Abstract
Background and Purpose
Statins are widely used in the primary and secondary prevention of ischemic stroke, but their effects on stroke-induced immunodeppression and post-stroke infections are elusive. We investigated effects of simvastatin treatment on stroke-induced splenic atrophy and lung susceptibility to bacterial infection in acute experimental stroke in mice.
Methods
Ischemic stroke was induced by transient occlusion of middle cerebral artery (MCAO) followed by reperfusion. In some experiments, splenectomies were performed 2 weeks prior to MCAO. Animals were randomly assigned to sham and MCAO groups treated subcutaneously with vehicle or simvastatin (20 mg/kg/day). Brain infarction, neurological function, brain interferon-γ expression, splenic atrophy and apoptosis, and lung infection were examined.
Results
Simvastatin reduced stroke-induced spleen atrophy and splenic apoptosis via increased mitochrondrial anti-apoptotic Bcl-2 expression and decreased pro-apoptotic Bax translocation from cytosol into mitochondria. Splenectomy reduced brain interferon-γ (3d) and infarct size (5d) after stroke and these effects were reversed by adoptive transfer of splenocytes. Simvastatin inhibited brain interferon-γ (3d) and reduced infarct volume and neurological deficits (5d) after stroke, and these protective effects were observed not only in naïve stroke mice but also in splenectomied stroke mice adoptively transferred with splenocytes. Simvastatin also decreased the stroke-associated lung susceptibility to spontaneous bacterial infection.
Conclusions
Results provide the first direct experimental evidence that simvastatin ameliorates stroke-induced peripheral immunodepression by attenuating spleen atrophy and lung bacterial infection. These findings contribute to a better understanding of beneficial effects of statins in the treatment of stroke.
Keywords: Brain ischemia, bacterial infection, immune response, statin, spleen
Introduction
Stroke is the third leading cause of death in the world, but treatment options for acute stroke remain limited. Cerebral ischemia not only produces local brain damage, but also triggers a profound systemic immunodepression or ‘stroke-induced immunodeficiency syndrome’, which predisposes patients after stroke to infections [1-3]. Infectious complications, predominantly chest and urinary tract infections, occur in many stroke patients within the first few days after stroke, and are a leading cause of death in patients with acute stroke [4,5]. The prognosis of stroke depends strongly on the incidence of infectious complications, in particular pneumonia. Pneumonia is the most common post-stroke infection, about half of pneumonia cases occur within the first 2 days after stroke onset, and almost all cases occur within the first week [4, 5]. Thus, therapeutic modification of post-stroke immunodepression and avoiding poststroke infections may represent a valuable approach to improving outcome in stroke patients [6].
Statins are inhibitors of the enzyme 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) and they are the most effective agents for lowering cholesterol in clinical practice for the treatment of cardiovascular diseases. Recently, it has become clear that statins also have pleiotropic effects on inflammation and immunity in addition to their lipid-lowering properties [7]. Previous studies have reported that statins have beneficial effects on various forms of experimental brain injury such as ischemic stroke [8,9], subarachnoid hemorrhage [10], and intracerebral hemorrhage [11,12]. Statins are widely used in the primary and secondary prevention of ischemic stroke in patients [13]. Pretreatment of experimental stroke with simvastatin significantly reduces brain infarct volume and improves neurological outcome [14]. Delayed treatment of experimental stroke with a statin starting 24 hours after stroke does not decrease lesion volume, but significantly improves functional outcome compared to animals with nontreated stroke [15, 16].
Post-stroke peripheral immunodepression is characterized by profound atrophy of secondary lymphatic organs (spleen, thymus and lymph node) that occur within the first few days after stroke [1, 2]. Several recent studies have reported that spleen contributes to neuroinflammation and the development of ischemic stroke in acute experimental stroke [17-21]. Splenectomy two weeks prior to middle cerebral artery occlusion (MCAO) in the rat significantly reduces neuroinflammation and brain damage [22]. To our knowledge, no experimental studies have addressed if and how statins affect stroke-induced immunodeppression and post-stroke infections. Thus, the present study was designed to investigate the effects of simvastatin on stroke-induced peripheral immunodeppression and post-stroke infections. We focused on investigation on the effects of simvastatin on stroke-induced splenic atrophy and lung susceptibility to bacterial infection.
Methods
Ethics Statement
All experimental procedures were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Animal Care and Use Committee of the Louisiana State University Health Science Center-Shreveport (IACUC approval number: 11010). Male C57BL/6J mice (8 to10 weeks old, 22 to 25g), obtained from Jackson Laboratories (Bar Harbor, Me), were used in the study.
Experimental Model of Stroke
Focal cerebral ischemia was induced by transient occlusion of the left middle cerebral artery (MCAO) with a 6-0 silicone-coated nylon monofilament (Doccol Corp.), as previously described [23]. Subgroups of mice were randomly selected to receive MCAO or sham procedures. In ischemic groups, animals were subjected to 60 minutes MCAO followed by reperfusion for indicated times. In the sham controls, the arteries were visualized but not disturbed. Rectal temperature was maintained at 37±0.5°C throughout the procedure from the start of the surgery until the animals recovered from anesthesia with a feed-back regulated heating pad. Regional cerebral blood flow (rCBF) was monitored by laser Doppler flowmetry (MSP300XP; ADInstruments Inc) as previously described [23]. Only animals that exhibited a reduction in CBF>85% during MCAO and a CBF recovery by >80% after 15 min of reperfusion were included in the study.
Splenectomy and Adoptive Transfer of Splenocytes
In some experiments, splenectomy was performed two weeks prior to MCAO using the published procedure [24]. Briefly, under anesthesia a left flank skin incision (~2.5 cm long) was made and the spleen was exposed and pulled onto the exterior surface of the peritoneum with blunt forceps. The splenic blood vessels were tied off with sutures and the spleen was removed. The abdominal wall and the skin incision were closed with sutures. Sham operations were also performed where the spleen was exteriorized and then reinserted into the abdominal cavity.
Splenocytes were prepared from spleens of male wild-type mice by forcing the tissues through a fine wire mesh. Cells were washed and resuspended in 2 ml Dulbecco’s Phosphate Buffered Saline (PBS) containing 10% fetal calf serum, 2 mM glutamine and 100 μg/ml of streptomycin and penicillin, and contaminating erythrocytes were lysed using 1× red cell lysis buffer (BD Biosciences). One hour after MCAO, isolated splenocytes (2 × 107 in 250 ul PBS) or equal volume of PBS were infused (3 minutes) into splenectomized or sham-splenectomized mice via the femoral vein.
Drug Administration
Simvastatin (Sigma Chemicals) was prepared as a 5 mg/ml stock as previously described [25]. Briefly, 5 mg of simvastatin was dissolved in 100 μl of ethanol and 150 μl of 0.1 N NaOH, incubated at 50°C for 2 h, then added water to 800 ul volume and the pH adjusted to 7 with 1N HCl, and final volume corrected to 1ml. This simvastatin stock solution was stored at -80°C and diluted with sterile saline immediately before use. Animals were randomized to receive subcutaneous (SC) injection of simvastatin 20 mg/kg/day or saline treatment for 5 days, with the first dose given immediately after MCAO. The doses of statin chosen are consistent with the primary bioactive dose used in vivo in mouse/rat studies (typically 10–100 mg/kg/day) [25-27].
Brain Infarct Measurement
On day 5 after MCAO, the mice were euthanized by deep anesthesia and then perfused transcardially with 30 ml phosphate buffered saline (PBS) followed by cold 4% paraformaldehyde (PFA) in PBS. The brains were collected, and incubated overnight in the 4% PFA solution and then transferred into 10%, 20% and 30% sucrose solution until they sank. Then, coronal sections (40-μm thick) were serially cut using a cryostat, starting from the frontal pole through the entire brain, and 5 coronal sections at 1 mm intervals (relative to bregam: +1, 0, -1, -2, -3 mm) were selected and stained with 0.1% crystal violet solution (pH 3.8) for 5 min. The areas of the uncorrected infarcted area and the total areas of both hemispheres were measured for each slice by a computerized NIH image analysis system. Corrected infarct area in a slice was calculated by subtracting the area of normal tissue in the ipsilateral hemisphere from the total area of the contralateral hemisphere. Total corrected infarct volume was then calculated by multiplying the area by the slice thickness and summing the volumes from all slices [28]. All measurements were performed by 2 blinded researchers and the mean of their results was calculated.
Neurological Scoring
The neurological scores were assessed by a 28-point neurological score test by an experimenter blinded to the experimental groups at 1d, 3d and 5d. Scoring was based on physical appearance and behavior using a scale previously developed to provide a precise assessment of focal neurologic damage [29]. The mice were scored in each of the following seven categories: body symmetry, gait, climbing, circling behavior, forelimb symmetry, compulsory circling, and sensory response. Each mouse was scored from 0 (no deficit) to 4 (severely affected) in each category, as previously described [29].
Detection of Stroke-induced Splenocyte Apoptosis
Fresh spleens from sham-operated and MCAO mice were collected and whole splenocytes were obtained by gentle grinding of spleens between frosted glass slides. The erythrocytes were lysed with BD Pharm Lyse™ Lysing Buffer (Cat. No. 555899), and single cell suspensions were collected by passing the tissue through a 100-μm nylon mesh. Collected splenocytes were washed twice in PBS, counted, and reconstituted in Ca2+-rich annexin V binding buffer (BD Pharmingen) at a concentration of 107 cells/mL. Cell suspension (100 μl) was stained with 2.5 μl annexin V-FITC and/or 1 μl propidium iodine (PI) for 15 min and adjusted to a total volume of 500 μl with binding buffer. Cells were then analyzed by flow cytometry with FACSCalibur (BD Biosciences), and annexin V+/PI- cells were considered as apoptotic cells. The percentage of apoptotic cells was calculated according to total annexin V+/PI- divided by total cells.
Analysis of THC-induced Splenocyte Apoptosis In Vitro
Fresh spleen cells were isolated from C57BL/6 mice as described above. THC-induced apoptosis in splenocytes was analyzed as described previously [30]. A single cell suspension in complete medium [RPMI 1640 medium (Sigma) supplemented with 5% FBS, 10 mM HEPES, 1 mM glutamine, 40 ug/ml gentamicin sulfate, and 50 uM 2-mercaptoethanol] was added to 24-well flat bottom plates (1×106 cells/well) and treated without or with simvastatin (25 μM) for 1h, followed by 10 μM delta-9-tetrahydrocannabinol (THC, Sigma) for additional 16h. After that, the cells were harvested and washed twice with PBS/0.2%BSA and stained with annexin V-FITC and PI for flow cytometry analysis.
Western blot analysis
Mice were transcardially perfused with ice-cold PBS and spleens were removed and splenocytes isolated as described above. The cytosolic and mitochondria fractions were prepared from the isolated splenocytes using a commercially available cytosol/mitochondria fractionation kit according to the manufacturer’s protocol (Millipore). Samples were stored at -80°C for further analysis. Antibodies were used as follows: monoclonal rabbit antibody against Bcl-2 (1:1000), Bax (1:1000), COX-V (1:1000) (All purchased from Cell Signaling) and mouse antibody against β-actin (Sigma). Immunopositive bands of horseradish peroxidase-conjugated secondary antibodies were detected with an ECL system (GE Healthcare) and exposure to ECL Hyperfilm.
Enzyme-linked immunosorbent assay (ELISA)
Mice were transcardially perfused with ice-cold PBS and the ipsilateral cerebral cortex was collected and immediately homogenized in five volumes of PBS with complete protease cocktail (Roche, Indianapolis, IN, USA) using a dounce homogenizer. After incubation on ice for 5 min, the extracts were cleared by centrifugation at 14000 r.p.m. for 10 min. The protein content of each extract was determined by the bradford protein assay (Bio-Rad). ELISA assays were performed using Mouse IFN-γ ELISA kit (Thermo scientific, Rockford, IL, USA) following the manufacturer’s instructions.
Histopathological Analysis of Lung Tissue
Mice were anesthetized and intracardially perfused with 30 ml PBS followed by 20 ml of 4% PFA. Lungs were removed, postfixed in 4% PFA for overnight, and then embedded in paraffin. 6-μm thick sections were obtained by microtome dissection and stained with hematoxylin/eosin (HE). 20 representative sections of lungs were chosen per animal and evaluated by two investigators, who were blinded to the treatment groups. The sections were graded according to the following criteria: no definite damage represented no histological changes or minor changes, including unequal distension of alveolar units, mild thickening of the alveolar septa, and perivascular and peribronchiolar edema. Definite damage was observed as lung consolidation, thickened alveolar septae, and the presence of intra-alveolar inflammatory infiltrates [31].
Microbiological Analysis of Blood and Lung Tissue
Mice were euthanized at indicated time points after MCAO and washed with 70% ethanol under sterile conditions. Blood was taken by cardiac puncture after thoracotomy, and lobes of lungs were collected, minced and homogenized under sterile conditions. Bacterial strains from lung tissue homogenates grown on blood agar plates were examined by our Clinical Pathology Laboratory at LSU Medical Center-Shreveport. For determination of colony-forming units (CFU), 100 μL of lung tissue homogenate or blood was serially diluted in PBS, plated onto blood agar plates (Merck), and incubated at 37 °C for 18 h [31], and then bacterial colonies were counted by two researchers blinded to treatment groups.
Statistical Analysis
Variables were analyzed by Student’s t-test (two-tailed) and a P<0.05 was considered statistically significant. Values are expressed as mean±SD (standard deviation) of at least three independent experiments. Animal survival rates between the groups were analyzed using the log-rank test.
Results
Simvastatin attenuates stroke-induced splenic atrophy and body weight loss
Splenic atrophy is an important feature of stroke-induced peripheral immunodepression and is characterized by reduced organ size and reduced number of spleen cells that occurred within the first few days after a stroke [20, 21]. The spleen weight and the number of splenocytes in vehicle-treated groups were significantly decreased at 3 and 5 days after MCAO, when compared with sham controls (Fig. 1a, b). The reduction and regain of body weight was measured as an index of general stress elicited by cerebral ischemia. In vehicle-treated animals, body weight was significantly decreased at 1 day and continued to decrease 5 days after MCAO (Fig. 1c). Simvastatin treatment significantly attenuated the splenic atrophy and body weight loss within the 5-day observation period (Fig. 1a-c).
Figure 1. Simvastatin attenuates stroke-induced splenic atrophy and body weight loss.
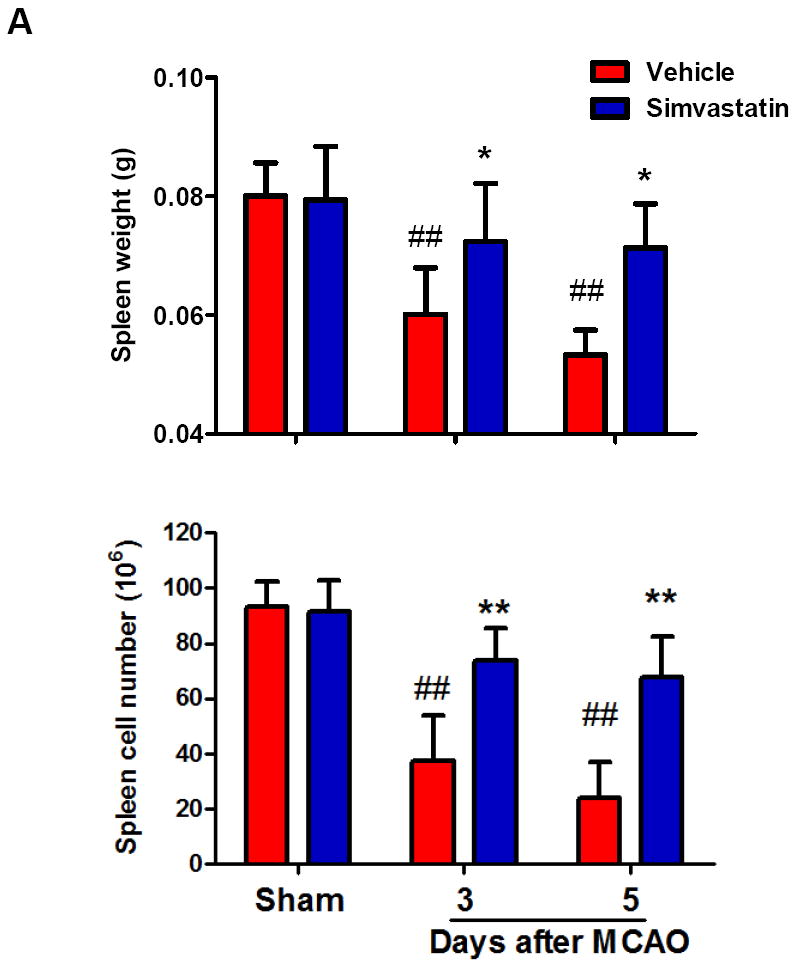
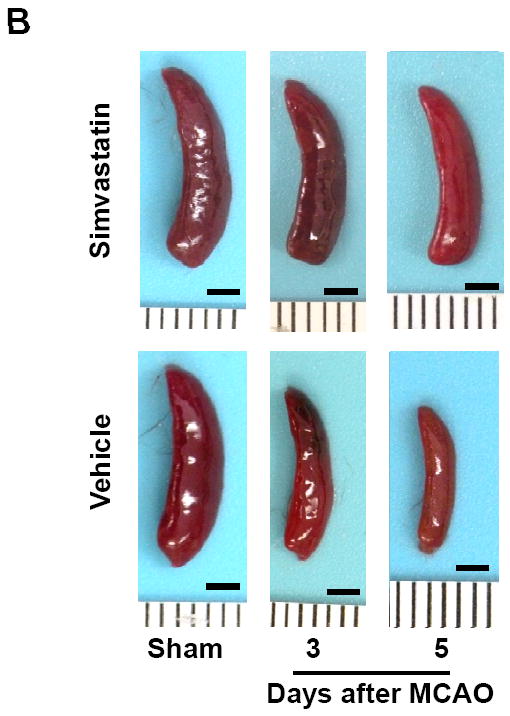
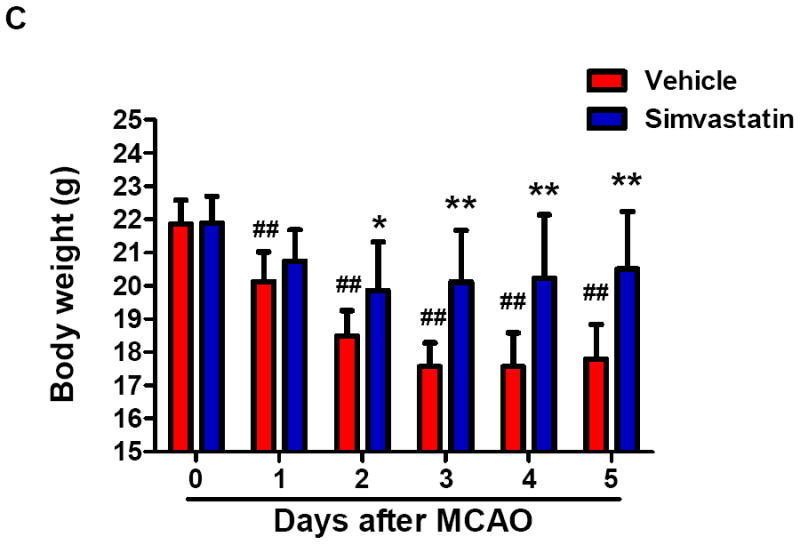
A, Quantification of spleen weights and cell numbers in sham control mice and MCAO mice treated with vehicle or simvastatin. B, Representative pictures of spleens in sham and MCAO mice at the indicated time points of MCAO. Scale bar = 2 mm. C, Quantification of body weight loss in the indicated groups. A & C: n=7-9 mice per group per time point; ## P<0.01 vs. respective sham control; * P<0.05, **P<0.01 vs. respective vehicle control at the same time point.
Simvastatin inhibits splenocyte apoptosis through mitochondrial pathway
Induction of apoptosis is one of the mechanisms leading to splenic atrophy in experimental stroke [20, 21]. Previous studies with mice have shown that splenocyte apoptosis was significantly induced as early as 12 h after transient MCAO [31], and the apoptosis was further increased over time (22-96 h) after stroke [20]. Thus, we investigated whether simvastatin reduced spleen atrophy through inhibition of splenocyte apoptosis at both the early (12h) and late (5d) time points after MCAO. Flow cytometry analysis was performed to identify and quantify splenocyte apoptosis. Annexin V+ PI− cells are considered apoptotic cells. The number of Annexin V+ PI–apoptotic cells from the spleens of the vehicle-treated mice was significantly increased at 12 h and further increased at 5 d after MCAO, but this increase at both time points was significantly inhibited by simvastatin treatment (Fig. 2a, 2b). Moreover, we determined whether simvastatin can directly act on spleen cells in vitro. The apoptosis of splenocytes isolated from C57Bl5 mice was induced by delta-9-Tetrahydrocannabinol (THC), a well-known immunosuppressive agent [30]. Treatment with THC (10 uM for 16 h) significantly increased apoptosis of splenocytes (Fig. 2c, 2d), consistent with published data [30]. Clearly, simvastatin significantly decreased THC-induced spleen cells apoptosis in vitro (Fig. 2c, 2d).
Figure. 2. Simvastatin attenuates splenocyte apoptosis through mitochondrial pathway.
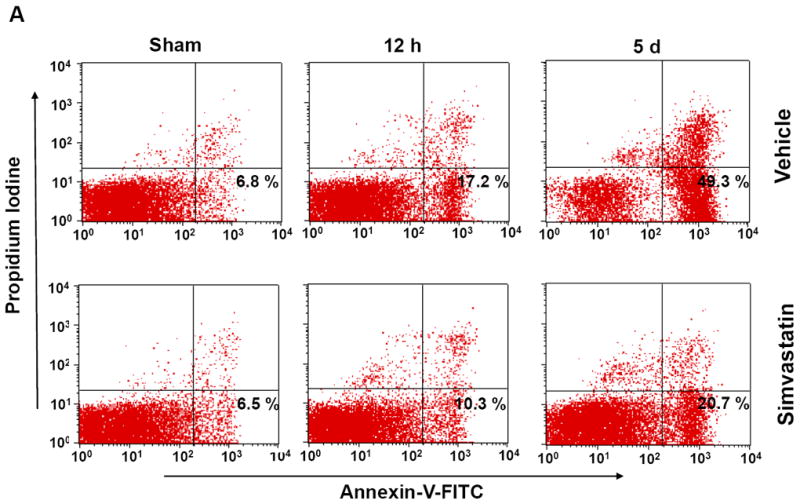
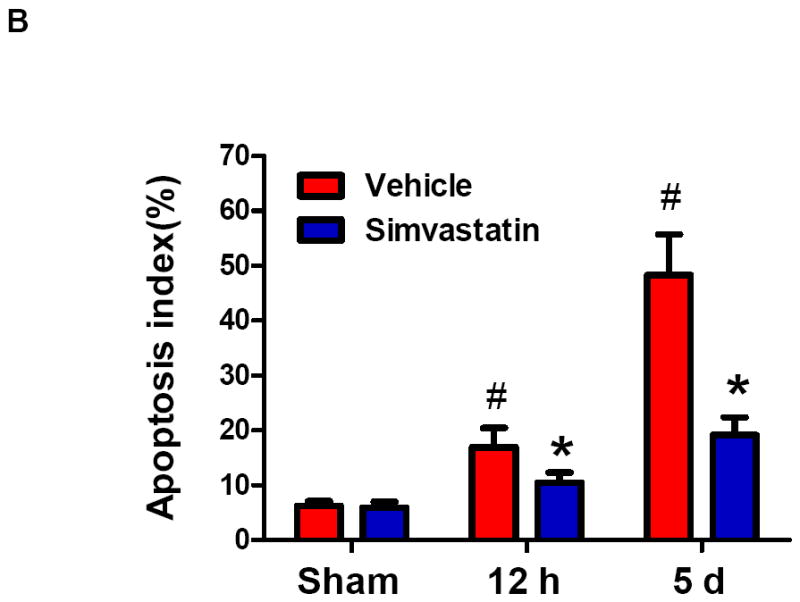

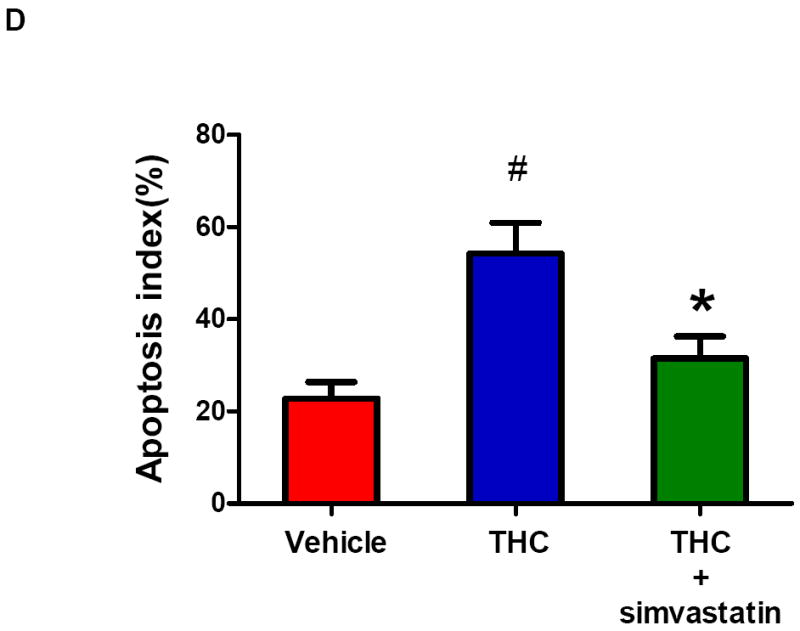
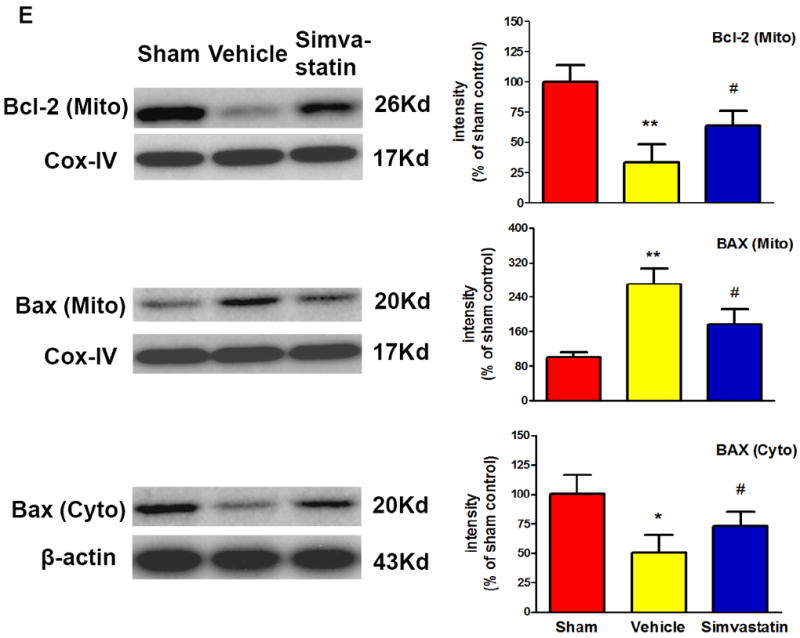
A, B Mice were euthanized 12 hours or 5 days after MCAO for splenic analysis. A, Representative flow cytometric dot plots for FITC-Annexin V and propidium iodine (PI) labeling in freshly isolated splenocytes from sham control mice and MCAO mice treated with vehicle or simvastatin. Annexin V+ and PI- cells were considered early apoptotic cells (lower right quadrant). B, Flow cytometric analysis of stroke-induced splenocyte apoptosis in the simvastatin- and vehicle- treated mice at the indicated time points after MCAO. The percentage of apoptotic cells was calculated from the ratio of apoptotic cells to total cells counted. Data represent the mean ± SD of four independent experiments. # P<0.05 vs. sham control; * P<0.05 vs. vehicle control. C, Representative flow cytometric dot plots showing THC-induced splenocyte apoptosis in vitro. D, Flow cytometric analysis of THC-induced splenocyte apoptosis in vitro. Data represent the mean ± SD of five independent experiments. #P<0.05 vs. Vehicle; *P<0.05 vs. the THC only group. E, Western blot analysis of the protein levels of mitochondrial Bcl-2, mitochondrial and cytosolic Bax in the splenic cells from sham control mice and MCAO mice treated with vehicle or simvastatin. β-actin and COX IV were used as loading controls for cytosolic and mitochondrial fractions, respectively. Data are presented as mean ± SD of four independent experiments (n=4 mice/group for each experiment). * P<0.05, ** P<0.01 vs. sham control; # P<0.05 vs. vehicle control.
Further, we examined the molecular mechanisms underlying the simvastatsin-mediated anti-apoptotic effects. The ratio of pro- apoptotic Bax to anti-apoptotic Bcl-2 proteins is considered as a major checkpoint in apoptosis [32]. The membrane and cytosolic fractions of the spleen cells were prepared from the sham or MCAO mice treated with vehicle or simvastatin, and the fractions were analyzed by Western blotting to measure the expression levels of these apoptosis regulatory proteins. The expression of Bcl-2 protein in mitochondria was markedly reduced in the vehicle-treated group, but this reduction was largely reversed in the simvastatin-treated group (Fig. 2e). Bax is mostly cytosolic and translocates to mitochondria following an apoptotic stimulus. The expression of Bax was significantly decreased in the cytosol by a concomitant increase in the mitochondria of the spleens from the vehicle-treated stroke group, but these changes were reversed by simvastatin treatment (Fig. 2e).
Simvastatin reduces brain IFNγ expression and brain injury contributed by splenocytes
We performed splenectomy or sham-operation 2 weeks prior to MCAO in C57Bl6 mice. The mean total infarct volume (5d) was 30.6 ± 7.9 mm3 in splenectomized animals and 54.7 ± 7.0 mm3 in sham controls (P < 0.01) (Fig. 3a). The 28-point neurological score (3d, 5d) was also improved in splenectomized animals (Fig. 3b), compared with sham controls. Furthermore, we demonstrated that adoptive transfer of isolated splenocytes into splenectomized mice abolished the stroke-protective effects of splenectomy on infarct volumes (Fig. 3a) and neurological score (Fig.3b). These data established a role of spleen in a mouse stroke model. Moreover, simvastatin treatment significantly decreased infarct volume (39.5 ± 5.9 mm3) (Fig. 3a) and improved neurological score (Fig. 3b) in splenectomized mice adoptively transferred with splenocytes. In addition, we observed that sivastatin treatment and splenectomy did not significantly alter regional cerebral blood flow (Suppl. Figure S1) and blood physiological parameters (Suppl. Table S1).
Figure 3. Simvastatin reduces brain IFNγ protein expression and brain damage contributed by splenic cells.
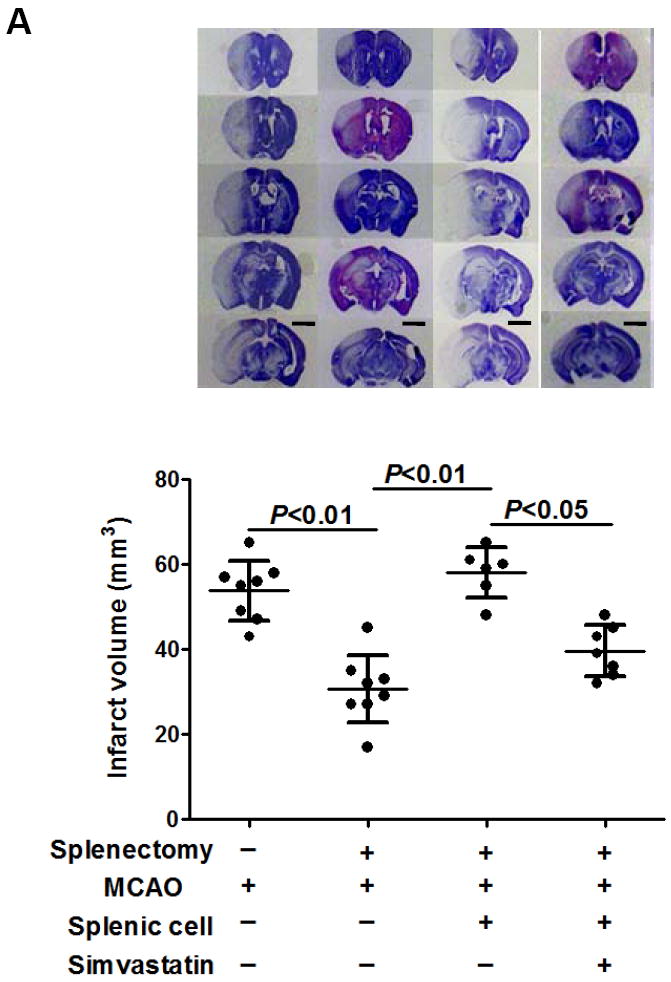
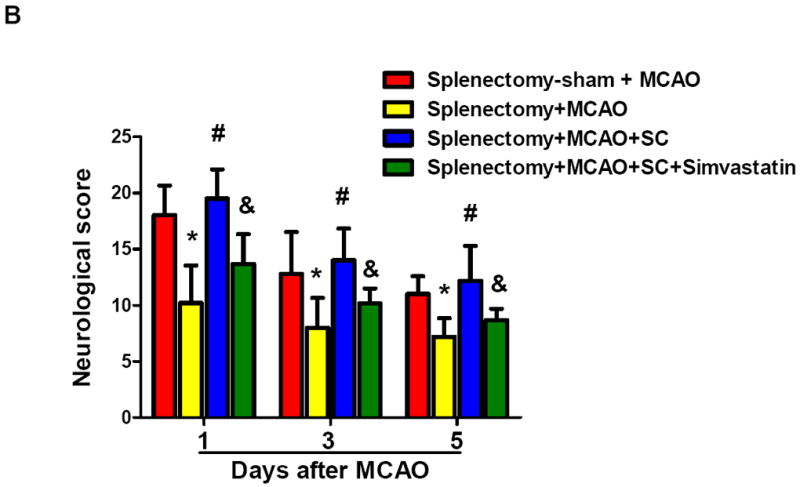
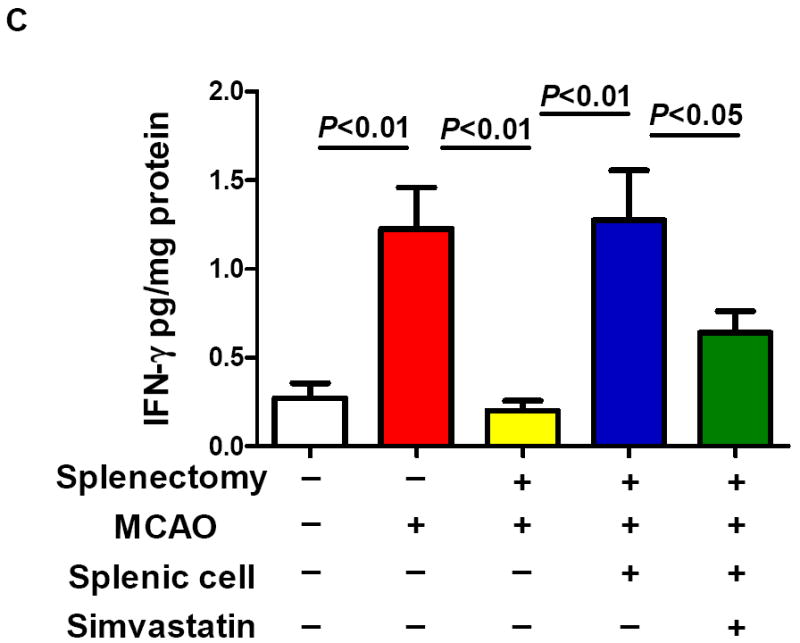
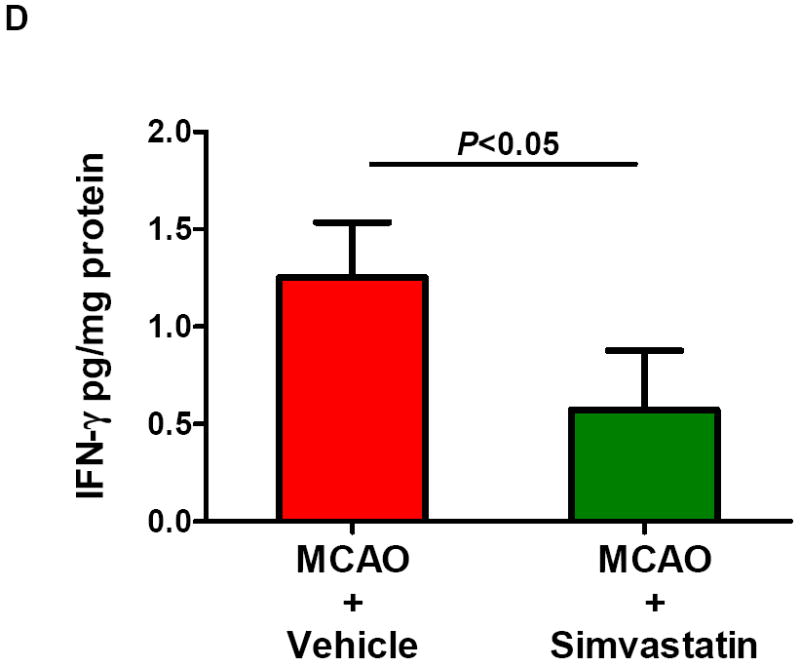
Splenectomy and adoptive transfer of splenocytes were performed as described in the Method. Stroke analysis was performed 5 days after MCAO. A, Representative images of cresyl violet-stained coronal brain sections (upper) and Quantitative analysis of infarct volumes (lower). Scale bar = 2 mm. B, The 28-point neurological scoring at indicated time points (n= 6-8 survived mice/group). *P<0.05 vs. sham-splenectomized MCAO mice; #P<0.05 vs. splenectomized MCAO mice, and &P<0.05 vs. splenectomized MCAO mice with adoptive transfer of splenic cells (SC) at the same time point. C and D, ELISA measurement of IFN-γ protein concentrations in the ipsilateral hemispheres from the indicated groups at 72h after MCAO. n=5 mice/group.
Next, we examined if and how simvastatin affects the IFNγ protein expression in the brain at 72 h after MCAO. This time point was chosen based on the published data showing that IFNγ protein levels were increased in the brain most prominently at 72h after MCAO in rats [17]. We demonstrated that IFNγ protein levels were increased in the brain of sham-splenectomied MCAO mice, but the increase was abolished in splenectomied MCAO mice (Fig. 3c). Furthermore, the IFNγ protein levels in the brain of splenectomied MCAO mice were regained by adoptive transfer of isolated splenocytes (Fig. 3c), and this was largely abolished by simvastatin treatment (Fig. 3c). In addition, simvastatin treatment significantly reduced the IFNγ protein levels in the brain of MCAO mice without a splenectomy or sham-splenectomy (Fig. 3d). Collectively, these data suggest that simvastatin protects the brain against ischemic injury contributed by spleen cells through modulating brain IFNγ expression.
Simvastatin reduces brain damage and mortality in acute experimental stroke
Previous work showed that simvastatin pre-treatment reduced brain injury at early time point (24 h) after transient MCAO in mice [14]. We further determined whether simvastatin protects brain against ischemic injury at a later time point after stroke. Brain infarction at 5 days after MCAO was measured on cresyl violet-stained coronal sections. Animal survival rate from the same experimental groups was evaluated daily in the 5-day observation period. Infarct volumes (5d) was 40.3 ± 10.1 mm3 in the simvastatin-treated animals and 53.2 ± 9.3 mm3 in vehicle-treated controls (P<0.05) (Fig. 4a). The 28-point neurological score (1, 3, 5d) were also significantly reduced in the simvastatin-treated animals (Fig. 4b), compared with vehicle-treated controls. Impressively, all experimental mice (n=9) survived in the simvastatin-treated stroke group, while only 7/13 (53.8%) of mice survived in the vehicle-treated stroke group over the 5-day observation period (P<0.05) (Fig. 4c).
Figure 4. Simvastatin reduces ischemic brain injury and animal mortality 5 days after MCAO.
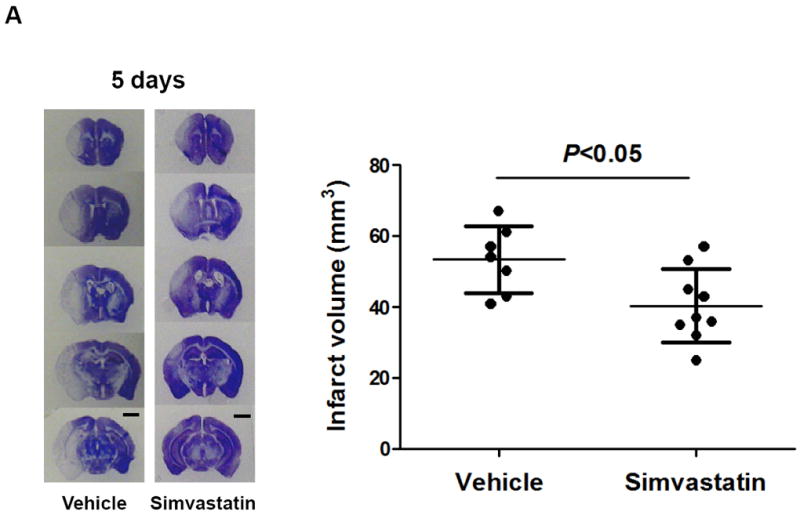
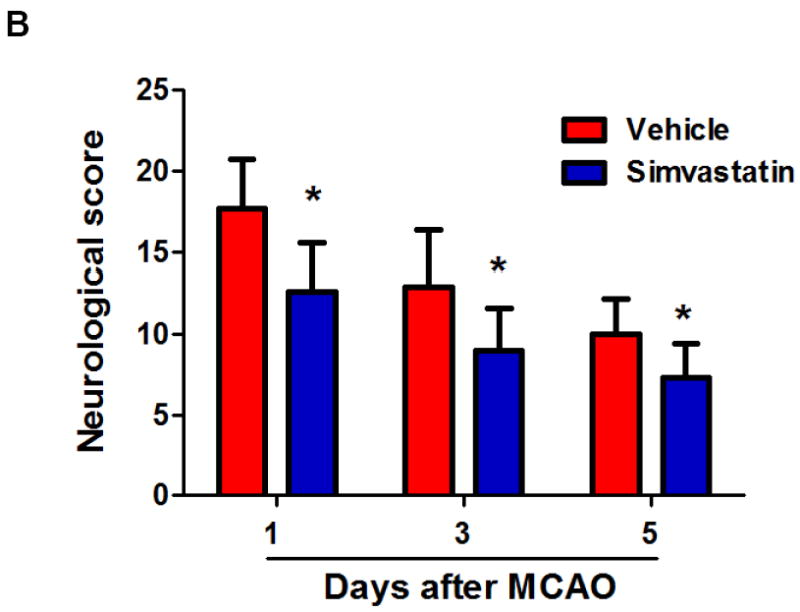
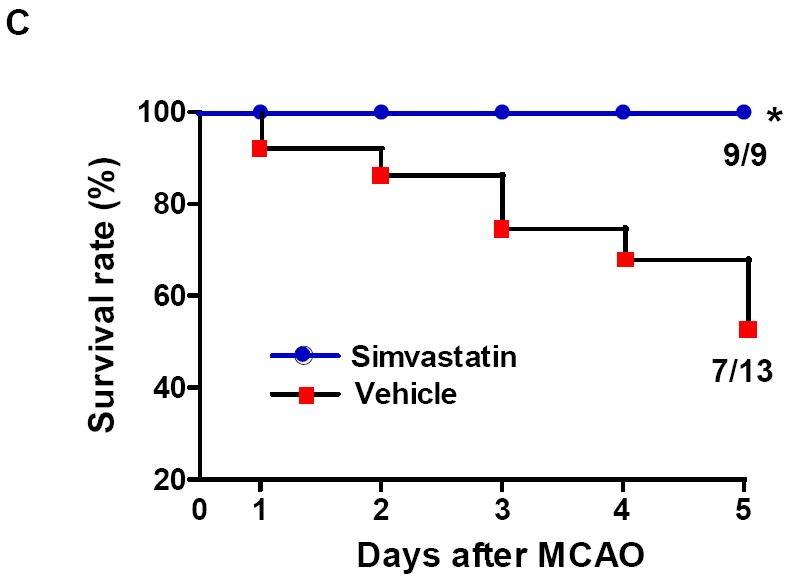
A, Representative images of cresyl violet-stained coronal brain sections (left panels) and Quantitative analysis of infarct volumes (right panels). Scale bar = 2 mm. B, The 28-point neurological scoring at indicated time points in the vehicle-treated and simvastatin-treated mice. n = 7-9 per group. *P<0.05 vs. vehicle-treated group at the same time point. C, Animal survival rate from the same experiments was recorded daily for 5 days after MCAO. n=9 for simvastatin- and n=13 for vehicle-treated mice. *p<0.05, log-rank test compared with vehicle-treated mice.
Simvastatin reduces lung susceptibility to spontaneous bacterial infection after stroke
Histological examination revealed typical signs of bacterial pneumonia, showing lung consolidation, thickened alveolar septa, and intra-alveolar inflammatory infiltrates [33], in the vehicle-treated stroke mice at 72 h after MCAO, but the damage to lung tissue was significantly attenuated in the simvastatin-treated stroke (n=5 per group, Fig. 5a). Moreover, lung sections of sham controls revealed no signs of pneumonia (n=4, Fig. 5a). Therefore, susceptibility to infection resulted from stroke but not from surgical stress. In addition, splenectomy did not increase lung susceptibility to bacterial infection 72 after MCAO (Fig. S2).
Figure 5. Simvastatin attenuates stroke-induced lung susceptibility to spontaneous bacterial infection.
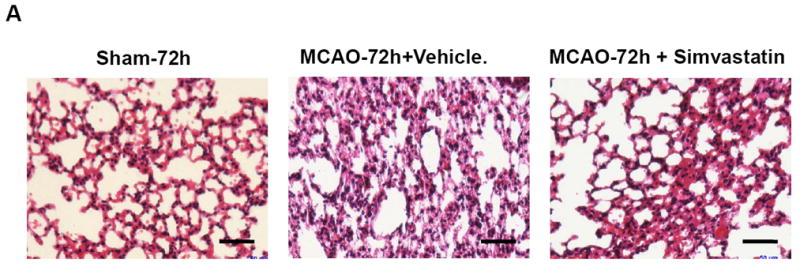
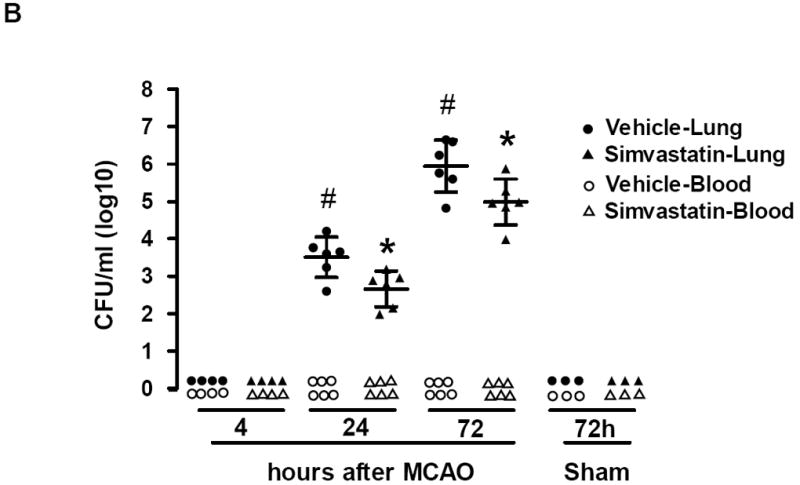
A, Representative images of HE-stained lung sections (6-μm thick) showing typical signs (thickening of alveolar walls and neutrophilic infiltrates) of pneumonia from MCAO (72h) but not from sham animals. Scale bar = 50 μm. B, Lungs and blood samples from sham and MCAO mice treated with vehicle or simvastatin were prepared for bacteriological analysis at the indicated time points after surgery. Data are expressed as CFU/ml (log 10) in blood or lung tissue homogenate. N=3-6 per group. # P<0.05 vs. vehicle control at 4h after MCAO; * P<0.05 vs. vehicle control at same time point.
Microbiological analysis revealed significant bacterial loads in lung cultures of all stroke mice at 24 and 72 h after MCAO (Fig. 5b). By examining bacteria grown on blood agar plates, we observed that bacterial cultures from lungs exhibited >95% gram-negative E. coli, consistent with other reports [31]. In contrast, lung cultures from sham-operated mice remained sterile at 72 h (Fig. 5b). In addition, no bacterial growth was observed in the blood cultures of either sham or MCAO mice, consistent with previous reports in C57Bl6 mice [31, 34]. Simvastatin significantly reduces bacterial growth in the lung tissue cultures (Fig. 5b). These data suggest that simvastatin attenuates stroke-induced lung susceptibility to bacterial infection.
Discussion
Stroke-induced peripheral immunodepression occurs mostly during the first few days after acute stroke and involves profound loss of immune cells, in particularly T- and B- cells, in secondary lymphatic organs (spleen, thymus and lymph node), leading to atrophy of these organs [19-21, 31]. The present data demonstrated a profound atrophy of spleen at 3 and 5 days of stroke in mice with a significant loss of body weight during the 5-day observation period. Further, our work reveals the potential mechanism by which simvastatin attenuates stroke-induced splenic atrophy, which is mediated, at least in part, through increased expression of mitochrondrial anti-apoptotic Bcl-2 protein and decreased translocation of proapoptotic Bax from the cytosol to mitochondria. These results should provide a molecular basis for the beneficial immune modulatory effects of simvastatin in the prevention of stroke-induced peripheral immunodepression.
The spleen is an important immune organ and has been implicated in the immune response to ischemic injury in several organs (e.g. liver, lungs, intestines) [35-37]. Previous studies have suggested that splenic atrophy in experimental stroke may contribute to brain injury possibly through 1) release of inflammatory mediators and 2) release of spleen-derived inflammatory cells into the circulation and migration into the brain, which exacerbate the brain inflammatory response and cause secondary brain damage [17-21, 38]. In accordance with this concept, splenectomy has been shown to have beneficial effects in animal models of ischemic stroke [17, 18, 22], hemorrhagic stroke [39], and traumatic brain injury [40]. The present data demonstrated that splenectomy two weeks prior to MCAO in mice significantly reduces infarct volume and neurological deficits 5 days after stroke. Further, the stroke-protective effect of splenectomy was abolished by adoptive transfer of splenocytes. These results indicate that spleen substantially contributes to brain injury in the mouse stroke model utilized in the present study. The inflammatory signals from the spleen to the ischemic brain have not been completely identified. Our previous data suggest that IFNγ plays an important role in acute experimental stroke, as IFNγ knockout mice show reduced infarct volume following transient MCAO [41]. Several studies suggest that IFNγ could play a role in the splenic response by exacerbating the inflammation associated with ischemic brain injury. Recently, Seifert et al [17, 18] reported that IFNγ was elevated early (24h) in spleens but later (72, 96h) in the brains of rats following MCAO, and the neuroprotection of splenectomy is most likely caused by the loss of IFNγ, because splenectomy reduced IFNγ expression in the brain after MCAO and that systemic administration of IFNγ reversed the protective effects of splenectomy. Moreover, intraventricular administration of IFNγ neutralizing antibodies three days post-MCAO protects the brain from stroke induced injury [42]. Collectively, these findings indicate that IFNγ may be one of the inflammatory signals originating from the spleen causing a delayed inflammatory response in the ischemic brain, supporting a role for spleen-derived IFNγ in stroke pathology. In this regard, the present study focused on investigating stroke-induced IFNγ expression in the brain and its modulation by adoptive transfer of splenocytes and by simvastatin treatment. The present data suggest that stroke-induced IFNγ expression in the brain is associated with the splenic response to cerebral ischemia. Simvastatin treatment reduced brain IFNγ (3d) and infarct volume and neurological deficits (5d) in acute experimental stroke, with a significant increase in animal survival over a 5-day period of observation. These protective effects by simvastatin were observed not only in naïve MCAO stroke mice but also in splenectomied MCAO mice adoptively transferred with splenocytes. These findings support the hypothesis that the splenic response to cerebral ischemia contributes to secondary brain injury and simvastatin attenuates ischemic brain injury contributed by spleen cells via modulating IFNγ expression in the brain in acute experimental stroke.
Immunodepression after stroke increases the susceptibility to infections, in particular pneumonia, the most relevant complication in stroke patients [1, 2]. In addition to cholesterol lowering effects, statins have anti-inflammatory and immunomodulatory properties, so called pleiotropic effects [13]. Despite some controversies exist, the treatment of patients with statins has been shown to improve both incidence and survival in acute ischemic stroke [13]. A recent clinical study shows that young patients with a first ischemic stroke who used statin poststroke had lower rates of new vascular events in a long-term follow-up [43]. However, whether statins can help to prevent stroke-induced infections is still debated in clinical practice [13, 44, 45]. Bacterial pneumonia is the most common cause of death in patients suffering from acute stroke and occur mostly within the first few days after stroke [4, 5]. In the present study, we analyzed the infection status of mice in the first 3 days after acute experimental stroke. In agreement with previous reports [19, 31, 34], the present data show that 60 min-MCAO resulted in no bacterial growth in blood samples and bacterial growth in 100% of lung tissue cultures obtained from C57Bl6 mice. Notably, simvastatin treatment significantly inhibited the bacterial growth in the lung tissue cultures. It is worthy of note that the incidence of poststroke infections may vary largely depending on not only stroke models but also mouse strains [19, 34]. Liesz et al [19] reported that mice with pure cortical infarcts in the coagulation model had no bacterial growth in blood and low-level bacterial growth in 30% of the lung tissue cultures; 30 min-MCAO resulted in no bacterial growth in blood samples and bacterial growth in 50% of lung tissue cultures. In contrast, 60% of the 90 min-MCAO mice had bacterial growth in blood cultures and 100% had growth in lung homogenates. Moreover, Schulte-Herbrüggen et al [34] reported that the susceptibility to poststroke infections in mice is strain dependent. They compared poststroke infections in mice from 129SV, C57/B6, and Balb/C strains subjected to a 60 min-MCAO. Three days after stroke, 129SV mice did not only develop bacterial chest infection, but also had a strongly increased susceptibility to bacteremia. In contrast, C57BL/6 and Balb/C mice acquired bacterial lung infections only, and these differences in susceptibility to infection did not correlate with infarct volumes. Therefore, careful interpretation of the results should be used when translating these experimental findings into clinical practice.
In summary, the results of the present study provide the first direct experimental evidence that simvastatin ameliorates stroke-induced peripheral immunodepression by attenuating spleen atrophy and lung bacterial infection. These findings contribute to a better understanding of beneficial effects of statins in the treatment of stroke, and also may provide a rationale for future investigation of translational applicability for administration of simvastatin prophylactically and/or therapeutically using clinically relevant time windows of acute ischemic stroke. This study has some limitations, including no studies of comparisons of simvastatin’s effects with other statins as well as the detailed mechanisms how statins modulate immune response after ischemic stroke; Thus, further studies are needed.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by National Institutes of Health grant HL087990 (GL) and HL026441 (DNG) and by a scientist development grant (0530166N, GL) from the American Heart Association.
Footnotes
Disclosures
None.
References
- 1.Dirnagl U, Klehmet J, Braun JS, Harms H, Meisel C, Ziemssen T, et al. Stroke-induced immunodepression: experimental evidence and clinical relevance. Stroke. 2007;38(2 Suppl):770–773. doi: 10.1161/01.STR.0000251441.89665.bc. [DOI] [PubMed] [Google Scholar]
- 2.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brait VH, Arumugam TV, Drummond GR, Sobey CG. Importance of T lymphocytes in brain injury, immunodeficiency, and recovery after cerebral ischemia. J Cereb Blood Flow Metab. 2012;32:598–611. doi: 10.1038/jcbfm.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westendorp WF, Nederkoorn PJ, Vermeij JD, Dijkgraaf MG, van de Beek D. Post-stroke infection: a systematic review and meta-analysis. BMC Neurol. 2011;11:110. doi: 10.1186/1471-2377-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katzan IL, Cebul RD, Husak SH, Dawson NV, Baker DW. The effect of pneumonia on mortality among patients hospitalized for acute stroke. Neurology. 2003;60:620–625. doi: 10.1212/01.wnl.0000046586.38284.60. [DOI] [PubMed] [Google Scholar]
- 6.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bu DX, Griffin G, Lichtman AH. Mechanisms for the anti-inflammatory effects of statins. Curr Opin Lipidol. 2011;22:165–170. doi: 10.1097/MOL.0b013e3283453e41. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, et al. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Zhang ZG, Ding GL, Jiang Q, Liu X, Meng H, et al. Multitargeted effects of statin-enhanced thrombolytic therapy for stroke with recombinant human tissue-type plasminogen activator in the rat. Circulation. 2005;12:3486–3494. doi: 10.1161/CIRCULATIONAHA.104.516757. [DOI] [PubMed] [Google Scholar]
- 10.Lynch JR, Wang H, McGirt MJ, Floyd J, Friedman AH, Coon AL, et al. Simvastatin reduces vasospasm after aneurysmal subarachnoid hemorrhage: Results of a pilot randomized clinical trial. Stroke. 2005;36:2024–2026. doi: 10.1161/01.STR.0000177879.11607.10. [DOI] [PubMed] [Google Scholar]
- 11.Jung KH, Chu K, Jeong SW, Han SY, Lee ST, Kim JY, et al. HMG-CoA reductase inhibitor, atorvastatin, promotes sensorimotor recovery, suppressing acute inflammatory reaction after experimental intracerebral hemorrhage. Stroke. 2004;35:1744–1749. doi: 10.1161/01.STR.0000131270.45822.85. [DOI] [PubMed] [Google Scholar]
- 12.Seyfried D, Han Y, Lu D, Chen J, Bydon A, Chopp M. Improvement in neurological outcome after administration of atorvastatin following experimental intracerebral hemorrhage in rats. J Neurosurg. 2004;101:104–107. doi: 10.3171/jns.2004.101.1.0104. [DOI] [PubMed] [Google Scholar]
- 13.Montecucco F, Quercioli A, Mirabelli-Badenier M, Viviani GL, Mach F. Statins in the treatment of acute ischemic stroke. Curr Pharm Biotechnol. 2012;13:68–76. doi: 10.2174/138920112798868737. [DOI] [PubMed] [Google Scholar]
- 14.Cakmak A, Yemişçi M, Köksoy C, Yazgan U, Dinçer D, Dalkara T. Statin pre-treatment protects brain against focal cerebral ischemia in diabetic mice. J Surg Res. 2007;138:254–258. doi: 10.1016/j.jss.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Zacharek A, Chen J, Cui X, Yang Y, Chopp M. Simvastatin Increases Notch Signaling Activity and Promotes Arteriogenesis After Stroke. Stroke. 2009;40:254–260. doi: 10.1161/STROKEAHA.108.524116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, et al. Atorvastatin induction of VEGF and bDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seifert HA, Leonardo CC, Hall AA, Rowe DD, Collier LA, Benkovic SA, et al. The spleen contributes to stroke induced neurodegeneration through interferon gamma signaling. Metab Brain Dis. 2012;27:131–141. doi: 10.1007/s11011-012-9283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seifert HA, Hall AA, Chapman CB, Collier LA, Willing AE, Pennypacker KR. A transient decrease in spleen size following stroke corresponds to splenocyte release into systemic circulation. J Neuroimmune Pharmacol. 2012;7:1017–1024. doi: 10.1007/s11481-012-9406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liesz A, Hagmann S, Zschoche C, Adamek J, Zhou W, Sun L, et al. The spectrum of systemic immune alterations after murine focal ischemia: immunodepression versus immunomodulation. Stroke. 2009;40:2849–2858. doi: 10.1161/STROKEAHA.109.549618. [DOI] [PubMed] [Google Scholar]
- 20.Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, et al. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol. 2006;176:6523–6531. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- 21.Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- 22.Ajmo CT, Jr, Vernon DO, Collier L, Hall AA, Garbuzova-Davis S, Willing A, et al. The spleen contributes to stroke-induced neurodegeneration. Neurosci Res. 2008;86:2227–2234. doi: 10.1002/jnr.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin R, Song Z, Yu S, Piazza A, Nanda A, Penninger JM, et al. Phosphatidylinositol-3-kinase gamma plays a central role in blood-brain barrier dysfunction in acute experimental stroke. Stroke. 2011;42:2033–2044. doi: 10.1161/STROKEAHA.110.601369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coligan JE. Current Protocols in Immunology. 1991:1.10.1–1.10.11. [Google Scholar]
- 25.Leung BP, Sattar N, Crilly A, Prach M, McCarey DW, Payne H, et al. A novel anti-inflammatory role for simvastatin in inflammatory arthritis. J Immunol. 2003;170:1524–1530. doi: 10.4049/jimmunol.170.3.1524. [DOI] [PubMed] [Google Scholar]
- 26.Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, et al. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286:1946–1949. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 27.Dimmeler SA, Aicher M, Vasa C, Mildner-Rihm K, Adler M, Tiemann H, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;08:391–397. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takano K, Tatlisumak T, Formato JE, Carano RA, Bergmann AG, Pullan LM. Glycine site antagonist attenuates infarct size in experimental focal ischemia. Postmortem and diffusion mapping studies. Stroke. 1997;28:1255–1262. doi: 10.1161/01.str.28.6.1255. [DOI] [PubMed] [Google Scholar]
- 29.Doyle KP, Yang T, Lessov NS, Ciesielski TM, Stevens SL, Simon RP, et al. Nasal administration of osteopontin peptide mimetics confers neuroprotection in stroke. J Cereb Blood Flow Metab. 2008;28:1235–1248. doi: 10.1038/jcbfm.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKallip RJ, Lombard C, Martin BR, Nagarkatti M, Nagarkatti PS. Delta(9)-tetrahydrocannabinol-induced apoptosis in the thymus and spleen as a mechanism of immunosuppression in vitro and in vivo. J Pharmacol Exp Ther. 2002;302:451–465. doi: 10.1124/jpet.102.033506. [DOI] [PubMed] [Google Scholar]
- 31.Prass K, Meisel C, Hoflich C, Braun J, Halle E, Wolf T, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke t helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 33.Wang Q, Teder P, Judd NP, Noble PW, Doerschuk CM. CD44 deficiency leads to enhanced neutrophil migration and lung injury in Escherichia coli pneumonia in mice. Am J Pathol. 2002;61:2219–2228. doi: 10.1016/S0002-9440(10)64498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schulte-Herbrüggen O, Klehmet J, Quarcoo D, Meisel C, Meisel A. Mouse strains differ in their susceptibility to poststroke infections. Neuroimmunomodulation. 2006;13:13–18. doi: 10.1159/000092109. [DOI] [PubMed] [Google Scholar]
- 35.Okuaki Y, Miyazaki H, Zeniya M, Ishikawa T, Ohkawa Y, Tsuno S, et al. Splenectomy reduced hepatic injury induced by ischemia/reperfusion in the rat. Liver. 1996;16:188–194. doi: 10.1111/j.1600-0676.1996.tb00726.x. [DOI] [PubMed] [Google Scholar]
- 36.Savas MC, Ozguner M, Ozguner IF, Delibas N. Splenectomy attenuates intestinal ischemia-reperfusion-induced acute lung injury. J Pediatr Surg. 2003;38:1465–1470. doi: 10.1016/s0022-3468(03)00497-4. [DOI] [PubMed] [Google Scholar]
- 37.Jiang H, Meng F, Li W, Tong L, Qiao H, Sun X. Splenectomy ameliorates acute multiple organ damage induced by liver warm ischemia reperfusion in rats. Surgery. 2007;141:32–40. doi: 10.1016/j.surg.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 38.Bao Y, Kim E, Bhosle S, Mehta H, Cho S. A role for spleen monocytes in post-ischemic brain inflammation and injury. J Neuroinflammation. 2010;7:92. doi: 10.1186/1742-2094-7-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee ST, Chu K, Jung KH, Kim SJ, Kim DH, Kang KM, et al. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain. 2008;131(Pt 3):616–629. doi: 10.1093/brain/awm306. [DOI] [PubMed] [Google Scholar]
- 40.Li M, Li F, Luo C, Shan Y, Zhang L, Qian Z, et al. Immediate Splenectomy Decreases Mortality and Improves Cognitive Function of Rats After Severe Traumatic Brain Injury. J Trauma. 2011;71:141–147. doi: 10.1097/TA.0b013e3181f30fc9. [DOI] [PubMed] [Google Scholar]
- 41.Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113:2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- 42.Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- 43.Putaala J, Haapaniemi E, Kaste M, Tatlisumak T. Statins after ischemic stroke of undetermined etiology in young adults. Neurology. 2011;77:426–430. doi: 10.1212/WNL.0b013e318227b1c2. [DOI] [PubMed] [Google Scholar]
- 44.van den Hoek HL, Bos WJ, de Boer A, van de Garde EM. Statins and prevention of infections: systematic review and meta-analysis of data from large randomised placebo controlled trials. BMJ. 2011;343:d7281. doi: 10.1136/bmj.d7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeh PS, Lin HJ, Chen PS, Lin SH, Wang WM, Yang CM, et al. Effect of statin treatment on three-month outcomes in patients with stroke-associated infection: a prospective cohort study. Eur J Neurol. 2012;19:689–695. doi: 10.1111/j.1468-1331.2011.03608.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


