007/s00405-003-0623-x. [DOI] [PubMed] [Google Scholar]
- 6.Svane-Knudsen V, Halkier-Sørensen L, Rasmussen G, Ottosen PD. Cholesteatoma: a morphologic study of stratum corneum lipids. Acta Otolaryngol. 2001;121:602–6. [PubMed] [Google Scholar]
- 7.Bujía J, Schilling V, Holly A, Stammberger M, Kastenbauer E. Hyperproliferation-associated keratin expression in human middle ear cholesteatoma. Acta Otolaryngol. 1993;113:364–8. doi: 10.3109/00016489309135826. [DOI] [PubMed] [Google Scholar]
- 8.Broekaert D, Coucke P, Leperque S, et al. Immunohistochemical analysis of the cytokeratin expression in middle ear cholesteatoma and related epithelial tissues. Ann Otol Rhinol Laryngol. 1992;101:931–8. doi: 10.1177/000348949210101109. [DOI] [PubMed] [Google Scholar]
- 9.Broekaert D, Cornille A, Eto H, et al. A comparative immunohistochemical study of cytokeratin and vimentin expression in middle ear mucosa and cholesteatoma, and in epidermis. Virchows Arch A Pathol Anat Histopathol. 1988;413:39–51. doi: 10.1007/BF00844280. [DOI] [PubMed] [Google Scholar]
- 10.Rolland JP, Lee KS, Khoudeir L, et al. Virtual skin biopsy with gabor domain optical coherence microscopy. Stud Health Technol Inform. 2012;173:398–404. [PubMed] [Google Scholar]
- 11.Ahn YC, Chung J, Wilder-Smith P, Chen Z. Multimodality approach to optical early detection and mapping of oral neoplasia. J Biomed Opt. 2011;16:076007. doi: 10.1117/1.3595850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roblyer D, Richards-Kortum R, Sokolov K, El-Naggar AK, Williams MD, Kurachi C, Gillenwater AM. Multispectral optical imaging device for in vivo detection of oral neoplasia. J Biomed Opt. 2008;13:024019. doi: 10.1117/1.2904658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muldoon TJ, Pierce MC, Nida DL, Williams MD, Gillenwater A, Richards-Kortum R. Subcellular-resolution molecular imaging within living tissue by fiber microendoscopy. Opt Express. 2007;15:16413–23. doi: 10.1364/oe.15.016413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muldoon TJ, Roblyer D, Williams MD, Stepanek VM, Richards-Kortum R, Gillenwater AM. Noninvasive imaging of oral neoplasia with a high-resolution fiber-optic microendoscope. Head Neck. 2012;34:305–12. doi: 10.1002/hed.21735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muldoon TJ, Thekkek N, Roblyer D, Maru D, Harpaz N, Potack J, Anandasabapathy S, Richards-Kortum R. Evaluation of quantitative image analysis criteria for the high-resolution microendoscopic detection of neoplasia in Barrett’s esophagus. J Biomed Opt. 2010;15:026027. doi: 10.1117/1.3406386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vila P, Park C, Pierce M, et al. Discrimination of benign and neoplastic mucosa with a high-resolution microendoscope (HRME) in head and neck cancer. Ann Surg Oncol. 2012 doi: 10.1245/s10434-012-2351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferguson LR, Denny WA. Genotoxicity of non-covalent interactions: DNA intercalators. Mutat Res. 2007;623:14–23. doi: 10.1016/j.mrfmmm.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 18.Polglase AL, McLaren WJ, Skinner SA, Kiesslich R, Neurath MF, Delaney PM. A fluorescence confocal endomicroscope for in vivo microscopy of the upper- and the lower-GI tract. Gastrointest Endosc. 2005;62:686–95. doi: 10.1016/j.gie.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Kiesslich R, Burg J, Vieth M, et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004;127:706–13. doi: 10.1053/j.gastro.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 20.Janssen PA, Selwood BL, Dobson SR, Peacock D, Thiessen PN. To dye or not to dye: a randomized, clinical trial of a triple dye/alcohol regime versus dry cord care. Pediatrics. 2003;111:15–20. doi: 10.1542/peds.111.1.15. [DOI] [PubMed] [Google Scholar]
- 21.Smouha EE, Bojrab DI. General considerations in cholesteatoma. In: Smouha EE, Borjab DI, editors. Cholesteatoma. Thieme; New York, NY: 2011. pp. 133–135. [Google Scholar]
- 22.Roger G, Denoyelle F, Chauvin P, Schlegel-Stuhl N, Garabedian EN. Predictive risk factors of residual cholesteatoma in children: a study of 256 cases. Am J Otol. 1997;18:550–8. [PubMed] [Google Scholar]
- 23.Habesoglu TE, Balak N, Habesoglu M, Zemheri E, Isik N, Elmaci I, Egeli E. Intracranial cholesteatoma - case report and critical review. Clin Neuropathol. 2009;28:440–4. doi: 10.5414/npp28440. [DOI] [PubMed] [Google Scholar]
- 24.Luzzati V, Masson F, Lerman LS. Interaction of DNA and proflavine: a small-angle x-ray scattering study. J Mol Biol. 3:634–9. doi: 10.1016/s0022-2836(61)80026-0. 196. [DOI] [PubMed] [Google Scholar]
- 25.Bragulla HH, Homberger DG. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J Anat. 2009;214(4):516–59. doi: 10.1111/j.1469-7580.2009.01066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ananthapadmanabhan KP, Lips A, Vincent C, et al. pH-induced alterations in stratum corneum properties. Int J Cosmet Sci. 2003;25:103–12. doi: 10.1046/j.1467-2494.2003.00176.x. [DOI] [PubMed] [Google Scholar]