Abstract
In the mammalian central nervous system (CNS), coupling of neurons by gap junctions (ie. electrical synapses) and the expression of the neuronal gap junction protein, connexin 36 (Cx36), transiently increase during early postnatal development. The levels of both subsequently decline and remain low in the adult, confined to specific subsets of neurons. However, following neuronal injury [such as ischemia, traumatic brain injury (TBI) and epilepsy], the coupling and expression of Cx36 rise. Here we summarize new findings on the mechanisms of regulation of Cx36-containing gap junctions in the developing and mature CNS and following injury. We also review recent studies suggesting various roles for neuronal gap junctions and, particularly, their role in glutamate-mediated neuronal death.
Keywords: connexin 36, gap junctions, glutamate excitotoxicity, stroke, brain trauma, development
Introduction
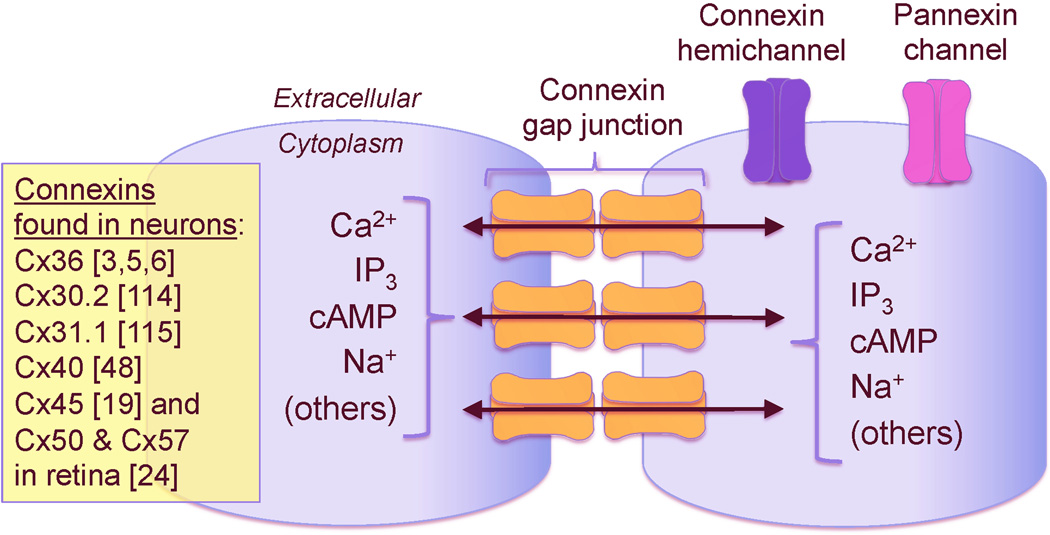
Gap junctions connect neighboring cells via intercellular channels that allow direct electrical communication as well as sharing of ions and small molecules (Figure 1) [1]. The channels are made of two hemichannels (one in each membrane) each consisting of six subunits known as connexins. The connexins, which are integral membrane proteins, were named according to their molecular weight [e.g., connexin 36 (Cx36) has a molecular weight of 36 kDa]. Connexins are encoded by a family with 20 and 21 genes in the mouse and human genomes, respectively [2]. In the rodent CNS eleven connexins are expressed, most of which are found in glial cells [3,4]. Cx36 is the main neuronal connexin [3,5,6], though others have been variably detected in mature neurons (Figure 1).
Figure 1. Schematic drawing of connexin gap junctions, connexin hemichannels and pannexin channels.
The gap junction channels are made of two hemichannels (one in each apposed membrane) and each hemichannel consists of six connexin subunits. Connexins forming the gap junction channels in neurons are indicated on the figure. Also shown are some of the molecules and ions that pass through gap junctions [1,111]. In addition to gap junctions, unopposed connexin hemichannels and pannexin channels (which, however, do not form gap junctions) also can be expressed by neurons [8,11,12].
In addition to gap junctions, unopposed hemichannels are also found in the nervous system. Different types of connexins may form the hemichannels [4,7], presumably including neuronal Cx36 [8]. Pannexins, which are vertebrate homologues of invertebrate gap junction proteins (innexins) also are expressed in glial cells and neurons [9–11]. They, however, exist solely as unopposed cell membrane channels and do not form gap junctions [12].
In the mammalian CNS, connexin gap junctions and hemichannels, as well as pannexin channels, display a complex distribution among various cell types and each has a unique developmental pattern of expression. They are involved in multiple aspects of CNS physiology. Their expression and function are regulated via numerous mechanisms, from transcriptional regulation to the regulation of gating (for reviews on distribution, regulation and roles of glial gap junctions and hemichannels see [4,7]; for reviews on pannexin channels, see [10,12]). Here, we will discuss neuronal gap junctions, their regulation and role during development, adulthood and injury in the rodent CNS. Our discussions will be focused largely on Cx36, since it is the best characterized and predominant neuronal connexin.
Properties of Cx36-containing gap junctions
Cx36, a 321 amino acid protein, is a member of the γ -subclass of connexin family [2]. The GJD2 gene (encoding Cx36) is located on mouse chromosome 2, in a region syntenic to human chromosome 15q14 [13,14]. The protein coding sequence of the Cx36 gene is highly conserved, with 98% amino acid identity among mouse, rat and human and 80% identity with the fish ortholog Cx35. When forcedly expressed in Xenopus oocytes and HeLa cells, Cx36 channels exhibit low unitary conductance (~10–15 pS) and low voltage sensitivity [15,16].
Electrical synapses containing Cx36/Cx35 gap junctions usually are formed at dendro-dendritic or dendro-somatic neuronal contacts, but they also have been observed between axon terminals [17]. They are dynamic structures, with channel turnover measured in minutes [18]. It seems that the Cx36-containing channels function homotypically [15]; though, they may be bihomotypic (ie. expressed in the same plaque) and form co-scaffolding with Cx45 channels and the protein zonula occludens-1 (ZO-1; see below) [19]. Cx36 can functionally compensate for the loss of Cx45 in the mouse retina, but not in the developing heart, suggesting tissue-specific activity of Cx36 gap junctions [20].
In various regions of rodent CNS and in fish Mauthner cells, Cx36/Cx35 often are found in “mixed” synapses (ie. electrical plus chemical) and are located in close proximity to NMDA receptors (NMDARs) [21–23] and AMPA receptors (AMPARs) [23,24]. Cx36/Cx35-containing gap junctions form complexes with a large number of intracellular proteins that result in them being anchored to the cytoskeleton. These complexes include structural and regulatory proteins that likely have roles in controlling channel activity, transport, assembly, localization and gene transcription [25]. For example, Cx36/Cx35 are associated and/or interact with a number of scaffolding proteins, including ZO-1, ZO-2, the transcription factor ZO-1-associated nucleic acid-binding protein, afadin (AF6) and multi-PDZ domain protein-1 [25–27]. The interaction of Cx36 with these proteins likely occurs simultaneously at individual gap junctions in the same neuron. A carboxy-terminal four amino acid motif (“SAYV”) of Cx36 is required for such interactions [25,27]. Interestingly, the same motif is needed for incorporation of Cx36 in electrical synapses [28], suggesting that these Cx36/scaffolding protein interactions are required for incorporation.
The Cx36/Cx35 complexes also include protein kinases, such as Ca2+/calmodulin-dependent protein kinase II (CaMKII). Binding to, co-localization with, and phosphorylation by CaMKII have been demonstrated for Cx36/Cx35 in mouse inferior olive neurons and synapses on the goldfish Mauthner cells [29,30]. This interaction may influence expression and/or stability of CaMKII, since CaMKII levels are reduced in neurons of Cx36 knockout mice [31]. In addition to CaMKII, Cx36/Cx35 are phosphorylated on multiple residues by cAMP-dependent protein kinase (PKA), cGMP-dependent protein kinase (PKG), protein kinase C (PKC) and casein kinase II [32–34].
Evidence indicates that individual neurons can be coupled to a highly variable number of neighboring neurons and a given neuron can be coupled with remarkably different degrees of conductance with each of its partners [22]. Further, the extent of interaction with CaMKII [29] and the phosphorylation state of Cx36 [35] can vary widely within a single neuron. These findings suggest that neuronal gap junction coupling (GJC) is controlled at the level of individual plaques. Such synapse-by-synapse regulation presumably permits cell-type-specific responses and depends upon the identity of signaling complexes assembled in individual gap junctions [35]. Remarkably similar interactions are observed between the above-described scaffolding/regulatory proteins and non-neuronal connexins (e.g., Cx43), suggesting a possibility that multisubunit complexes represent a core feature of GJC among multiple cell types [36].
Changes in neuronal GJC during development and following neuronal injury
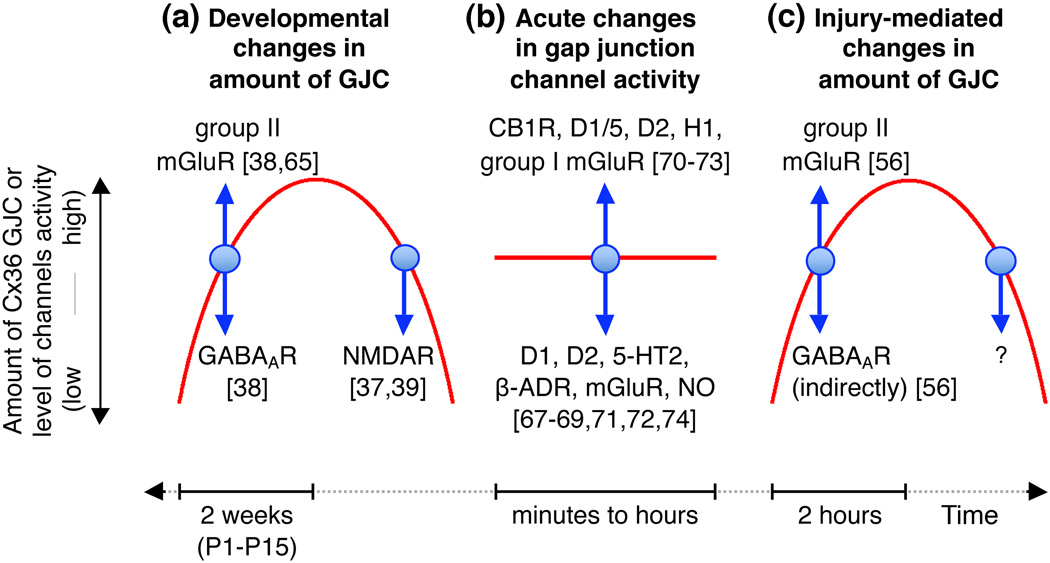
Transient coupling of neurons by gap junctions is a general phenomenon in the developing mammalian CNS: it has been documented in different CNS regions and rodent species (Figure 2a) [1]. In most CNS regions, including the cortex and hypothalamus, the incidence of neuronal GJC (measured using electrotonic and/or dye coupling approaches) and the expression of Cx36 increase during the first two postnatal weeks and then decrease by the end of postnatal weeks three to four [5,37,38]. In some regions, such as the spinal cord, coupling increases during late embryonic development followed by uncoupling and Cx36 down-regulation during the first postnatal days [5,39,40].
Figure 2. Neuronal GJC in the CNS.
This figure schematically illustrates conclusions from studies on the role of neurotransmitters and their receptors in (a) developmental, (b) acute, and (c) injury-mediated changes in neuronal GJC. In a and c, red lines represent the increase (upward phase) and decrease (downward phase) in the amount of neuronal GJC and expression of Cx36 during development and neuronal injury; note the differences in duration of developmental (ie. 2 weeks; [37–39,65]) and injury-mediated (ie. 2 hours; [56]) increases in GJC. In b, the red line represents background activity of multiple neuronal gap junctions relative to which modulatory changes in the activity occur. In all figures, blue arrows show the direction of change in GJC after activation of the indicated neurotransmitter receptors. Abbreviations: α -ADR, α -adrenoreceptor; CB1R, cannabinoid type 1 receptor; D1, D2 and D1/5, dopamine receptors; GABAAR, GABAA receptor; H1, histamine receptor; 5-HT2, serotonin receptor; mGluR, metabotropic glutamate receptor; NMDAR, NMDA receptor; NO, nitric oxide; P1 and P15, postnatal days 1 and 15.
The extent of developmental coupling is highly variable among different anatomical locations. For example, at its peak, the incidence of neuronal GJC is 60–100% in the rat cortex, hippocampus, dentate gyrus, striatum and spinal cord [39–44]. However, it is significantly lower (16–40%) in the ventrobasal thalamus, hypothalamus and between genioglossal motoneurons [38,45,46]. In addition, in the developing CNS, coupling is not restricted to cells of the same type, so that glutamatergic neurons (including pyramidal cells) are coupled to interneurons [6,42] and neurons may be coupled to glial cells [47]. However, connexins other than Cx36 also form gap junctions between developing neural cells (e.g., Cx26 and Cx40 [48,49]) and may contribute to the extent of coupling in the developing CNS.
During developmental uncoupling, not only the total levels of neuronal GJC and Cx36 expression decrease, but the remaining coupling reallocates. This results in elimination of Cx36-containing gap junctions from cortical and hippocampal pyramidal neurons, uncoupling of neurons from glial cells, and restriction of Cx36 GJC almost exclusively to homogeneous neuronal subpopulations (reviewed in [50,51]). However, in some neuronal subtypes (e.g., in the cortex, thalamic reticular nucleus [TRN] and mesencephalic trigeminal nucleus) developmental uncoupling does not occur [42,52,53]. This suggests different regulation and function of gap junctions in those neurons.
Neuronal gap junctions and Cx36 are observed in every major region of the mature CNS, albeit at lower levels than during development [50]. Cx36 expression and GJC transiently increase following a wide range of neuronal injuries, including ischemia [54–56], spinal cord injury and TBI [57–59], retinal injury [60], epilepsy [61,62] and inflammation [63] (Figure 2c). A key difference with the pattern of expression during development is the timing of expression. Whereas in development the rise and fall of Cx36 GJC occurs on a time scale of weeks, following injury the increase occurs during 1–2 hours post-injury with a decline in the subsequent 24–48 hours [56,58,59,62,63]. This temporal difference suggests utilization of different regulatory mechanisms for neuronal GJC in development versus injury.
Regulation of neuronal GJC
Developmental regulation
The expression of Cx36 initiates while chemical synaptic transmission is not yet established [64]. However, chemical neurotransmitter receptors apparently play a role in the developmental increase in neuronal GJC. Specifically, in the rat and mouse hypothalamus and cortex, this increase is controlled by an interplay between the activity of group II metabotropic glutamate receptors (mGluRs) and GABAA receptors (GABAARs) [38]. Chronic (2 week) activation of group II mGluRs augments, and inactivation prevents, the developmental increases in neuronal coupling and Cx36 expression. The situation is opposite for GABAARs. The regulation by group II mGluRs is via cAMP/PKA-dependent signaling, while regulation by GABAARs is via developmental depolarization and Ca2+/PKC-dependent signaling. Acetylcholine, GABAB and other classes of glutamate receptors presumably are not involved in these regulatory mechanisms [38].
Evidence to date indicates that the developmental regulation of Cx36 gene expression relies upon regulation of both gene transcription and protein translation. The receptor-dependent developmental increase in mouse Cx36 expression is transcription-dependent and requires a neuron-restrictive silencer element in the Cx36 gene promoter. However, the receptor-dependent developmental decrease of Cx36 requires sequences within the 3’-untranslated region of the Cx36 mRNA, suggesting post-transcriptional regulation [38]. In addition, the acute (ie. within 1hr) activation of group II mGluRs in developing mouse cortical neurons induces only a transient increase (over a 24 hour period) in Cx36 protein without changing Cx36 mRNA levels [65], implicating translational mechanisms.
Temporally, the developmental uncoupling of neurons overlaps with the major period of chemical synapse formation and increased synaptic activity [66]. Not surprisingly, the uncoupling also is regulated by the neurotransmitter glutamate. However, this regulation occurs via activation of NMDARs (Figure 2a) [37,39] and involves Ca2+/cAMP response element binding protein (CREB)-dependent down-regulation of Cx36 gene expression [37].
The net developmental changes in neuronal GJC likely are determined by the cumulative effects of all of these mechanisms. The variations among different CNS regions in the timing of coupling and uncoupling presumably can be explained by interregional differences in aspects of these mechanisms, such as receptor and synaptic activity and timing of the excitation/inhibition switch for GABAARs. Ultimately, activity of neurotransmitter receptors most likely modifies, rather than determines, the magnitude and timing of developmental changes in neuronal GJC, during the period when both electrical and chemical mature synaptic pathways are being established. It is also likely that the developmental increase and decrease in neuronal GJC reflect, the rising and declining contribution of gap junctions, respectively, in specific developmental processes (discussed further below).
Acute modulation of neuronal GJC
In developing and, particularly, adult mammalian CNS, activation of neurotransmitter receptors also results in acute modulation of neuronal GJC (Figure 2b). This modulation may involve changes in channel opening probability as well as alteration of connexin protein synthesis, trafficking, assembly, disassembly and degradation. The purpose of acute modulation presumably is in rapid modification of neuronal connectivity and signaling in response to transient changes in chemical synaptic activity.
A variety of neurotransmitters and transmitter receptors can acutely modulate neuronal GJC. For example, dye coupling between rat cortical neurons is reduced within minutes following activation of D1 and D2 dopamine receptors (D1Rs and D2Rs, respectively), α -adrenoreceptors, serotonin (5-HT2) receptors and elevation of nitric oxide (NO) [67]. Activation of α -adrenoreceptors also decreases electrotonic coupling between rat hippocampal interneurons [68] and NO uncouples striatal neurons [69]. However, coupling of hypothalamic neurons increases following activation of H1 histamine receptors [70]. Moreover, there is evidence for bidirectional regulation of dye coupling in the rat striatum and nucleus accumbens by dopamine; specifically, activation of D1Rs decreases, whereas D2Rs increases, GJC [71].
In the mammalian retina under dark conditions, various types of neurons are extensively coupled to each other by Cx36-containing gap junctions [72]. In response to light, amacrine cells release dopamine and NO, which activate a number of intracellular pathways and induce PKA-, PKG- and protein phosphatase 2A (PP2A)-dependent phosphorylation or dephosphorylation of Cx36 [32–35]. In general, the coupling between retinal neurons is increased by activation of D2Rs, and decreased via actions of D1Rs and NO [72]. However, the regulation of coupling varies across the population of retinal cells, where both increased and decreased coupling in response to light has been observed for different groups of neurons. Moreover, light has varying effects on the conductance of Cx36 gap junctions in the same neuron, depending on the level of brightness [72].
As discussed above, Cx36 channels often are found in “mixed” synapses that also have a glutamatergic component [21–23]. Chemical glutamatergic synapses are known for their activity-dependent modifications: long-term potentiation (LTP) and long-term depression (LTD). The conductance in electrical synapses may also undergo activity-dependent modifications. In the “mixed” goldfish Mauthner cell synapses, afferent stimulation initiates a chain of events (including an activation of group I mGluRs, cannabinoid type 1 receptors and D1R/D5Rs) that induces simultaneous LTP of electrical (Cx35) and glutamatergic (ionotropic receptor-dependent) synaptic transmission [73]. In contrast, in neurons of the rat TRN, activation of mGluRs induces LTD of electrical synapses [74]. Furthermore, Cx36 knockout impairs LTP of chemical synapses in the mouse hippocampus [75] and cortex [76], but not LTD in the cortex [76]. Overall, it is clear that close and mutually dependent interactions exist between electrical and chemical synaptic transmissions.
Regulation of neuronal GJC in the injured CNS
The cellular mechanisms underlying the regulation of neuronal GJC during neuronal injury is an emerging area of interest, and holds potential translational value for the development of novel therapeutic targets for neuroprotection. A recent study investigated GJC in several models of neuronal injury, including focal cortical ischemia in adult mice and in vitro models (including ischemia, hypoosmotic shock, and hyperactivity) [56]. In all models, neuronal GJC and/or Cx36 expression showed significant increases two hours post-injury (Figure 2c). These events coincide with the period of massive glutamate release from injured cells [77,78]. As during development [38], the injury-mediated elevation in coupling and Cx36 were prevented by inactivation of group II mGluRs, suggesting a role for these receptors in the expansion of neuronal GJC following injury [56]. However, compared to during development, GABAARs were only indirectly involved in down-regulating Cx36 expression following injury, presumably via inhibition of electrical activity. Further, the increase in Cx36 following injury involves post-transcriptional mechanisms, since Cx36 mRNA levels do not change despite increased protein levels.
Physiological roles of neuronal GJC
Studies to date suggest multiple roles for neuronal GJC during development. Observations of the developmental changes in GJC and Cx36 expression, and the use of knockdown, knock-in and mutational approaches support the involvement of neuronal GJC in synaptogenesis [48], neuronal differentiation [79], migration [80] and neural circuit formation and maturation [81,82]. It is believed that the contribution of gap junctions to these phenomena is via passage of Ca2+, metabolites and second messengers between the cells that provides coordination of metabolic and transcriptional activities in developing neurons [83]. In addition, gap junctions contribute via generation of the highly synchronized excitatory electrical activity. This network-driven activity is a hallmark of the developing brain [64] and often involves cooperation between gap junctions and chemical neurotransmitter receptors [43,81].
Cx36 knockout mice exhibit almost complete (~95%) loss of neuronal GJC [84–86]. Studies in these mice suggested that the primary role of neuronal gap junctions in the mature CNS is the modulation and/or generation of synchronized oscillatory activity. The Cx36 knockout mice demonstrate deficient neuronal synchronization, including reduced synchronization between cortical interneurons [85], reduced high-frequency [87] and β -oscillations in the hippocampus [86], and loss of synchronized activity in the inferior olivary nucleus [88], TRN [89], inferior olive [90] and cerebellum [91]. However, evidence exists that Cx36 GJC can mediate desynchronization under particular conditions [92].
A wide range of phenotypes in Cx36 knockout mice have been documented, such as deficits in circadian activity [93], motor control [84,90], rod-based vision [94,95], brain reward system [96] and some forms of behavior [31] and learning and memory [97,98]. These deficits likely result from the loss of synchronized activity in neurons in the corresponding CNS regions. However, additional mechanisms may also be active. This is particularly true for the retina, where the function of GJC presumably is in shaping the visual signal-transduction pathways; thus, the visual deficits in knockout mice likely result from the interruption in signaling rather than from desynchronization of retinal cells [72]. Some observed phenotypes may possibly result from alterations in electrical-chemical synapse interactions as Cx36 knockout affects the expression of excitatory and inhibitory postsynaptic activity in the striatum [99], neuronal responses to dopamine [99], NMDAR subunit expression [75,100] and LTP in the hippocampus and cortex [75,76].
Role of neuronal GJC in cell death
Programmed cell death is a critical process in the developing CNS that helps to establish the final number of neurons and, thus, regulates neuronal circuit formation [101]. The activity of NMDARs contributes to cell survival versus death decisions during development; NMDAR activity above or below a specific level results in neuronal cell death [102,103]. In addition, glutamate-dependent excitotoxicity (which is mostly caused by hyperactivation of NMDARs) plays critical role in neuronal death in the mature CNS following injury, including ischemic stroke, TBI and epilepsy [104,105]. Multiple studies support a role for gap junctions in cell death/survival during development, glutamate-mediated excitotoxicity and neuronal injury (discussed below). However, whether Cx36 GJC is pro-death or pro-survival for neurons remains controversial, given reports that provide support for both activities.
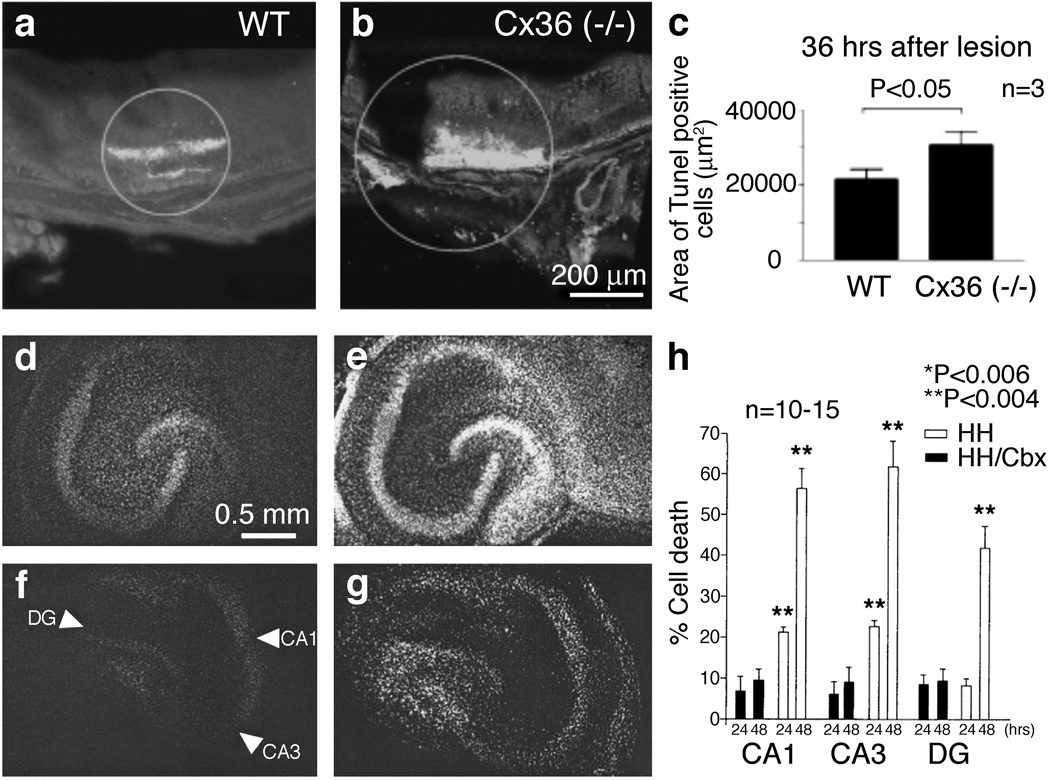
As shown previously [106], glutamate-induced neuronal death in mouse neuronal cortical cultures was augmented by blockade of gap junctions with non-selective GJC blockers. Another study specifically addressed the role of Cx36-containing gap junctions in neuronal death in the mouse retina following infrared laser photocoagulation [60]. Secondary neuronal loss was most prominent between 24 and 48 hours after lesioning and was dramatically increased by gap junction blockers (non-selective and relatively selective for Cx36) and genetic knockout of Cx36 (Figure 3a–c). Such findings suggest that Cx36 contributes to the survival and resistance against damage of retinal cells [60].
Figure 3. Is neuronal GJC pro-death or pro-survival?
(a–c) Infrared laser photocoagulation in mouse retina caused retinal trauma, resulting in substantial neuronal death in the retina of wild-type (WT) mice (a) [60]. Such neuronal death was significantly higher in Cx36 knockout (−/−) mice (b). Micrographs of retinas showing TUNEL staining (that detects apoptotic death; a,b) and quantitation (c) are shown. The analysis was done 36 hours after the laser lesioning. Because elimination of Cx36 enhances the level of neuronal death, these data suggest that neuronal GJC is pro-survival [60]. (d–h) Hypoxic-hypoglycemic insult (that models ischemia) induces substantial neuronal death that is seen in organotypic rat hippocampal slices 24 (d) and 48 (e) hours after the insult [110]. Such neuronal death is dramatically reduced by carbenoxolone (a non-specific blocker of GJC) at both 24 (f) and 48 (g) hours post-injury. Images of slices stained with propidium iodide (that detects cell death; d–g) and quantitation of cell death in three main hippocampal regions (CA1, CA3, and dentate gyrus, DG; h) are shown. HH, hypoxia-hypoglycemia; HH/Cbx, hypoxia-hypoglycemia plus carbenoxolone (Cbx, 120 µmol/L) treatment. The three main hippocampal regions are marked by arrowheads in panel f. Together with studies by other groups, demonstrating that pharmacological blockade and knockout of Cx36 GJC dramatically reduce neuronal death in models of ischemia and brain trauma [56,100,107], the data presented in d–h suggest that neuronal GJC is pro-death [110]. Adapted, with permission, from [60] (a–c) and [110] (d–h).
Other studies support the opposite conclusion: that Cx36 GJC has a critical role in promoting neuronal death. For example, as discussed above, a prolonged activation of group II mGluRs augmented the developmental increase in neuronal GJC and Cx36 expression [38]. However, it also amplified NMDAR-mediated excitotoxicity. Blockade of group II mGluRs had the exact opposite effects. This suggests that not only do group II mGluRs control the developmental increase in GJC, but via this increase they also contribute significantly to death decisions in developing neurons [38]. As also discussed, group II mGluRs control the injury-mediated boost in neuronal GJC [56]. Predictably, inactivation of group II mGluRs reduced injury-mediated neuronal death, as demonstrated with the use of several different injury models [56,107]
In additional studies, NMDAR-mediated excitotoxicity was induced in the forebrain of adult wild-type mice by systemic administration of NMDA [100]. This excitotoxicity was prevented by co-administration of mefloquine, a blocker of GJC. Mefloquine treatment also reduced ischemic neuronal death [100] and the secondary neuronal death caused by controlled cortical impact in mice, a model of TBI [107]. Essentially identical neuroprotection was provided by Cx36 knockout [100,107]. Because in those studies [100,107], mefloquine did not have any additional neuroprotection in Cx36 knockout mice, the conclusion was made that neuroprotection by mefloquine is via blockade of Cx36 GJC. Other studies support a role for neuronal GJC in NMDAR- and injury-mediated neuronal death [108–110] (Figure 3d–h).
Conflicting reports of cell death and survival roles for many non-neuronal gap junctions also have been published. The possible reasons for this controversy have been summarized [111]. These reasons include the use of various cell death models and cell types, poor specificity of gap junction blockers, as well as the presence of connexin channels/hemichannels and pannexin channels. These differences and challenges confound attempts at drawing general conclusion about gap junctions and cell death causality. It may be that the contribution of GJC to cell death (or the prevention thereof) is so highly contextual that it is essentially impossible to make sweeping generalizations regarding this phenomenon.
However, this does not diminish the potential utility of understanding the functional role of the injury-mediated increase in neuronal GJC, when considering strategies to minimize neuronal death following stroke, TBI or other insults. Given that increased GJC appears to be, on the surface, detrimental to survival of the organism following injury, an open question is: Why does it occur? One possibility is that increased GJC following injury is compensatory and provides a mode of communication between neurons at a time when chemical synaptic transmission is compromised. A second possibility is that regulatory mechanisms that elevate GJC during development are inadvertently activated by injury. A more nuanced model, that takes into account the apparently conflicting reports of the pro-death and pro-survival roles of GJC, is that the magnitude of injury is the key determinant of the pathologic outcomes of GJC. That is, whether an increase in coupling is beneficial or detrimental to the organism’s survival depends on the severity of neuronal injury. At low, sub-lethal levels of injury, the coupling may support neuronal survival by promoting the spread of pro-survival signals. However, with severe neuronal injury, gap junctions mediate the spread of pro-death signals that results in widespread neuronal demise. Therefore, the contribution of Cx36 gap junctions to cell death, documented in multiple models of brain injury, may simply be an unfortunate by-product of a generally pro-survival mechanism that evolved to cope with moderate injury.
A modified model for the mechanism of glutamate-dependent excitotoxicity
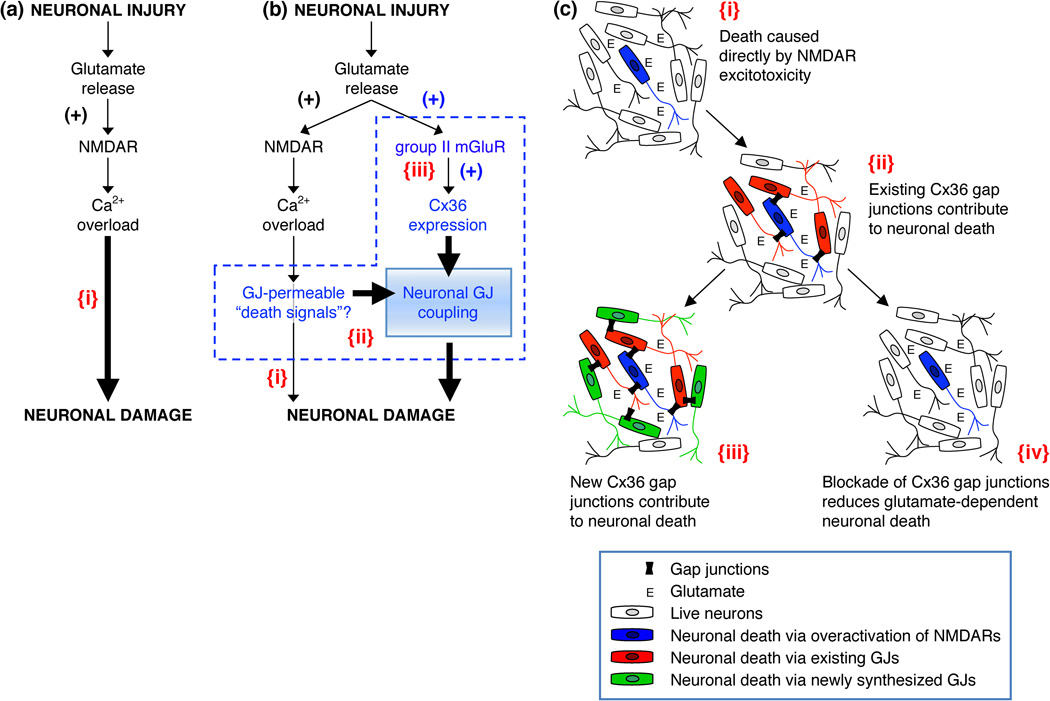
Brain injuries, such as those that occur following TBI and stroke, are characterized by two distinct areas: core and penumbra [77,78]. The core is the region directly subjected to physical impact (ie. as occurs in TBI) or anoxia (ie. during stroke) and shows almost complete loss of neural cells due to necrosis (acute cell death). The penumbra, located immediately outside the core, suffers from secondary injury and shows signs of apoptosis (delayed cell death). It has been suggested previously [104,105] that an underlying mechanism for secondary neuronal death in the penumbra is excessive release of glutamate from injured cells that causes glutamate-dependent excitotoxicity. The excitotoxic mechanisms of glutamate are well-characterized. They include hyperactivation of glutamate receptors (primarily NMDARs), massive influx of Ca2+ ions, and over-activation of multiple Ca2+-dependent signaling pathways, all potentially fatal for neurons [104,105] (Figure 4a{i}). As such, the development of NMDAR antagonists for the purposes of neuroprotection was a rational approach for limiting cell death following brain injury. However, the majority of NMDAR antagonists that have been tested in clinical trials failed to demonstrate clinical benefit [112].
Figure 4. Glutamate-dependent excitotoxicity during neuronal injury.
(a) Traditional model of the mechanisms for glutamate-dependent excitotoxicity [104,105]. (b) Alternative model of the mechanisms of glutamate-dependent excitotoxicity [56]. (c) This figure illustrates key points of the alternative model. In all figures: {i}, Neuronal death caused directly by overactivation of NMDARs; {ii}, Existing neuronal gap junctions (GJs) contribute substantially to neuronal death caused by overactivation of NMDARs [100,109]; {iii}, New neuronal gap junctions are induced by activation of group II mGluRs and also contribute to glutamate-dependent neuronal death [56]; {iv}, Pharmacological [100] or genetic [56,100] blockade of neuronal GJC reduces glutamate-dependent neuronal death. In a,b: the sign (+) indicates the increase in receptor activity or expression of Cx36. In b: a possibility is illustrated that some Ca2+-dependent molecules (or Ca2+ itself) serve as gap junction-permeable death signals (b{ii}) [111]; Ca2+ overload also directly induces neuronal death (b{i}), however, this is not the main driver of neuronal death, rather the key determinant for expansion of cell death is neuronal GJC (b{ii,iii}). Adapted, with permission, from [56] (a and b).
Based on observations of the crucial role of neuronal GJC in NMDAR- and injury-mediated neuronal death, an alternative model for mechanisms of glutamate-dependent excitotoxicity was proposed recently, centered on neuronal gap junctions (Figure 4b,c) [56]. According to this model, the hyperactivation of NMDARs per se is not the main driver of glutamate-dependent neuronal death following injury. Rather, the key determinant for expansion of cell death is the presence and extent of Cx36 GJC in the injured CNS regions. While NMDAR hyperactivity triggers neurodegeneration, in the absence of neuronal gap junctions this neuronal death is restricted to a limited number of neurons, which for various reasons may be especially sensitive to excitotoxicity (as discussed in [109]) (Figure 4b{i}, which is the same as Figure 4a{i}). However, in the presence of neuronal GJC, the expanse of neuronal death is extended to nearly all coupled neurons, expanding the breadth of NMDAR-initiated neuronal death (Figures 4b{ii}, 4c{ii}). In this respect, the background level of neuronal GJC, normally found in many mature brain regions [1], would be an important determinant for sensitivity of neurons to NMDAR-mediated excitotoxicity. In addition, the increase in Cx36 GJC, that results from activation of group II mGluRs [56], is a significant, underlying driver of expansive neuronal death (Figures 4b{iii}, 4c{iii}).
The simplest mechanistic explanation for the contribution of neuronal GJC to cell death is that it allows propagation of “pro-death” molecules between the coupled neurons (Figure 4b{ii}). For example, NMDAR-, AMPAR-, inflammation- and apoptosis-dependent signals such as Ca2+, Na+ and inositol-1,4,5-trisphosphate [111] could all serve as “death signals” to bystander cells linked to dying cells by gap junctions. A second possibility, and one that has been suggested recently for non-neuronal connexins, is via channel-independent mechanisms. According to this model, connexins may control cell death via direct or indirect regulation of transcriptional programs and apoptotic pathways (reviewed in [111]). However, these two models are by no means mutually exclusive. Clarification of the biochemical events that underlie Cx36-dependent neuronal death awaits additional experimentation (see Box 1).
Box 1. Outstanding questions.
-
! !
The Cx36 knockout mice do not appear to have obvious abnormalities in CNS structure, development, and neuronal properties [86]. Does this argue for a limited role of Cx36 in development or are there mechanisms that compensate for the loss of Cx36 GJC?
-
! !
Initial translational up-regulation of Cx36 during development can lead to persistent, transcriptional activation of the gene. What are the regulatory mechanisms and how are they coordinated?
-
! !
What are the specific molecular mechanisms that result in increased Cx36 expression following neuronal injury?
-
! !
Are the effects of Cx36 GJC on cell death dependent upon the channel function of gap junctions, or do non-channel functions contribute?
-
! !
If Cx36 gap junctions contribute to cell death in a channel-dependent way, what specific molecules or ions pass through the gap junctions to cause the spread of neuronal death following injury?
-
! !
If Cx36 gap junctions contribute to a cell death in a non-channel-dependent way, what specific death signaling pathways and molecules are involved?
-
! !
Does the influence of Cx36 GJC on cell death or survival depend upon the magnitude of the insult?
-
! !
Would blockade of Cx36 GJC in humans be effective in limiting the extent of neuronal death following injury from stroke, TBI or epilepsy?
Multiple lines of evidence also indicate participation of hemichannels, non-Cx36-containing gap junctions, and pannexin-containing channels in injury-induced neuronal death [9,58,113]. However, given the substantial neuroprotection provided by pharmacological blockade and knockout of Cx36 GJC, or the inactivation of the mechanisms controlling the injury-dependent increase in the coupling (Figure 4c{iv}) [56,100,107,109], Cx36-containing gap junctions seem to be a primary determinant of injury-mediated neuronal death.
Concluding remarks
A growing body of evidence indicates that neuronal (Cx36-containing) gap junctions are highly dynamic structures. They are regulated by and interact with chemical synapses. The quantity of neuronal GJC changes during development and following neuronal injury, reflecting their critical role in those events. Particularly, neuronal gap junctions play an important role in cell death/survival mechanisms. Studies to date support both a pro-death and pro-survival role for Cx36 GJC, which might possibly be explained by different experimental approaches; consistent with this interpretation is the possibility that Cx36 GJC may be protective at moderate levels of brain injury, but act detrimentally following more severe insult. Recent work suggests that Cx36 GJC plays a key role in glutamate-mediated cell death. Such findings potentially open-up novel approaches for neuroprotection prior to, and in the acute period following, brain injury.
Acknowledgment
Preparation of this review was supported by grants from the National Institute of Neurological Disorders and Stroke (R01NS064256 and R21NS076925) to A.B.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eugenin EA, et al. The role of gap junction channels during physiologic and pathologic conditions of the human central nervous system. J Neuroimmune Pharmacol. 2012;7:499–518. doi: 10.1007/s11481-012-9352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beyer EC, Berthoud VM. Chapter 1: The family of connexin genes. In: Harris A, Locke D, editors. Connexins: A Guide. Humana Press; 2009. pp. 3–26. [Google Scholar]
- 3.Rash JE, et al. Connexin composition in apposed gap junction hemiplaques revealed by matched double-replica freeze-fracture replica immunogold labeling. J Membr Biol. 2012;245:333–344. doi: 10.1007/s00232-012-9454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giaume C, Theis M. Pharmacological and genetic approaches to study connexin-mediated channels in glial cells of the central nervous system. Brain Res Rev. 2010;63:160–176. doi: 10.1016/j.brainresrev.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Belluardo N, et al. Expression of connexin36 in the adult and developing rat brain. Brain Res. 2000;865:121–138. doi: 10.1016/s0006-8993(00)02300-3. [DOI] [PubMed] [Google Scholar]
- 6.Venance L, et al. Connexin expression in electrically coupled postnatal rat brain neurons. Proc Natl Acad Sci U S A. 2000;97:10260–10265. doi: 10.1073/pnas.160037097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saez JC, et al. Cell membrane permeabilization via connexin hemichannels in living and dying cells. Exp Cell Res. 2010;316:2377–2389. doi: 10.1016/j.yexcr.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 8.Schock SC, et al. ATP release by way of connexin 36 hemichannels mediates ischemic tolerance in vitro. Biochem Biophys Res Commun. 2008;368:138–144. doi: 10.1016/j.bbrc.2008.01.054. [DOI] [PubMed] [Google Scholar]
- 9.Bargiotas P, et al. Pannexins in ischemia-induced neurodegeneration. Proc Natl Acad Sci U S A. 2011;108:20772–20777. doi: 10.1073/pnas.1018262108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacVicar BA, Thompson RJ. Non-junction functions of pannexin-1 channels. Trends Neurosci. 2010;33:93–102. doi: 10.1016/j.tins.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Thompson RJ, et al. Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science. 2008;322:1555–1559. doi: 10.1126/science.1165209. [DOI] [PubMed] [Google Scholar]
- 12.Sosinsky GE, et al. Pannexin channels are not gap junction hemichannels. Channels. 2011;5:193–197. doi: 10.4161/chan.5.3.15765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Condorelli DF, et al. Cloning of a new gap junction gene (Cx36) highly expressed in mammalian brain neurons. Eur J Neurosci. 1998;10:1202–1208. doi: 10.1046/j.1460-9568.1998.00163.x. [DOI] [PubMed] [Google Scholar]
- 14.Sohl G, et al. The murine gap junction gene connexin36 is highly expressed in mouse retina and regulated during brain development. FEBS Lett. 1998;428:27–31. doi: 10.1016/s0014-5793(98)00479-7. [DOI] [PubMed] [Google Scholar]
- 15.Al-Ubaidi MR, et al. Functional properties, developmental regulation, and chromosomal localization of murine connexin36, a gap-junctional protein expressed preferentially in retina and brain. J Neurosci Res. 2000;59:813–826. doi: 10.1002/(SICI)1097-4547(20000315)59:6<813::AID-JNR14>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 16.Srinivas M, et al. Functional properties of channels formed by the neuronal gap junction protein connexin36. J Neurosci. 1999;19:9848–9855. doi: 10.1523/JNEUROSCI.19-22-09848.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamzei-Sichani F, et al. Gap junctions on hippocampal mossy fiber axons demonstrated by thin-section electron microscopy and freeze fracture replica immunogold labeling. Proc Natl Acad Sci U S A. 2007;104:12548–12553. doi: 10.1073/pnas.0705281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flores CE, et al. Trafficking of gap junction channels at a vertebrate electrical synapse in vivo. Proc Natl Acad Sci U S A. 2012;109:E573–E582. doi: 10.1073/pnas.1121557109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, et al. Connexin45-containing neuronal gap junctions in rodent retina also contain connexin36 in both apposing hemiplaques, forming bihomotypic gap junctions, with scaffolding contributed by zonula occludens-1. J Neurosci. 2008;28:9769–9789. doi: 10.1523/JNEUROSCI.2137-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frank M, et al. Neuronal connexin-36 can functionally replace connexin-45 in mouse retina but not in the developing heart. J Cell Sci. 2010;123:3605–3615. doi: 10.1242/jcs.068668. [DOI] [PubMed] [Google Scholar]
- 21.Nagy JI. Evidence for connexin36 localization at hippocampal mossy fiber terminals suggesting mixed chemical/electrical transmission by granule cells. Brain Res. 2012 doi: 10.1016/j.brainres.2012.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoge GJ, et al. The extent and strength of electrical coupling between inferior olivary neurons is heterogeneous. J Neurophysiol. 2011;105:1089–1101. doi: 10.1152/jn.00789.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamzei-Sichani F, et al. Mixed electrical-chemical synapses in adult rat hippocampus are primarily glutamatergic and coupled by connexin-36. Frontiers in neuroanatomy. 2012;6:13. doi: 10.3389/fnana.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puller C, et al. ZO-1 and the spatial organization of gap junctions and glutamate receptors in the outer plexiform layer of the mammalian retina. J Neurosci. 2009;29:6266–6275. doi: 10.1523/JNEUROSCI.5867-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, et al. The effector and scaffolding proteins AF6 and MUPP1 interact with connexin36 and localize at gap junctions that form electrical synapses in rodent brain. Eur J Neurosci. 2012;35:166–181. doi: 10.1111/j.1460-9568.2011.07947.x. [DOI] [PubMed] [Google Scholar]
- 26.Ciolofan C, et al. Association of connexin36 and zonula occludens-1 with zonula occludens-2 and the transcription factor zonula occludens-1-associated nucleic acid-binding protein at neuronal gap junctions in rodent retina. Neuroscience. 2006;140:433–451. doi: 10.1016/j.neuroscience.2006.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, et al. Direct association of connexin36 with zonula occludens-2 and zonula occludens-3. Neurochem Int. 2009;54:393–402. doi: 10.1016/j.neuint.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Helbig I, et al. In vivo evidence for the involvement of the carboxy terminal domain in assembling connexin 36 at the electrical synapse. Mol Cell Neurosci. 2010;45:47–58. doi: 10.1016/j.mcn.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flores CE, et al. Variability of distribution of Ca(2+)/calmodulin-dependent kinase II at mixed synapses on the mauthner cell: colocalization and association with connexin 35. J Neurosci. 2010;30:9488–9499. doi: 10.1523/JNEUROSCI.4466-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alev C, et al. The neuronal connexin36 interacts with and is phosphorylated by CaMKII in a way similar to CaMKII interaction with glutamate receptors. Proc Natl Acad Sci U S A. 2008;105:20964–20969. doi: 10.1073/pnas.0805408105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zlomuzica A, et al. Behavioral alterations and changes in Ca/calmodulin kinase II levels in the striatum of connexin36 deficient mice. Behav Brain Res. 2012;226:293–300. doi: 10.1016/j.bbr.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 32.Kothmann WW, et al. Connexin 35/36 is phosphorylated at regulatory sites in the retina. Vis Neurosci. 2007;24:363–375. doi: 10.1017/S095252380707037X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel LS, et al. Regulation of gap junction coupling through the neuronal connexin Cx35 by nitric oxide and cGMP. Cell Commun Adhes. 2006;13:41–54. doi: 10.1080/15419060600631474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urschel S, et al. Protein kinase A-mediated phosphorylation of connexin36 in mouse retina results in decreased gap junctional communication between AII amacrine cells. J Biol Chem. 2006;281:33163–33171. doi: 10.1074/jbc.M606396200. [DOI] [PubMed] [Google Scholar]
- 35.Kothmann WW, et al. Dopamine-stimulated dephosphorylation of connexin 36 mediates AII amacrine cell uncoupling. J Neurosci. 2009;29:14903–14911. doi: 10.1523/JNEUROSCI.3436-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herve JC, et al. Gap junctional channels are parts of multiprotein complexes. Biochim Biophys Acta. 2012;1818:1844–1865. doi: 10.1016/j.bbamem.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 37.Arumugam H, et al. NMDA receptors regulate developmental gap junction uncoupling via CREB signaling. Nat Neurosci. 2005;8:1720–1726. doi: 10.1038/nn1588. [DOI] [PubMed] [Google Scholar]
- 38.Park W-M, et al. Interplay of chemical neurotransmitters regulates developmental increase in electrical synapses. J Neurosci. 2011;31:5909–5920. doi: 10.1523/JNEUROSCI.6787-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mentis GZ, et al. Increased incidence of gap junctional coupling between spinal motoneurones following transient blockade of NMDA receptors in neonatal rats. J Physiol. 2002;544:757–764. doi: 10.1113/jphysiol.2002.028159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pastor AM, et al. Increased electrotonic coupling in spinal motoneurons after transient botulinum neurotoxin paralysis in the neonatal rat. J Neurophysiol. 2003;89:793–805. doi: 10.1152/jn.00498.2002. [DOI] [PubMed] [Google Scholar]
- 41.Connors BW, et al. Coupling between neurons of the developing rat neocortex. J Neurosci. 1983;3:773–782. doi: 10.1523/JNEUROSCI.03-04-00773.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyer AH, et al. In vivo labeling of parvalbumin-positive interneurons and analysis of electrical coupling in identified neurons. J Neurosci. 2002;22:7055–7064. doi: 10.1523/JNEUROSCI.22-16-07055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strata F, et al. A pacemaker current in dye-coupled hilar interneurons contributes to the generation of giant GABAergic potentials in developing hippocampus. J Neurosci. 1997;17:1435–1446. doi: 10.1523/JNEUROSCI.17-04-01435.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Venance L, et al. Electrical and chemical transmission between striatal GABAergic output neurones in rat brain slices. J Physiol. 2004;559:215–230. doi: 10.1113/jphysiol.2004.065672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee SC, et al. Electrical and chemical synapses between relay neurons in developing thalamus. J Physiol. 2010;588:2403–2415. doi: 10.1113/jphysiol.2010.187096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mazza E, et al. Anatomical and electrotonic coupling in developing genioglossal motoneurons of the rat. Brain Res. 1992;598:127–137. doi: 10.1016/0006-8993(92)90176-a. [DOI] [PubMed] [Google Scholar]
- 47.Alvarez-Maubecin V, et al. Functional coupling between neurons and glia. J Neurosci. 2000;20:4091–4098. doi: 10.1523/JNEUROSCI.20-11-04091.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Personius KE, et al. Reduced gap junctional coupling leads to uncorrelated motor neuron firing and precocious neuromuscular synapse elimination. Proc Natl Acad Sci U S A. 2007;104:11808–11813. doi: 10.1073/pnas.0703357104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu YC, et al. Preferential electrical coupling regulates neocortical lineage-dependent microcircuit assembly. Nature. 2012;486:113–117. doi: 10.1038/nature10958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Connors BW. Chapter 6: Electrical signaling with neuronal gap junctions. In: Harris A, Locke D, editors. Connexins: A Guide. Humana Press; 2009. pp. 143–164. [Google Scholar]
- 51.Hestrin S, Galarreta M. Electrical synapses define networks of neocortical GABAergic neurons. Trends Neurosci. 2005;28:304–309. doi: 10.1016/j.tins.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 52.Parker PR, et al. Stability of electrical coupling despite massive developmental changes of intrinsic neuronal physiology. J Neurosci. 2009;29:9761–9770. doi: 10.1523/JNEUROSCI.4568-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Curti S, et al. Synergy between electrical coupling and membrane properties promotes strong synchronization of neurons of the mesencephalic trigeminal nucleus. J Neurosci. 2012;32:4341–4359. doi: 10.1523/JNEUROSCI.6216-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Pina-Benabou MH, et al. Blockade of gap junctions in vivo provides neuroprotection after perinatal global ischemia. Stroke. 2005;36:2232–2237. doi: 10.1161/01.STR.0000182239.75969.d8. [DOI] [PubMed] [Google Scholar]
- 55.Oguro K, et al. Global ischemia-induced increases in the gap junctional proteins connexin 32 (Cx32) and Cx36 in hippocampus and enhanced vulnerability of Cx32 knock-out mice. J Neurosci. 2001;21:7534–7542. doi: 10.1523/JNEUROSCI.21-19-07534.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y, et al. Neuronal gap junction coupling is regulated by glutamate and plays critical role in cell death during neuronal injury. J Neurosci. 2012;32:713–725. doi: 10.1523/JNEUROSCI.3872-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang Q, et al. Nerve injury induces gap junctional coupling among axotomized adult motor neurons. J Neurosci. 2000;20:674–684. doi: 10.1523/JNEUROSCI.20-02-00674.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frantseva MV, et al. Specific gap junctions enhance the neuronal vulnerability to brain traumatic injury. J Neurosci. 2002;22:644–653. doi: 10.1523/JNEUROSCI.22-03-00644.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohsumi A, et al. Alteration of gap junction proteins (connexins) following lateral fluid percussion injury in rats. Acta Neurochir Suppl. 2006;96:148–150. doi: 10.1007/3-211-30714-1_33. [DOI] [PubMed] [Google Scholar]
- 60.Striedinger K, et al. Loss of connexin36 increases retinal cell vulnerability to secondary cell loss. Eur J Neurosci. 2005;22:605–616. doi: 10.1111/j.1460-9568.2005.04228.x. [DOI] [PubMed] [Google Scholar]
- 61.Gajda Z, et al. Involvement of gap junctions in the manifestation and control of the duration of seizures in rats in vivo. Epilepsia. 2003;44:1596–1600. doi: 10.1111/j.0013-9580.2003.25803.x. [DOI] [PubMed] [Google Scholar]
- 62.Perez Velazquez JL, et al. Modulation of gap junctional mechanisms during calcium-free induced field burst activity: a possible role for electrotonic coupling in epileptogenesis. J Neurosci. 1994;14:4308–4317. doi: 10.1523/JNEUROSCI.14-07-04308.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garrett FG, Durham PL. Differential expression of connexins in trigeminal ganglion neurons and satellite glial cells in response to chronic or acute joint inflammation. Neuron Glia Biol. 2008;4:295–306. doi: 10.1017/S1740925X09990093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ben-Ari Y. Developing networks play a similar melody. Trends Neurosci. 2001;24:353–360. doi: 10.1016/s0166-2236(00)01813-0. [DOI] [PubMed] [Google Scholar]
- 65.Song J-H, et al. Regulation of connexin 36 expression during development. Neurosci Lett. 2012;513:17–19. doi: 10.1016/j.neulet.2012.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kandler K, Katz LC. Relationship between dye coupling and spontaneous activity in developing ferret visual cortex. Dev Neurosci. 1998;20:59–64. doi: 10.1159/000017299. [DOI] [PubMed] [Google Scholar]
- 67.Roerig B, Feller MB. Neurotransmitters and gap junctions in developing neural circuits. Brain Res Brain Res Rev. 2000;32:86–114. doi: 10.1016/s0165-0173(99)00069-7. [DOI] [PubMed] [Google Scholar]
- 68.Zsiros V, Maccaferri G. Noradrenergic modulation of electrical coupling in GABAergic networks of the hippocampus. J Neurosci. 2008;28:1804–1815. doi: 10.1523/JNEUROSCI.4616-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O'Donnell P, Grace AA. Cortical afferents modulate striatal gap junction permeability via nitric oxide. Neuroscience. 1997;76:1–5. doi: 10.1016/s0306-4522(96)00433-2. [DOI] [PubMed] [Google Scholar]
- 70.Yang QZ, Hatton GI. Histamine H1-receptor modulation of inter-neuronal coupling among vasopressinergic neurons depends on nitric oxide synthase activation. Brain Res. 2002;955:115–122. doi: 10.1016/s0006-8993(02)03374-7. [DOI] [PubMed] [Google Scholar]
- 71.O'Donnell P, Grace AA. Dopaminergic modulation of dye coupling between neurons in the core and shell regions of the nucleus accumbens. J Neurosci. 1993;13:3456–3471. doi: 10.1523/JNEUROSCI.13-08-03456.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bloomfield SA, Volgyi B. The diverse functional roles and regulation of neuronal gap junctions in the retina. Nat Rev Neurosci. 2009;10:495–506. doi: 10.1038/nrn2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cachope R, et al. Potentiation of electrical and chemical synaptic transmission mediated by endocannabinoids. Neuron. 2007;56:1034–1047. doi: 10.1016/j.neuron.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Landisman CE, Connors BW. Long-term modulation of electrical synapses in the mammalian thalamus. Science. 2005;310:1809–1813. doi: 10.1126/science.1114655. [DOI] [PubMed] [Google Scholar]
- 75.Wang Y, Belousov AB. Deletion of neuronal gap junction protein connexin 36 impairs hippocampal LTP. Neurosci Lett. 2011;502:30–32. doi: 10.1016/j.neulet.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Postma F, et al. Electrical synapses formed by connexin36 regulate inhibition- and experience-dependent plasticity. Proc Natl Acad Sci U S A. 2011;108:13770–13775. doi: 10.1073/pnas.1100166108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guyot LL, et al. Real-time measurement of glutamate release from the ischemic penumbra of the rat cerebral cortex using a focal middle cerebral artery occlusion model. Neurosci Lett. 2001;299:37–40. doi: 10.1016/s0304-3940(01)01510-5. [DOI] [PubMed] [Google Scholar]
- 78.Stoffel M, et al. Release of excitatory amino acids in the penumbra of a focal cortical necrosis. J Neurotrauma. 2002;19:467–477. doi: 10.1089/08977150252932415. [DOI] [PubMed] [Google Scholar]
- 79.Hartfield EM, et al. Connexin 36 expression regulates neuronal differentiation from neural progenitor cells. PLoS One. 2011;6:e14746. doi: 10.1371/journal.pone.0014746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cina C, et al. Expression of connexins in embryonic mouse neocortical development. J Comp Neurol. 2007;504:298–313. doi: 10.1002/cne.21426. [DOI] [PubMed] [Google Scholar]
- 81.Hanganu IL, et al. Cellular mechanisms of subplate-driven and cholinergic input-dependent network activity in the neonatal rat somatosensory cortex. Cereb Cortex. 2009;19:89–105. doi: 10.1093/cercor/bhn061. [DOI] [PubMed] [Google Scholar]
- 82.Maher BJ, et al. Experience-dependent maturation of the glomerular microcircuit. Proc Natl Acad Sci U S A. 2009;106:16865–16870. doi: 10.1073/pnas.0808946106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kandler K, Katz LC. Coordination of neuronal activity in developing visual cortex by gap junction-mediated biochemical communication. J Neurosci. 1998;18:1419–1427. doi: 10.1523/JNEUROSCI.18-04-01419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Zeeuw CI, et al. Deformation of network connectivity in the inferior olive of connexin 36-deficient mice is compensated by morphological and electrophysiological changes at the single neuron level. J Neurosci. 2003;23:4700–4711. doi: 10.1523/JNEUROSCI.23-11-04700.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Deans MR, et al. Synchronous activity of inhibitory networks in neocortex requires electrical synapses containing connexin36. Neuron. 2001;31:477–485. doi: 10.1016/s0896-6273(01)00373-7. [DOI] [PubMed] [Google Scholar]
- 86.Hormuzdi SG, et al. Impaired electrical signaling disrupts gamma frequency oscillations in connexin 36-deficient mice. Neuron. 2001;31:487–495. doi: 10.1016/s0896-6273(01)00387-7. [DOI] [PubMed] [Google Scholar]
- 87.Maier N, et al. Reduction of high-frequency network oscillations (ripples) and pathological network discharges in hippocampal slices from connexin 36-deficient mice. J Physiol. 2002;541:521–528. doi: 10.1113/jphysiol.2002.017624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Long MA, et al. Rhythmicity without synchrony in the electrically uncoupled inferior olive. J Neurosci. 2002;22:10898–10905. doi: 10.1523/JNEUROSCI.22-24-10898.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Landisman CE, et al. Electrical synapses in the thalamic reticular nucleus. J Neurosci. 2002;22:1002–1009. doi: 10.1523/JNEUROSCI.22-03-01002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Placantonakis DG, et al. Continuous electrical oscillations emerge from a coupled network: a study of the inferior olive using lentiviral knockdown of connexin36. J Neurosci. 2006;26:5008–5016. doi: 10.1523/JNEUROSCI.0146-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marshall SP, et al. Altered olivocerebellar activity patterns in the connexin36 knockout mouse. Cerebellum. 2007;6:287–299. doi: 10.1080/14734220601100801. [DOI] [PubMed] [Google Scholar]
- 92.Vervaeke K, et al. Rapid desynchronization of an electrically coupled interneuron network with sparse excitatory synaptic input. Neuron. 2010;67:435–451. doi: 10.1016/j.neuron.2010.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Long MA, et al. Electrical synapses coordinate activity in the suprachiasmatic nucleus. Nat Neurosci. 2005;8:61–66. doi: 10.1038/nn1361. [DOI] [PubMed] [Google Scholar]
- 94.Deans MR, et al. Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron. 2002;36:703–712. doi: 10.1016/s0896-6273(02)01046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guldenagel M, et al. Visual transmission deficits in mice with targeted disruption of the gap junction gene connexin36. J Neurosci. 2001;21:6036–6044. doi: 10.1523/JNEUROSCI.21-16-06036.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lassen MB, et al. Brain stimulation reward is integrated by a network of electrically coupled GABA neurons. Brain Res. 2007;1156:46–58. doi: 10.1016/j.brainres.2007.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Allen K, et al. Gap junctions between interneurons are required for normal spatial coding in the hippocampus and short-term spatial memory. J Neurosci. 2011;31:6542–6552. doi: 10.1523/JNEUROSCI.6512-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Frisch C, et al. Stimulus complexity dependent memory impairment and changes in motor performance after deletion of the neuronal gap junction protein connexin36 in mice. Behav Brain Res. 2005;157:177–185. doi: 10.1016/j.bbr.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 99.Cummings DM, et al. Neuronal coupling via connexin36 contributes to spontaneous synaptic currents of striatal medium-sized spiny neurons. J Neurosci Res. 2008;86:2147–2158. doi: 10.1002/jnr.21674. [DOI] [PubMed] [Google Scholar]
- 100.Wang Y, et al. Neuronal gap junctions are required for NMDA receptor-mediated excitotoxicity: implications in ischemic stroke. J Neurophysiol. 2010;104:3551–3556. doi: 10.1152/jn.00656.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nijhawan D, et al. Apoptosis in neural development and disease. Annu Rev Neurosci. 2000;23:73–87. doi: 10.1146/annurev.neuro.23.1.73. [DOI] [PubMed] [Google Scholar]
- 102.Adams SM, et al. Pronounced cell death in the absence of NMDA receptors in the developing somatosensory thalamus. J Neurosci. 2004;24:9441–9450. doi: 10.1523/JNEUROSCI.3290-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Johnston MV, et al. Mechanisms of hypoxic neurodegeneration in the developing brain. Neuroscientist. 2002;8:212–220. doi: 10.1177/1073858402008003007. [DOI] [PubMed] [Google Scholar]
- 104.Arundine M, Tymianski M. Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell Mol Life Sci. 2004;61:657–668. doi: 10.1007/s00018-003-3319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hazell AS. Excitotoxic mechanisms in stroke: an update of concepts and treatment strategies. Neurochem Int. 2007;50:941–953. doi: 10.1016/j.neuint.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 106.Ozog MA, et al. Blocked gap junctional coupling increases glutamate-induced neurotoxicity in neuron-astrocyte co-cultures. J Neuropathol Exp Neurol. 2002;61:132–141. doi: 10.1093/jnen/61.2.132. [DOI] [PubMed] [Google Scholar]
- 107.Belousov AB, et al. Neuronal gap junctions play a role in the secondary neuronal death following controlled cortical impact. Neurosci Lett. 2012;524:16–19. doi: 10.1016/j.neulet.2012.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cusato K, et al. Gap junctions mediate bystander cell death in developing retina. J Neurosci. 2003;23:6413–6422. doi: 10.1523/JNEUROSCI.23-16-06413.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.de Rivero Vaccari JC, et al. Gap junctions are required for NMDA receptor-dependent cell death in developing neurons. J Neurophysiol. 2007;98:2878–2886. doi: 10.1152/jn.00362.2007. [DOI] [PubMed] [Google Scholar]
- 110.Frantseva MV, et al. Ischemia-induced brain damage depends on specific gap-junctional coupling. J Cereb Blood Flow Metab. 2002;22:453–462. doi: 10.1097/00004647-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 111.Decrock E, et al. Connexin-related signaling in cell death: to live or let die? Cell Death Differ. 2009;16:524–536. doi: 10.1038/cdd.2008.196. [DOI] [PubMed] [Google Scholar]
- 112.Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002;1:383–386. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 113.Davidson JO, et al. Connexin hemichannel blockade improves outcomes in a model of fetal ischemia. Ann Neurol. 2012;71:121–132. doi: 10.1002/ana.22654. [DOI] [PubMed] [Google Scholar]
- 114.Kreuzberg MM, et al. Expression of connexin30.2 in interneurons of the central nervous system in the mouse. Mol Cell Neurosci. 2008;37:119–134. doi: 10.1016/j.mcn.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 115.Dere E, et al. Connexin31.1 deficiency in the mouse impairs object memory and modulates open-field exploration, acetylcholine esterase levels in the striatum, and cAMP response element-binding protein levels in the striatum and piriform cortex. Neuroscience. 2008;153:396–405. doi: 10.1016/j.neuroscience.2008.01.077. [DOI] [PubMed] [Google Scholar]