Abstract
Infants are exposed to the endocrine disruptor bisphenol A (BPA) through breast milk and baby formula. Detoxication by conjugation of BPA may be limited in infants. We demonstrate BPA exposure in 11 neonates and 1 young infant, but find no evidence of a low capacity for BPA conjugation.
Keywords: Neonate, Biomarker, Endocrine Disruptor, Environment, Exposure
An estimated 5-6 billion pounds of the hormonally active compound Bisphenol A (BPA) are produced globally per year.1 Human exposure is virtually universal, with over 90% of the United States population over age 5 years having detectable urinary concentrations of BPA.2 Human health concerns arise from the estrogen mimetic properties of BPA that can confer a variety of health impacts across the lifespan. Prior analyses have demonstrated that BPA exposure can occur in neonates via maternal sources (including breast milk) as well as leaching from packaging of liquid formula or polycarbonate baby bottles.3, 4
BPA is detoxicated to its inactive form primarily via glucuronidation and rapidly excreted in urine (t1/2 < 6 hours).5 Glucuronidation is limited in newborns relative to older children and adults, leading to concern that significant quantities of free BPA (the toxicologically active compound) may exist in the neonate and have deleterious long term developmental effects.6-8 Thus, the balance of free BPA and BPA-glucuronide is critical for determining exposure risk in neonates.
Data on specific BPA and BPA-glucuronide urinary concentrations in newborns and young children are sparse. Furthermore, progress in BPA quantification has been impeded by sample contamination and the lack of specific and sensitive quantitative methods that can independently measure free BPA and BPA-glucuronide at environmental levels of exposure.9 We have examined the quantitative levels of urinary free BPA and BPA-glucuronide in a population of healthy newborns using a highly sensitive high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) method that greatly reduces exogenous sources of contamination.10
Methods
In January and February 2012, mothers and healthy, full-term neonates (≥ 37 0/7 weeks gestation) were recruited from the Newborn Nursery at the Johns Hopkins Hospital. Recruitment and follow up protocols were approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board, and participant informed consent was obtained. Newborns were excluded if they were large or small size for gestational age, were noted to have intrauterine growth restriction, had an APGAR score of less than 5 at 5 minutes of age, had delayed voiding or stooling (occurring greater than 24 hours after birth), had blood incompatibility (ABO or Rh) with their mother, were admitted to the Neonatal Intensive Care Unit (NICU) for management of hyperbilirubinemia, or had other risk factors for hyperbilirubinemia (i.e., cephalohematoma, polycythemia). Neonates born to mothers with documented tobacco use in pregnancy, a positive urine toxicology screen (for cocaine, marijuana, heroin, or methadone) at delivery, and/or anti-epileptic drug use in pregnancy were also excluded. Infants with hyperbilirubinemia not requiring a NICU admission were included. We further limited recruitment to infants who were to receive pediatric primary care at the Johns Hopkins’ Harriet Lane Primary Care Clinic.
Urine samples were collected using BPA-free pediatric urine collection bags (U-Bag, Hollister, Inc. Libertyville, IL) during the neonates’ regular well-child care visits (occurring at or greater than one week of age). Immediately upon collection, each urine sample was transported to the lab on ice, transferred to a precleaned glass vial, and stored at −80 °C until analysis. Samples were analyzed for free BPA and BPA-glucuronide using HPLC-MS/MS according to a modified previously published method.10 Free BPA and BPA-glucuronide were derivatized with dansyl chloride and measured directly, with d6-BPA and d6-BPA-glucuronide as internal standards, eliminating the need for enzymatic hydrolysis and extraction steps prior to sample analysis. The limit of detection was 0.02 ng/mL and the limit of quantification was 0.1 ng/mL.
Results
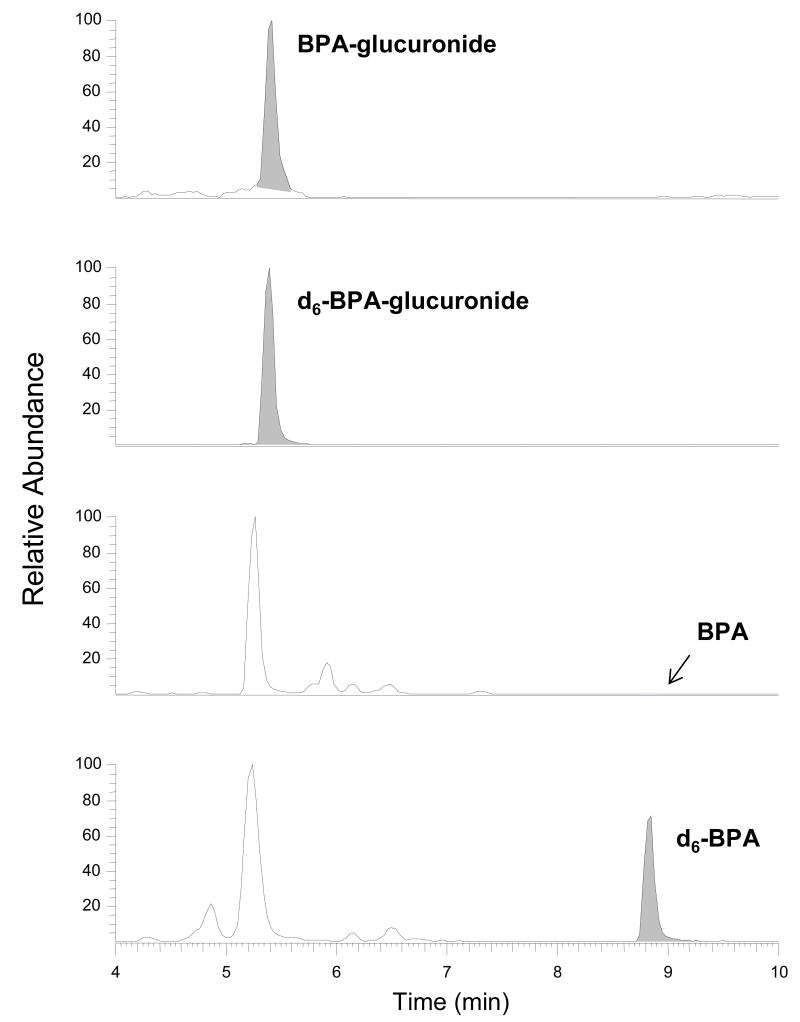
A total of 12 mothers and their babies were enrolled into the study. The median age at the time of urine collection was 17 days (Table). The average concentration of BPA-glucuronide, as measured in all of the duplicate urine samples, was 0.87 ± 0.51 ng/mL (median: 0.66 ng/mL). Free BPA was not found in any of the urine samples with the exception of one sample (subject 6) whose replicate sample was a non-detect (Figure).
Table 1.
Urinary Concentrations of Free BPA and BPA-glucuronide for 12 Infants
| Free BPA (ng/mL) |
BPA-Glucuronide (ng/mL) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Subject | Age (days) |
Sex | Feeding Type1 | Formula Type |
Replicate 1 |
Replicate 2 |
Replicate 1 |
Replicate 2 |
| 1 | 7 | M | Formula | Liquid | <0.1 | <0.1 | 0.65 | 0.75 |
| 2 | 9 | F | Formula | Liquid | <0.1 | <0.1 | 0.48 | 0.42 |
| 3 | 11 | F | Breast Milk | - | <0.1 | <0.1 | 0.54 | 0.54 |
| 4 | 13 | F | Formula | Liquid | <0.1 | <0.1 | 0.67 | 0.65 |
| 5 | 14 | M | Formula | Liquid | <0.1 | <0.1 | 0.55 | 0.51 |
| 6 | 16 | M | Formula + Breast Milk | Powder | 0.85 | <0.1 | 2.21 | 1.80 |
| 7 | 17 | F | Formula | Powder | <0.1 | <0.1 | 1.39 | 1.27 |
| 8 | 18 | M | Formula | Powder | <0.1 | <0.1 | 1.24 | 0.85 |
| 9 | 21 | M | Breast Milk | - | <0.1 | <0.1 | 0.26 | 0.37 |
| 10 | 22 | M | Formula | Powder | <0.1 | <0.1 | 0.93 | 1.16 |
| 11 | 25 | F | Formula2 + Breast Milk | - | <0.1 | <0.1 | 0.35 | 0.39 |
| 12 | 44 | M | Formula + Breast Milk | Powder | <0.1 | <0.1 | 1.63 | 1.23 |
On the day of sample collection.
Formula type not recorded
Figure.
Representative HPLC-MS/MS Chromatogram of Neonatal Urine Sample. Peaks Representing Bisphenol A (BPA) and BPA-Glucuronide Were Identified and Quantitated by the Addition of Internal Standards (d6-BPA and d6-BPA-glucuronide) into the Urine Samples.
Questionnaire data revealed that 10 of 12 newborns had some formula intake served in plastic bottles; 5 consumed formula made from powder and 4 drank liquid or “ready to feed” formula, which requires no addition of water.
Discussion
A critical issue for the assessment of health risks from BPA exposure is the balance of free BPA and its glucuronide conjugate. The presumptive estrogenic effects of BPA are only mediated by the availability of free BPA, with BPA-glucuronide viewed as a biologically inactive derivative. This study explored the potential problem of free BPA exposure in newborns under the age of 6 weeks. Decreased hepatic glucuronidation has been documented in full term and premature neonates relative to older children and adults6 and is manifested clinically by hyperbilirubinemia in the immediate newborn period.
In a previous study, free BPA was detected in 92% of urines from 42 premature infants in a Neonatal Intensive Care Unit (NICU), suggesting a period of reduced BPA metabolic capacity at birth.11 Thus, we hypothesized that free BPA would be present in the urine of most, if not all infants in our study. Free BPA concentrations were also quantified in 3% of 45 full term infants in Germany, but because median exposure levels in the population were below the method limit of quantification (0.45 μg/L), it is not possible to determine whether infant physiology was responsible for the low detection frequency of free BPA.12 Besides the German study, we know of no other published BPA biomarker data for full term healthy infants.
The detection of BPA-glucuronide in all infants demonstrates universal exposure to BPA in our study population. We were surprised to determine that BPA-glucuronide was the only detectable BPA compound in the urine of these newborns. These data fundamentally challenge our prior assumptions of the toxicology of this environmental contaminant. Further research is needed to understand whether the absence of detectable concentrations of free BPA in our study population reflects early development of one or more enzyme isoforms responsible for the formation of BPA-glucuronide, or whether at very low levels of exposure to BPA, enzyme activity in neonatal tissues is sufficient to quickly inactivate BPA.
Acknowledgments
We are grateful to the late Alison S. Geyh, PhD, for her instrumental support of this project and her contributions to study concept and design. We would like to thank Pamela Donohue, ScD (Department of Pediatrics, Johns Hopkins University School of Medicine), for her contribution to study concept and design. We also thank the Pediatric Nurse Practitioners in the Full Term Nursery at the Johns Hopkins Hospital for screening patients for eligibility.
Supported by the Johns Hopkins Center for a Livable Future and the National Institutes of Health (P01 ES006052, P30 ES003819, Contract N01-CO-12400, and training grant T32 ES007141). Neither sponsor had a role in the study design, collection, analysis, or interpretation of data, the writing of the report or the decision to submit the paper for publication. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the United States Government. The authors declare no conflicts of interest.
Abbreviations
- BPA
bisphenol A
- HPLC-MS/MS
high performance liquid chromatography-tandem mass spectrometry
- NICU
Neonatal Intensive Care Unit
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.U.S. Centers for Disease Control and Prevention. Department of Health and Human Services [Accessed July 31, 2012];Fourth National Report on Human Exposure to Environmental Chemicals. 2009 Available from: http://www.cdc.gov/exposurereport/pdf/FourthReport.pdf.
- 2.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Toxicology Program (Center for the Evaluation of Risks to Human Reproduction) NTP-CERHR monograph on the potential human reproductive and developmental effects of bisphenol A. NIH; 2008. Publication No. 08 – 5994. [Google Scholar]
- 4.Ackerman LK, Noonan GO, Heiserman WM, Roach JA, Limm W, Begley TH. Determination of bisphenol A in U.S. infant formulas: updated methods and concentrations. J Agric Food Chem. 2010;58:2307–2313. doi: 10.1021/jf903959u. [DOI] [PubMed] [Google Scholar]
- 5.Volkel W, Colnot T, Csanady GA, Filser JG, Dekant W. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chem Res Toxicol. 2002;15:1281–1287. doi: 10.1021/tx025548t. [DOI] [PubMed] [Google Scholar]
- 6.Alcorn J, McNamara PJ. Pharmacokinetics in the newborn. Adv Drug Deliv Rev. 2003;55:667–686. doi: 10.1016/s0169-409x(03)00030-9. [DOI] [PubMed] [Google Scholar]
- 7.Edginton AN, Ritter L. Predicting plasma concentrations of bisphenol A in children younger than 2 years of age after typical feeding schedules, using a physiologically based toxicokinetic model. Environ Health Perspect. 2009;117:645–652. doi: 10.1289/ehp.0800073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perera F, Vishnevetsky J, Herbstman JB, Calafat AM, Xiong W, Rauh V, et al. Prenatal bisphenol a exposure and child behavior in an inner-city cohort. Environ Health Perspect. 2012;120:1190–1194. doi: 10.1289/ehp.1104492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dekant W, Volkel W. Human exposure to bisphenol A by biomonitoring: methods, results and assessment of environmental exposures. Toxicol Appl Pharmacol. 2008;228:114–134. doi: 10.1016/j.taap.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Fox SD, Falk RT, Veenstra TD, Issaq HJ. Quantitation of free and total bisphenol A in human urine using liquid chromatography-tandem mass spectrometry. J Sep Sci. 2011;34:1268–1274. doi: 10.1002/jssc.201100087. [DOI] [PubMed] [Google Scholar]
- 11.Calafat AM, Weuve J, Ye X, Jia LT, Hu H, Ringer S, et al. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ Health Perspect. 2009;117:639–644. doi: 10.1289/ehp.0800265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volkel W, Kiranoglu M, Fromme H. Determination of free and total bisphenol A in urine of infants. Environ Res. 2011;111:143–148. doi: 10.1016/j.envres.2010.10.001. [DOI] [PubMed] [Google Scholar]