Abstract
Background and Purpose
Patient-reported outcome measures have been found useful in many disciplines but have received limited evaluation after stroke. The current study investigated the relationship that patient-reported measures have with standard impairment and disability scales after stroke.
Methods
Patients with motor deficits after stroke were scored on standard assessments including NIH Stroke Scale (NIHSS), modified Rankin Scale (mRS), and Fugl-Meyer motor scale (FM), and on two patient-reported measures, the hand function domain of the Stroke Impact Scale (SIS), which documents difficulty of hand motor usage, and the amount of use portion of the Motor Activity Log (MAL), which records amount of arm motor usage.
Results
The 43 participants had mild disability (median mRS=2), moderate motor deficits (FM=46 ± 22), and mild cognitive/language deficits. The two patient-reported outcome measures, SIS and MAL, were sensitive to the presence of arm motor deficits. Of 21 patients classified as having minimal or no impairment or disability by the NIHSS or mRS (score of 0-1), 15 (71%) reported difficulty with hand movements by the SIS score or reduced arm use by the MAL score. Furthermore, of 14 patients with a normal exam, 10 (71%) reported difficulty with hand movements or reduction in arm use.
Conclusions
Patient-reported measures were a unique source of insight into clinical status in the current population. Motor deficits were revealed in a majority of patients classified by standard scales as having minimal or no disability, and in a majority of patients classified as having no deficits.
Keywords: Patient-reported measures, arm function, ceiling effect
Valid determination of outcome after stroke is important in both clinical practice and in research trials. Scores on outcome measures are often used to determine the need for follow-up care, the appropriateness of an intervention for a specific patient, and to assess effects of therapy. Selecting the appropriate measurement tool from the large number available represents a major challenge.1
Numerous outcome measures are available to assess the effect of stroke at all levels of the International Classification of Functioning, Disability and Health.1, 2 The modified Rankin Scale (mRS), a global measure of activity, and the National Institute of Health Stroke Scale (NIHSS), a global measure of body structure/function, are two frequently used outcome measures in stroke clinical trials.3, 4 Measurement of outcome after stroke with these two scales is often dichotomized, with patients scoring 0 or1 on either scale classified as having “minimal or no disability”.5, 6 Such individuals may not be viewed as having continued deficits warranting intervention.
However, recent studies emphasize that mild deficits after stroke can have a large impact on long-term patient function.7, 8 It therefore becomes important to have a means to measure mild post-stroke deficits. Some common measures of post-stroke function such as the Functional Independence Measure and the Barthel Index have been criticized for having a ceiling effect with mild stroke9-11, i.e., a normal score can be reached despite persistence of deficits. The mRS has been praised for showing less ceiling effect than the Barthel Index12, 13 and advocated for identifying milder post-stroke dysfunction.14 However, questions remain regarding the extent of deficits present in individuals with favorable scores on mRS, particularly for upper extremity (UE) function.
Weakness in the UE is highly prevalent after stroke, affecting up to 76% of patients15 and contributing to decreased function and quality of life.16, 17 The primary hypothesis tested in the current study was that sensitive, patient-reported measures of UE motor status would reveal symptoms in a majority of patients classified as having minimal or no impairment or disability after stroke based on standard scales, i.e., that NIHSS and mRS have a ceiling effect for UE motor symptoms that can be overcome by use of patient-reported measures. One might expect that an arm specific measure of motor impairment would better UE capture deficits in individuals with mild stroke. Therefore, the UE Fugl-Meyer (FM) was also examined due to its frequent use in stroke research studies and because the presence of a ceiling effect for this measure has been debated in the literature.18, 19
Two patient-reported outcome measures were used to test this hypothesis. Patient-reported outcomes are sensitive to change, are the gold standard for many social and emotional consequences of brain injury, can reveal disability with high accuracy, and have been found useful for individual patient management.20, 21 Recent initiatives in the U.K. and U.S. increasingly emphasize use of patient-reported outcomes.22, 23 However, patient-reported outcome measures have not been used as frequently after stroke, likely because common post-stroke deficits could potentially confound accurate scoring on these measures (e.g. aphasia, neglect, cognitive change, depression). The two patient-reported outcome measures used herein were the hand domain of the Stroke Impact Scale (SIS), which measures difficulty of hand use24 and the Motor Activity Log (MAL), which assesses the amount of affected arm use.25 While both the SIS and MAL have been shown to be reliable and valid24, 26, 27, it is currently not known whether these measures provide additional information beyond the mRS and the NIHSS for assessing UE outcome after stroke. In the current analysis, the content of the SIS and MAL was characterized, then the distribution of SIS and MAL scores was examined among patients with minimal or no disability (NIHSS and mRS scores of 0-1). Secondary analyses examined SIS and MAL scores among patients with no disability or neurological deficits (NIHSS and mRS scores of 0), and among patients with no UE motor impairment, as determined by a normal score on the FM scale.
Methods
The current report is for consecutive patients who were seen at a university hospital over a 30 month period and who were screened for a number of different clinical trials. The current analysis consists of patients with a radiologically confirmed ischemic stroke that produced UE motor deficits; and whose exam showed alert state (NIHSS questions 1a-c score all = 0), absent or mild aphasia (NIHSS question 9 score = 0-1), and absent or mild neglect (NIHSS question 11 score = 0-1). Patients gave consent in accordance with Institutional Review Board approval.
Each patient underwent a one hour assessment that included a standard neurological exam, assessment of handedness28 and arm tone29, and completion of the mRS30, NIHSS31, UE FM motor scale32, and Purdue pegboard test33. The mRS is a global measure of activity/disability after stroke, ranging from 0 to 6, with lower scores indicating less disability. The NIHSS is a global measure of impairment and body structure/function, ranging from 0 to 42, with lower scores indicating less impairment. The UE FM motor scale is a measure of UE motor impairment, ranging from 0 to 66, with higher scores indicating less impairment. Purdue pegboard testing measured the number of pegs placed during a 30-second trial, separately for each hand, with higher values indicating less impairment.
In addition, two patient-reported measures of UE motor status were obtained. First, the hand function domain of the SIS24 was completed. The hand domain documents an individual's perception of difficulty of hand motor usage across five questions, each scored from 1 to 5 with a higher score indicating less difficulty (see Supplemental materials), with the final SIS score calculated as the mean of these five questions. Next, the amount of use portion of the MAL25 was completed. Of 30 available questions, the 10 most pertinent to distal upper extremity function were selected. This scale records amount of hand motor usage, each scored from 0 to 5, with a higher score indicating greater arm usage (see Supplemental materials), with the final MAL score calculated as the mean of these 10 questions.
For both of the patient-reported outcome measures (SIS, MAL), the relationship with standard scales (mRS, NIHSS, FM, and Purdue pegboard) was measured using non-parametric statistical methods (Spearman's r). Alpha was set at 0.05, and was reduced to 0.0125 when correcting for multiple comparisons. To address the main study hypothesis, the distribution of SIS and MAL scores was examined among subjects with minimal or no disability (score of 0-1 on NIHSS or on mRS).5 To address secondary hypotheses related to ceiling effects among standard scales, this analysis was repeated among subjects with no neurological deficits (score of 0 on NIHSS), no disability (score of 0 on mRS), or no arm motor impairment (score of 66 on the FM scale).
Results
Of the 43 participants, 22 presented with left-sided motor symptoms and 21 with right-sided symptoms. Gender was 24 male and 19 female. Median time post-stroke was 118 days (range 5 days-9.4 years). Arm tone was normal in 23 patients and increased in 20 patients. Based on NIHSS subscores, 13 patients had mild sensory deficits, 4 had mild aphasia, and 6 had mild hemineglect. All patients were right-handed except for three who were ambidextrous and one who was left-handed.
Stroke severity was mild to moderate. Median mRS score was 2 (range 0-4) and median NIHSS score was 2 (range 0-13, Table 1). Participants had moderate motor impairment (UE FM score mean ± SD: 46 ± 22, range 8-66) and deficits in paretic hand dexterity (the mean number of pegs moved by the stroke-affected hand on the Purdue pegboard test was 45% of the opposite hand, see Table 1). Patients reported substantial difficulty with, and decreased use of, the affected UE (Table 1): the mean SIS score (2.6 ± 1.7) reflected “somewhat” to “very difficult” paretic hand use, and the mean MAL score (2.4 ± 2.2) indicated that paretic arm use was “rarely” to “half as much” as prior to stroke.34
Table 1.
Participant characteristics
| Measure | Mean | Range | Normal value |
|---|---|---|---|
| Time post-stroke (days) | 301 ± 594 | 5-3441 | |
| Age (years) | 60 ± 14 | 29-91 | |
| Deltoid strength, affected side (MRC scale) | 3.8 ± 1.9 | 0-5 | 5 |
| Hand interossei strength, affected side (MRC scale) | 3.0 ± 2.2 | 0-5 | 5 |
| Tibialis anterior strength, affected side (MRC scale) | 3.7 ± 2.0 | 0-5 | 5 |
| NIHSS score | 2 | 0-13 | 0 |
| Fugl-Meyer arm motor score | 46 ± 22 | 8-66 | 66 |
| Purdue pegboard, # pegs, stroke-affected hand | 5 ± 6 | 0-18 | 14 |
| opposite hand | 10 ± 4 | 1-17 | 14 |
| ratio (affected/opposite) | 0.45 ± 0.45 | 0-1.3 | 0.95* |
| Modified Rankin score | 2 | 0-4 | 0 |
| SIS score | 2.6 ± 1.7 | 1-5 | 5 |
| MAL score | 2.4 ± 2.2 | 0-5 | 5 |
Both the SIS and the MAL correlated strongly with scores on standard scales (Table 2). For example, the MAL score had a correlation coefficient ≥ 0.9 in relation to the mRS, UE FM motor score, and Purdue pegboard performance with the stroke-affected hand. SIS and MAL scores did not correlate with age but had a moderate correlation with time post-stroke. Note that SIS scores were strongly correlated with MAL scores (r=.97, p<.0001).
Table 2.
Correlations between patient-reported assessments and objective measures
| Self-assessment scale | Correlates with | r | p |
|---|---|---|---|
| Stroke Impact Scale | Modified Rankin score | -0.92 | <.0001 |
| NIHSS score | -0.85 | <.0001 | |
| Fugl-Meyer arm motor score | 0.89 | <.0001 | |
| Purdue pegboard | |||
| stroke-affected hand | 0.90 | <.0001 | |
| opposite hand | 0.40 | 0.008 | |
| ratio (affected/opposite) | 0.90 | <.0001 | |
| Age (years) | -0.05 | 0.76 | |
| Time post-stroke | 0.38 | 0.01 | |
| Motor Activity Log | Modified Rankin score | -0.91 | <.0001 |
| NIHSS score | -0.87 | <.0001 | |
| Fugl-Meyer arm motor score | 0.91 | <.0001 | |
| Purdue pegboard | |||
| stroke-affected hand | 0.90 | <.0001 | |
| opposite hand | 0.37 | 0.02 | |
| ratio (affected/opposite) | 0.89 | <.0001 | |
| Age (years) | -0.03 | 0.8 | |
| Time post-stroke | 0.35 | 0.02 |
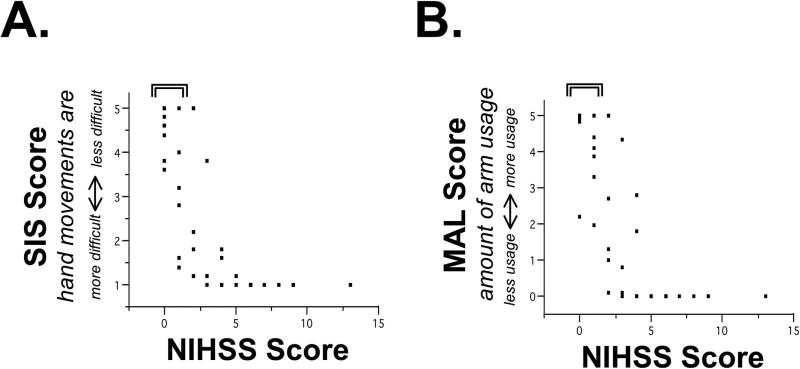
Both of the patient-reported outcome measures were sensitive to the presence of arm motor deficits in individuals classified as having minimal or no disability (Figure 1 and Table 3). Of 21 patients classified as having minimal or no impairment or disability by the NIHSS or mRS, 15 (71%) reported either difficulty with hand movements as defined by an abnormal SIS score (<5) or reduced use of the stroke-affected arm as defined by an abnormal MAL score (<5). For example, of the 18 patients with NIHSS score 0 or 1, 14 reported difficulty with hand movements, and 8 reported reduced use of the stroke-affected arm. In patients with minimal or no impairment or disability by the NIHSS or mRS, time post-stroke did not correlate with mean MAL (r=-0.179, p=0.477) or with mean hand SIS (r=-0.028, p=0.912).
Figure 1. A range of arm symptoms is present among participants classified as having minimal or no impairment.
Relationship between the NIHSS and (A) the Stroke Impact Scale (SIS) hand domain, reflecting difficulty of hand use, and (B) the Motor Activity Log (MAL) amount of arm use. The bracket indicates patients with an NIHSS score of 0-1, i.e., minimal or no neurologic impairment5, among whom a range of SIS and MAL scores are present.
Table 3.
Patient-reported assessments provide insights above the ceiling of objective measures
| Of subjects with | |||||
|---|---|---|---|---|---|
| Little or no impairment by NIHSS (score=0-1) | Normal NIHSS (score = 0) | Little or no disability by mRS (score=0-1) | Normal mRS (score=0) | Normal UE FM (score=66) | |
| N | 18 | 11 | 16 | 4 | 11 |
| Percent describing difficulty with hand movements after stroke (i.e., SIS Score <5) | 78% | 73% | 63% | 25% | 64% |
| Percent describing reduced arm use after troke (i.e., MAL Score <5) | 44% | 27% | 32% | 0% | 27% |
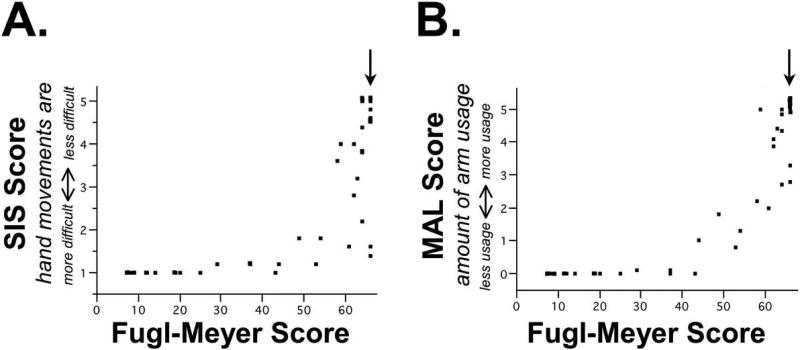
Furthermore, SIS and MAL were sensitive to the presence of arm motor deficits in patients classified by standard scales as having no neurological deficits or disability. Overall, of the 14 patients who had either a normal NIHSS score, normal mRS score, or normal FM score, 10 (71%) reported difficulty with hand movements as defined by an abnormal SIS score (<5) or reduced use of the stroke-affected arm as defined by an abnormal MAL score (<5). For example, 11 patients had an NIHSS score of 0. Of these, 8 (73%) reported difficulty with hand movements as defined by an abnormal SIS score and 3 (27%) had reduced use of the stroke-affected arm as defined by an abnormal MAL score. Only four patients had a mRS score of 0, limiting interpretation of this analysis. One might expect that a scale specifically focused on UE impairment would have high sensitivity to difficulty and amount of hand use. However, of 11 patients with a normal arm motor exam (FM score=66), 7 (64%) reported difficulty with hand movements and 3 (27%) reported reduced arm use, suggesting a similar ceiling effect for the FM scale (Figure 2 and Table 3).
Figure 2. A range of arm symptoms is present among participants classified as having no arm motor impairment.
Relationship between UE FM motor score and (A) the SIS, and (B) the MAL. The arrow indicates patients with no motor impairment, i.e., a normal UE FM score of 66, among whom a range of SIS and MAL scores are present.
It has been proposed that measures of hand dexterity may provide additional insight into stroke-related deficits beyond the mRS35, and so this was explored in the current study using the Purdue pegboard test as a measure of dexterity. Overall, the Purdue pegboard test was sensitive to arm motor deficits, but less so than the SIS. For individuals with an NIHSS score of 0 or 1, 53% demonstrated deficits on the Purdue pegboard test (ratio of affected hand/opposite hand <0.9) compared with 78% who reported deficits on the SIS. Similar results were seen with mRS, where 38% of the 16 individuals with an mRS score of 0 or 1 demonstrated deficits on the Purdue pegboard test versus 63% who showed an abnormal SIS score.
A floor effect was present in 14 (33%) of the 42 patients. Specifically, the lowest SIS score of 1 was present in 13 (30%), while the lowest MAL score of 0 was present in 14 (33%). For these 14, median NIHSS score was 6, median mRS was 4, and mean UE FM was 17 ± 11.
Discussion
This study hypothesized that two patient-reported measures would provide additional insights into clinical status beyond what is provided by the NIHSS and mRS. The current findings support the hypothesis, with the SIS and MAL each describing symptoms in a majority of patients classified by the NIHSS and mRS as having minimal or no impairment or disability, indeed in a majority of patients having a normal exam. The same effect was seen with the UE FM. A majority of patients with no measurable UE motor impairment continued to report difficulty with hand movements. Objective testing with the Purdue pegboard test also demonstrated persistent hand impairment in individuals with minimal or no impairment or disability on the NIHSS and mRS, similar to a previous report35, but the SIS was consistently more sensitive. Given the increased appreciation that mild deficits after stroke have an important effect on patient function7, 8, the current results suggest that patient-reported measures such as SIS and MAL are a unique source of insights for assessing effects of stroke in this population.
The SIS and the MAL each correlated with a number of objective measures, confirming prior reports regarding the validity of these two scales.24, 26 The strong correlation that SIS and MAL had with each other suggests a high degree of overlap between perceived difficulty and amount of UE use after stroke. Among those individuals with little to no abnormality on objective measures, the SIS score identified abnormalities in the majority, but MAL in a minority, of cases (Table 3). This constellation of findings suggests that among individuals with mild deficits after stroke, the amount of affected UE use remains relatively good even though perceived difficulty persists.
The NIHSS, mRS, and FM scale each demonstrated a ceiling effect that was addressed by the patient-reported measures. Thus, patient-reported measures provided evidence of ongoing problems with UE function in a majority (68%) of participants characterized by standard scales as having minimal or no disability. This was also true of participants who had a normal exam, where the SIS or MAL provided evidence for persistent symptoms in 71% of patients. Using an assessment that accurately measures deficits is central to understanding disease as well as to characterizing effects of a prescribed or investigational intervention.36 The current results suggest that, at least in the population studied, inclusion of patient-reported outcome measures may be important to most accurately capture treatment effects in clinical trials, which is concordant with a recent report from the United States Food and Drug Administration that highlighted the role of such measures.37 Patient-reported outcome measures have been used to screen, monitor progress, and to facilitate patient-centered care. They can improve communication and thereby improve compliance.21 However, patient-reported outcomes such as the MAL and SIS are by nature subjective, and the validity of such measures continues to be debated.38 Additionally, these measures have not been widely adopted in stroke studies in part because common sequelae of stroke such as aphasia and neglect can confound self-reported assessments. Major challenges exist for broader implementation of patient-reported assessments after stroke, such as implementation during acute hospitalization when fatigue is significant, or in the presence of severe cognitive or communication deficits.20
The two patient-reported outcomes demonstrated a floor effect among participants with a more severe stroke, however. Thus the lowest MAL score, indicating that the affected arm was not used for any activity, was present in one third of participants, among whom median mRS score was 4, consistent with moderately severe disability, and among whom mean FM score was 17, consistent with severe arm motor deficits. These findings suggest that, at this more severe end of the stroke spectrum, patient-reported measures of difficulty and amount of UE use may provide little additional information.
The difficulty with performance of skilled tasks with the paretic hand reported by individuals with little or no impairments may reflect deficits in more qualitative or cognitive aspects of motor control. Previous studies have reported deficits in skilled performance of some tasks in individuals considered to have minimal to no persistent motor deficits.39, 40 These deficits generally emerge when sensitive measures of task performance are used such as reach kinematics or force profiles, but such measures require equipment not always available in a clinical environment. Self-report measures may provide a more practical way to gain insight into deficits with high sensitivity when such equipment is not available. Another possible contributor to the reported difficulty with UE skilled task performance in the setting of minimal impairment might be abnormalities of attention, as successful performance of such movements requires greater attention after stroke.41, 42 The allocation of greater attentional resources to motor task performance after stroke may prevent an individual from directing attention to other simultaneous tasks41 and thereby result in a perception of overall greater task difficulty. Voluntary motor control arises from the interaction of a number of cognitive and perceptual brain circuits43, and likely a combination of factors influences each subject's perception of difficulty of arm movement.
A strength of the current study is that results were present across a wide range of times post-stroke. A limitation of the study is that deficits were overall mild to moderate, and individuals with substantial aphasia or neglect were excluded, and so the extent to which current results generalize to the broader stroke population remains uncertain. Potentially, such patients could be studied in future studies by use of proxy raters.44 Also, the current study employed a cross-sectional design, and a longitudinal study would be useful to better understand the extent to which patient-reported assessments provide unique information into the process of behavioral recovery. Sensory function may play an important role in one's perception of arm function after stroke. The current study did not include a sufficient number of individuals with sensory deficits among the mildly impaired cohort (only 3 out of 16 participants with a mRS score of 0 or 1 had mild sensory deficits) to determine the role of sensation in the relationships reported. Finally, depression was not measured but could be an important covariate. Nonetheless, the current results suggest that including patient-reported outcome measures may be important to deriving the most complete understanding of motor deficits after stroke, at least among patients with milder stroke.
Supplementary Material
Acknowledgments
Sources of funding: This study was supported by funds provided by the National Center of Research Resources, 5M011 RR-00827-29 and NS059909, US Public Health Service.
Footnotes
Conflict of interest: Dr. Cramer has received grant and consulting fees from GlaxoSmithKline, and consulting fees from Pfizer/Cogstate and Microtransponder.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barak S, Duncan PW. Issues in selecting outcome measures to assess functional recovery after stroke. NeuroRx. 2006;3:505–524. doi: 10.1016/j.nurx.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levin MF, Kleim JA, Wolf SL. What do motor “recovery” and “compensation” mean in patients following stroke? Neurorehabil Neural Repair. 2009;23:313–319. doi: 10.1177/1545968308328727. [DOI] [PubMed] [Google Scholar]
- 3.Quinn TJ, Dawson J, Walters MR, Lees KR. Functional outcome measures in contemporary stroke trials. Int J Stroke. 2009;4:200–205. doi: 10.1111/j.1747-4949.2009.00271.x. [DOI] [PubMed] [Google Scholar]
- 4.Savitz SI, Benatar M, Saver JL, Fisher M. Outcome analysis in clinical trial design for acute stroke: Physicians’ attitudes and choices. Cerebrovasc Dis. 2008;26:156–162. doi: 10.1159/000139663. [DOI] [PubMed] [Google Scholar]
- 5.The national institute of neurological disorders and stroke rt-pa stroke study group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 6.Bath PM, Lees KR, Schellinger PD, Altman H, Bland M, Hogg C, et al. Statistical analysis of the primary outcome in acute stroke trials. Stroke. 2012;43:1171–1178. doi: 10.1161/STROKEAHA.111.641456. [DOI] [PubMed] [Google Scholar]
- 7.Smith EE, Fonarow GC, Reeves MJ, Cox M, Olson DM, Hernandez AF, et al. Outcomes in mild or rapidly improving stroke not treated with intravenous recombinant tissue-type plasminogen activator: Findings from get with the guidelines-stroke. Stroke. 2011;42:3110–3115. doi: 10.1161/STROKEAHA.111.613208. [DOI] [PubMed] [Google Scholar]
- 8.Khatri P, Conaway MR, Johnston KC. Ninety-day outcome rates of a prospective cohort of consecutive patients with mild ischemic stroke. Stroke. 2012;43:560–562. doi: 10.1161/STROKEAHA.110.593897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlsson GE, Moller A, Blomstrand C. Consequences of mild stroke in persons <75 years -- a 1-year follow-up. Cerebrovasc Dis. 2003;16:383–388. doi: 10.1159/000072561. [DOI] [PubMed] [Google Scholar]
- 10.Duncan PW, Samsa GP, Weinberger M, Goldstein LB, Bonito A, Witter DM, et al. Health status of individuals with mild stroke. Stroke. 1997;28:740–745. doi: 10.1161/01.str.28.4.740. [DOI] [PubMed] [Google Scholar]
- 11.Edwards DF, Hahn M, Baum C, Dromerick AW. The impact of mild stroke on meaningful activity and life satisfaction. J Stroke Cerebrovasc Dis. 2006;15:151–157. doi: 10.1016/j.jstrokecerebrovasdis.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Balu S. Differences in psychometric properties, cut-off scores, and outcomes between the barthel index and modified rankin scale in pharmacotherapy-based stroke trials: Systematic literature review. Curr Med Res Opin. 2009;25:1329–1341. doi: 10.1185/03007990902875877. [DOI] [PubMed] [Google Scholar]
- 13.Banks JL, Marotta CA. Outcomes validity and reliability of the modified rankin scale: Implications for stroke clinical trials: A literature review and synthesis. Stroke. 2007;38:1091–1096. doi: 10.1161/01.STR.0000258355.23810.c6. [DOI] [PubMed] [Google Scholar]
- 14.Weimar C, Kurth T, Kraywinkel K, Wagner M, Busse O, Haberl RL, et al. Assessment of functioning and disability after ischemic stroke. Stroke. 2002;33:2053–2059. doi: 10.1161/01.str.0000022808.21776.bf. [DOI] [PubMed] [Google Scholar]
- 15.Rathore S, Hinn A, Cooper L, Tyroler H, Rosamond W. Characterization of incident stroke signs and symptoms: Findings from the atherosclerosis risk in communities study. Stroke. 2002;33:2718–2721. doi: 10.1161/01.str.0000035286.87503.31. [DOI] [PubMed] [Google Scholar]
- 16.Mayo NE, Wood-Dauphinee S, Cote R, Durcan L, Carlton J. Activity, participation, and quality of life 6 months poststroke. Arch Phys Med Rehabil. 2002;83:1035–1042. doi: 10.1053/apmr.2002.33984. [DOI] [PubMed] [Google Scholar]
- 17.Nichols-Larsen DS, Clark PC, Zeringue A, Greenspan A, Blanton S. Factors influencing stroke survivors’ quality of life during subacute recovery. Stroke. 2005;36:1480–1484. doi: 10.1161/01.STR.0000170706.13595.4f. [DOI] [PubMed] [Google Scholar]
- 18.Gladstone DJ, Danells CJ, Black SE. The fugl-meyer assessment of motor recovery after stroke: A critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16:232–240. doi: 10.1177/154596802401105171. [DOI] [PubMed] [Google Scholar]
- 19.Lin JH, Hsu MJ, Sheu CF, Wu TS, Lin RT, Chen CH, et al. Psychometric comparisons of 4 measures for assessing upper-extremity function in people with stroke. Phys Ther. 2009;89:840–850. doi: 10.2522/ptj.20080285. [DOI] [PubMed] [Google Scholar]
- 20.Barrett AM. Rose-colored answers: Neuropsychological deficits and patient-reported outcomes after stroke. Behav Neurol. 2010;22:17–23. doi: 10.3233/BEN-2009-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snyder CF, Aaronson NK, Choucair AK, Elliott TE, Greenhalgh J, Halyard MY, et al. Implementing patient-reported outcomes assessment in clinical practice: A review of the options and considerations. Qual Life Res. 2012;21:1305–1314. doi: 10.1007/s11136-011-0054-x. [DOI] [PubMed] [Google Scholar]
- 22.Department of Health . Equity and excellence: Liberating the nhs. London: 2010. [Google Scholar]
- 23. [November 1, 2012];Promis. Dynamic tools to measure health outcomes from the patient perspective. http://nihpromis.org/.
- 24.Duncan P, Wallace D, Lai S, Johnson D, Embretson S, Laster L. The stroke impact scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30:2131–2140. doi: 10.1161/01.str.30.10.2131. [DOI] [PubMed] [Google Scholar]
- 25.Taub E, Miller N, TA N, Cook E, Fleming W, Nepomuceno C, et al. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993;74:347–354. [PubMed] [Google Scholar]
- 26.Uswatte G, Taub E, Morris D, Light K, Thompson PA. The motor activity log-28: Assessing daily use of the hemiparetic arm after stroke. Neurology. 2006;67:1189–1194. doi: 10.1212/01.wnl.0000238164.90657.c2. [DOI] [PubMed] [Google Scholar]
- 27.van der Lee JH, Beckerman H, Knol DL, de Vet HC, Bouter LM. Clinimetric properties of the motor activity log for the assessment of arm use in hemiparetic patients. Stroke. 2004;35:1410–1414. doi: 10.1161/01.STR.0000126900.24964.7e. [DOI] [PubMed] [Google Scholar]
- 28.Oldfield R. The assessment and analysis of handedness: The edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 29.Bohannon RW, Smith MB. Interrater reliability of a modified ashworth scale of muscle spasticity. Phys Ther. 1987;67:206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 30.Rankin J. Cerebral vascular accidents in patients over the age of 60. Ii. Prognosis. Scott Med J. 1957;2:200–215. doi: 10.1177/003693305700200504. [DOI] [PubMed] [Google Scholar]
- 31.Brott T, Adams H, Olinger C, Marler J, Barsan W, Biller J, et al. Measurements of acute cerebral infarction: A clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 32.Fugl-Meyer A, Jaasko L, Leyman I, Olsson S, S S. The post-stroke hemiplegic patient: A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 33.Mathiowerz V, Weber K, Kashman N, Volland G. Adult norms for the nine hole ped test of finger dexterity. Occupational Therapy Journal of Research. 1985;5:24–38. doi: 10.5014/ajot.39.6.386. [DOI] [PubMed] [Google Scholar]
- 34.Spreen O, Strauss E. A compendium of neuropsychological tests. Oxford University Press; New York: 1991. [Google Scholar]
- 35.Dhamoon MS, Lazar RM, Marshall RS. Impairment versus activity limitation after incident ischaemic stroke. Int J Stroke. 2010;5:132–133. doi: 10.1111/j.1747-4949.2010.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duncan P, Jorgensen H, Wade D. Outcome measures in acute stroke trials: A systematic review and some recommendations to improve practice. Stroke. 2000;31:1429–1438. doi: 10.1161/01.str.31.6.1429. [DOI] [PubMed] [Google Scholar]
- 37.Food and Drug Administration Patient-reported outcome measures: Use in medical product development to support labeling claims. 2009.
- 38.Magasi S, Ryan G, Revicki D, Lenderking W, Hays RD, Brod M, et al. Content validity of patient-reported outcome measures: Perspectives from a promis meeting. Qual Life Res. 2012;21:739–746. doi: 10.1007/s11136-011-9990-8. [DOI] [PubMed] [Google Scholar]
- 39.Platz T, Bock S, Prass K. Reduced skilfulness of arm motor behaviour among motor stroke patients with good clinical recovery: Does it indicate reduced automaticity? Can it be improved by unilateral or bilateral training? A kinematic motion analysis study. Neuropsychologia. 2001;39:687–698. doi: 10.1016/s0028-3932(01)00005-7. [DOI] [PubMed] [Google Scholar]
- 40.Platz T, Prass K, Denzler P, Bock S, Mauritz KH. Testing a motor performance series and a kinematic motion analysis as measures of performance in high-functioning stroke patients: Reliability, validity, and responsiveness to therapeutic intervention. Arch Phys Med Rehabil. 1999;80:270–277. doi: 10.1016/s0003-9993(99)90137-5. [DOI] [PubMed] [Google Scholar]
- 41.Dennis A, Bosnell R, Dawes H, Howells K, Cockburn J, Kischka U, et al. Cognitive context determines dorsal premotor cortical activity during hand movement in patients after stroke. Stroke. 2011;42:1056–1061. doi: 10.1161/STROKEAHA.110.597880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meehan SK, Randhawa B, Wessel B, Boyd LA. Implicit sequence-specific motor learning after subcortical stroke is associated with increased prefrontal brain activations: An fmri study. Hum Brain Mapp. 2011;32:290–303. doi: 10.1002/hbm.21019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shumway-Cook A, Woollacott M. Motor control. Translating research into clinical practice. 4th Ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2007. [Google Scholar]
- 44.Carod-Artal FJ, Egido JA. Quality of life after stroke: The importance of a good recovery. Cerebrovasc Dis. 2009;27(Suppl 1):204–214. doi: 10.1159/000200461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.