Abstract
The neurotransmitter serotonin (5-hydroxytryptamine; 5-HT) exerts a pleiomorphic function in the modulation of information processing, through the activation of multiple receptor families. In particular, stimulation of 5-HT1A and 5-HT2A receptors leads to sensorimotor gating impairments and perceptual perturbations. Previous evidence has shown that chronic deprivation of l-tryptophan (TRP), the precursor of 5-HT, results in marked reductions of 5-HT brain levels, as well as neuroplastic alterations in 5-HT1A and 5-HT2A expression and/or signaling. Building on these premises, in the present study we tested whether a prolonged TRP deprivation may differentially impact the roles of these receptors in the regulation of the prepulse inhibition (PPI) of the acoustic startle reflex, a dependable index of gating. Male Sprague-Dawley rats were fed for 14 days with either a regimen with negligible TRP content (TR−) or the same diet supplemented of TRP (TR+). At the end of this schedule, rats were treated with the prototypical 5-HT1A receptor agonist 8-OH-DPAT (62.5–250 μg/kg, subcutaneous, s.c.) or the 5-HT2 receptor agonist DOI (.25–1 mg/kg, s.c.). Notably, the PPI deficits induced by 8-OH-DPAT in TR− rats were significantly milder than those observed in their TR+ counterparts; these effects were fully prevented by the 5-HT1A antagonist WAY-100135 (10 mg/kg, intraperitoneal). Conversely, TRP deprivation did not affect the PPI-disrupting properties of DOI. These findings suggest that prolonged 5-HT depletion attenuates the influence of 5-HT1A, but not 5-HT2 receptors on sensorimotor gating, confirming the distinct mechanisms of these two targets in PPI regulation.
Keywords: Serotonin, Tryptophan, 5-HT1A, 5HT2, Prepulse inhibition, Startle
1.1 INTRODUCTION
The neurotransmitter serotonin (5-hydroxytryptamine; 5-HT) plays a pivotal role in the emotional processing of contextual information (Merens et al, 2007; Harmer, 2008). Perturbations of 5-HT homeostasis have been shown to result in abnormalities of sensorimotor gating (Rigdon and Weatherspoon, 1992; Kehne et al, 1996; Sipes and Geyer, 1997), the ability to filter out irrelevant stimuli and generate saliency maps (Braff and Geyer, 1990). This endophenotypic alteration, albeit not inherently pathological, is featured in a number of psychiatric disorders, including schizophrenia and obsessive-compulsive disorder (for a general overview, see Braff et al, 2001). Accordingly, these conditions have been associated with alterations of 5-HTergic signaling (Roth and Meltzer, 1995; Baumgarten and Grozdanovic, 1998). One of the best-validated paradigms to measure gating integrity is afforded by the prepulse inhibition (PPI) of the acoustic startle reflex, corresponding to the attenuation of the startle response that occurs when the evoking burst is preceded by a weak prestimulus (Hoffman and Ison, 1980). Rich evidence has shown that 5-HT controls sensorimotor gating through a complex set of mechanisms, which are mediated by different receptors. The multifaceted role of 5-HT in the modulation of gating is highlighted by the fact that PPI deficits have been associated to both an excessive release of 5-HT and depletion of this neurotransmitter (Padich et al, 1996; Martinez and Geyer, 1997; Fletcher et al, 2001; Prinssen et al, 2002). In parallel with this dichotomy, PPI is significantly reduced in rats by activation of either 5-HT1A or 5-HT2A receptors. The gating impairments induced by the prototypical 5-HT1A agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) are likely to reflect the stimulation of 5-HT1A somatodendritic autoreceptors in the raphe nuclei, which leads to a decrease in 5-HT release in the forebrain (Sipes and Geyer, 1995). Conversely, the 5-HT2 receptor agonist 2,5-dimethoxy-4-iodoamphetamine (DOI) is posited to reduce PPI through activation of 5-HT2A postsynaptic receptors in the ventral pallidum (Sipes and Geyer, 1997). This functional divergence suggests that neuroplastic changes of the 5-HTergic system may yield differential effects on the modulatory influence exerted by 5-HT1A and 5-HT2A receptors on sensorimotor gating. An efficient way to test this possibility is to study the adaptive responsiveness of these two receptors with respect to PPI following the reduction of 5-HT levels. Such an analysis, however, cannot be conclusively performed with pharmacological inhibitors of 5-HT synthesis or release, since these compounds have been shown to produce intrinsic PPI deficits (Rigdon and Weatherspoon, 1992; Fletcher et al., 2001; Prinssen et al, 2002). A more suitable and naturalistic alternative to this approach is afforded by the dietary restriction of l-tryptophan (TRP), the amino acid precursor of 5-HT. We previously reported that rats chronically fed with a TRP-deficient diet exhibit a marked reduction (35–45%) of brain 5-HT levels (Fadda et al, 2000), which did not result in significant modifications of baseline PPI (Bortolato et al, 2008). Building on these premises, in the present study we analyzed the impact of long-term TRP deprivation on the distinct role of 5-HT1A and 5-HT2A receptors on PPI, to further qualify the differential functions of these targets in the modulation of sensorimotor gating.
2. EXPERIMENTAL PROCEDURES
2.1 Animals
A total of 221 adult male Sprague Dawley (SD) rats (Harlan, Italy) weighing 225–250 g, were used in this study. Animals were housed under standard laboratory conditions with free access to food and water and were maintained on a reversed light/dark schedule. Behavioral testing occurred during the dark phase. Experiments were performed in accordance with the European Communities Council Directive (86/609/EEC) for the Care and Use of Laboratory Animals and were approved by the Animal Care Committee guidelines of experimental animals.
2.2 Diet
Animals were fed with one of two different diets. One group was fed for 14 days with a TRP-deficient diet based on maize flour (TR−), which contains a very low amount of TRP with respect to the other amino acids (Bortolato et al, 2008). The second group was fed for the equivalent period with a balanced diet (TR+) that had an identical composition, with the sole exception that 180 mg of TRP/100 g of maize flour were added in place of an equivalent amount of sucrose.
2.3 Drugs
The following drugs were used: 8-OH-DPAT, WAY-100135, and DOI (Sigma-Aldrich, Milan, Italy). All substances were weighed out as salts, dissolved in saline and administered in an injection volume of 2 ml/kg.
2.4 Apparatus
The apparatus for the detection of the startle reflexes (Med Associates, St. Albans, VT) consisted of four standard cages placed in sound-attenuated chambers with fan ventilation. Each cage was a Plexiglas cylinder of 9-cm diameter mounted on a piezoelectric accelerometric platform connected to an analogue digital converter. Background noise and acoustic bursts were conveyed by two separate speakers placed close to the startle cage so as to produce a variation of sound within 1 dB across it. Both speakers and startle cages were connected to a main personal computer, which detected and analyzed all chamber variables by means of custom software. Acoustic stimuli were monitored and balanced before each testing session through a digital sound level meter (Extech Instruments, Waltham, MA), while the mechanical response of each cage was set and equalized in all chambers via a 10-Hz spinner calibrator (Med Associates).
2.5 Experimental procedure
Seven days after arrival in the animal facilities, rats were exposed to a brief baseline startle session consisting of a randomized sequence of 12- to 40-ms bursts of 115 dB (with background noise at 70 dB) interposed with three trials in which a 82-dB pre-stimulus preceded the same pulse by 100 ms. A total of four rats, exhibiting very high or very low baseline startle values (more than two standard deviations above or below the overall population median values for each experiment) were excluded from the study. Experimental groups were then established by stratified sampling based on the average startle values for each rat. Throughout the study, no significant differences in mean startle and percent PPI baseline values were found by 1-way analysis of variance (ANOVA). Each group was randomly assigned to a diet (TR− or TR+) and treatment combination. On each of the five days prior to the experiment (days 10–14 of the diet), each rats was handled for 5 min. Animals were tested for startle and PPI on the fourteenth day of the diet. Testing was conducted as previously described (Frau et al, 2006). Each session consisted of three consecutive sequences of trials (periods). Unlike the first and the third periods, during which rats were presented with only five pulse-alone trials of 115 dB, the second period consisted of a pseudorandom sequence of 50 trials, including 12 pulse-alone trials, 30 trials of pulse preceded by 74, 78, or 82-dB prepulses (ten for each level of prepulse loudness) and eight no-stimulus trials where only the background noise was delivered. Intertrial intervals were selected randomly between 10 and 15 s. The duration of pulses and prepulses was 80 and 40 ms, respectively. Prepulse–pulse delay amounted to 100 ms. Each startle session lasted about 30 min. At the end of a session, cages were thoroughly cleaned with 1% acetic acid solution and dried. Baseline PPI testing was performed the day before the beginning of either TR− or TR+ diet. Experiments were performed twenty-four h after the end of either 14-day regimen. Throughout the diet period, rats were handled daily (5 min/day), to minimize stress. No evident signs of distress were observed in TRP-deprived rats.
2.6 Data analyses
Startle amplitude values were calculated as the difference between peak-to-peak voltage during a time window of 80 ms after stimulus onset and peak-to-peak voltage in the 80-ms time window before stimulus onset. For each animal, the mean startle amplitudes for the whole session were compared by ANOVA, as required by the specific experimental design. The %PPI was calculated with the following formula: 100 − [(mean ASR amplitude for prepulse - pulse trials/mean ASR amplitude for pulse - alone trials)] x 100. Normality and homoscedasticity of data distribution were verified by using the Kolmogorov-Smirnov and Bartlett’s tests. Data were analyzed in multifactor ANOVAs (with specific design and comparisons noted below for each experiment) with the different combinations of diet and treatments as factors. For %PPI analyses, data relative to different prepulse levels were collapsed, since no interactions were found between prepulse levels and other factors throughout the study. Post-hoc analyses were performed using Tukey’s test with Spjøtvoll-Stoline correction for unequal N wherever appropriate. Alpha was set at .05.
3. RESULTS
3.1 Effects of 8-OH-DPAT on gating characteristics of TRP-deprived rats
In the first experiment, we tested whether different doses (62.5–250 μg/kg, subcutaneous, s.c.) of 8-OH-DPAT could significantly affect PPI in TR− rats as compared to rats fed with TR+ chow. Each experimental group included 9–11 rats, for a total of 38 TR+ and 39 TR− animals. Both the drug and its vehicle (0.9% saline solution) were administered 10 min prior to the placement of the rats in the startle chambers.
Startle analysis was performed by two-way ANOVA, with diet and treatment as independent factors. TR− rats exhibited a significantly lower startle reflex {main diet effect: [F(1,69) = 6.96, p < .01]}; however, no main effects for treatment [F(3,69) = .48, NS] or diet x treatment interaction [F(3,69) = .98, NS] were identified (Fig. 1A).
Fig. 1.
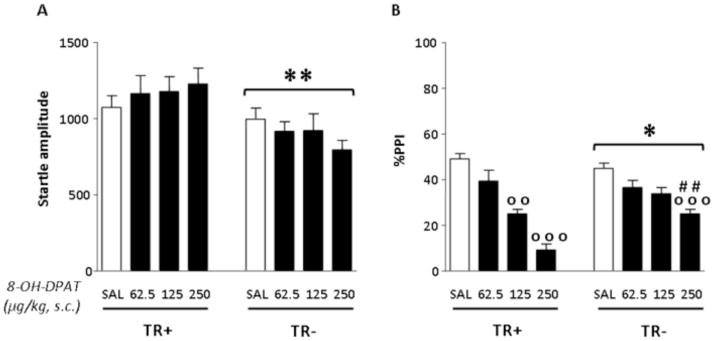
Effects of 8-OH-DPAT on startle amplitude and %PPI in rats after 14 days of either control (TR+) or TRP-poor (TR−) diet. n=9–11/group. Treatment and diet conditions are indicated below the horizontal axis. Results are expressed as mean ± SEM. 8-OH-DPAT doses are given in μg/kg (s.c.). *, p < .05, **, p < .01, main effects for diet; oo, p < .01, ooo, p < .001 in comparison with rats treated with SAL (diet x treatment interaction); ##, p < .01 in comparison with TR+ diet group (diet x treatment interaction). For further details, see text.
PPI analyses, performed with the same statistical design, revealed significant main effects for the diet [F(1,68) = 5.19, p < .05], as well as 8-OH-DPAT [F(3,68) = 44.51, p < .001] and their interaction [F(3,68) = 6.25, p < .001]. As shown in Fig. 1B, post-hoc comparisons confirmed the lack of significant differences in PPI between saline-treated TR+ and TR− rats (Bortolato et al, 2008). Furthermore, Tukey’s test revealed that the 250 μg/kg dose of the 5-HT1A receptor agonist significantly reduced PPI in both groups (p < .001 for both comparisons vs saline-treated controls); notably, this effect was significantly more pronounced in TR+ than TR− rats (p < .01). Post-hoc analyses also revealed that the 125 μg/kg dose disrupted PPI in TR+ (p < .01 in comparison with saline-treated TR+ animals), but not in their TR− counterparts.
Previous evidence has shown that 8-OH-DPAT is also a potent 5-HT7 receptor agonist (Bard et al, 1993); thus, to ascertain that the PPI variations induced by this compound in TR− rats were actually mediated by 5-HT1A receptors, we studied the effect of this compound in combination with the prototypical antagonist for this receptor, WAY-100135 (10 mg/kg, IP), in both TR− and TR+ rats (n=8–10 for each treatment group, for a total of 38 TR+ and 38 TR− rats).
Analyses were run by 3-way ANOVAs, with pre-treatment, treatment and diet as factors. Significant effects were found for diet [F(1,68) = 22.28, p < .001] and pre-treatment [F(1,68) = 13.84, p < .001], but not treatment [F(1,68) = 1.58, NS]; these results were interpreted to signify that startle amplitude was reduced in both TR− rats and animals treated with WAY-100135 (Fig. 2A). No significant diet x pre-treatment [F(1,68) = .07, NS] or diet x pre-treatment x treatment interactions [F(1,68) = 2.03, NS] were found.
Fig. 2.
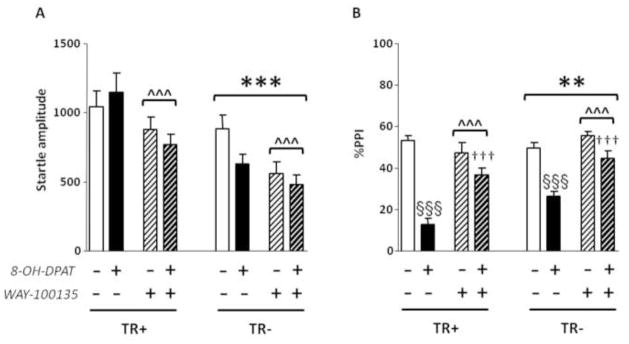
Effects of 8-OH-DPAT (250 μg/kg, s.c.) and WAY-100135 (10 mg/kg, i.p.) on startle amplitude and %PPI in rats after 14 days of either control diet (TR+) or TRP-poor (TR−) diet. n=8–10/group. Treatment and diet conditions are indicated below the horizontal axis. Results are expressed as mean ± SEM. **,p < .01; ***, p < .001, main effects for diet; ^^^, p < .001, main effects for pre-treatment. §§§, p < .001, in comparison with rats treated with SAL + SAL (pre-treatment x treatment interaction); †††, p < .001, in comparison with rats treated with SAL + 8-OH-DPAT (pre-treatment x treatment interaction). For further details, see text.
PPI analysis showed significant main effects for all three factors {diet: [F(1,67) = 8.60, p < .01]; pre-treatment: [F(1,67) = 22.95, p < .001]; treatment: [F(1,67) = 93.08, p < .001]. Moreover, a significant pre-treatment x treatment interaction [F(1,67) = 22.55, p < .001] indicated that WAY-100135 antagonized the PPI disruption caused by 250 μg/kg dose of 8-OH-DPAT (p < .001 for comparisons between SAL + SAL and SAL + 8-OH-DPAT and between SAL + 8-OH-DPAT and WAY-100135 + 8-OH-DPAT) in both TR+ and TR− rats (Fig. 2B).
3.2 Effects of DOI in gating characteristics of TRP-deprived rats
We finally tested the effects of DOI (.25–1 mg/kg, s.c.) on the regulation of startle and PPI in TR− and TR+ rats. Similarly to the previous experiment, both the drug and its vehicle ( .9% saline solution) were administered 10 min before rats were placed in the cage for behavioral testing. Each treatment group included 8 rats, for a total of 32 TR+ rats and 32 TR− rats.
Analyses of startle magnitude were performed with a 2-way ANOVA, with diet and treatment as categorical factors (Fig. 3A). No significant difference in startle amplitude was detected between animals fed with TR+ and TR− diets [(F(1,56) = .11; NS]. A significant effect for treatment [(F(3,56) = 5.93; p < .01] was found to depend on significant differences between the dose of .25 and the dose of .1 (p < .05; Tukey’s). No significant diet x treatment interactions were found [F(3.56) = .45, NS].
Fig. 3.
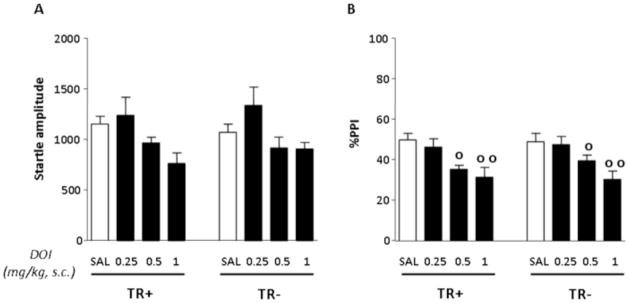
Effects of DOI on startle amplitude and %PPI in rats after 14 days of of either control (TR+) or TRP-poor (TR−) diet. n=8/group. Treatment and diet conditions are indicated below the horizontal axis. Results are expressed as mean ± SEM. DOI doses are given in mg/kg (s.c.). o, p < .05, oo, p < .01, main effects for treatment. For further details, see text.
PPI analyses were conducted with a similar statistical design, and revealed that DOI produced a dose-dependent impairment of gating [(F(3,56) = 10.41, p < .001; p < .05 and p < .01 for comparisons between SAL and DOI .5 and 1 mg/kg, respectively; Tukey]; however, this reduction was not affected by either diet {diet x treatment interaction: [(F(3,56) = .22, NS]}. Finally, no significant main effect for diet was detected [(F(1,56) = .11; NS] (Fig. 3B).
4. DISCUSSION
The major finding of the present study is that chronic dietary deprivation of TRP, the precursor of 5-HT, significantly decreased the PPI-disrupting ability of the prototypical 5-HT1A receptor agonist 8-OH-DPAT; conversely, the same regimen did not elicit significant effects on the PPI deficits induced by the 5-HT2A agonist DOI. These results are in agreement with previous reports, documenting that prolonged TRP deprivation reduced the magnitude of other outcomes of 8-OH-DPAT (d’Souza et al, 2004; Zhang et al, 2004).
Taken together, the present results suggest that the mechanisms of gating regulation mediated by 5-HT1A may be directly influenced by the brain content of 5-HT. Indeed, previous reports have documented that PPI is impaired by the activation of somatodendritic 5-HT1A autoreceptors within the raphe nuclei (Sipes and Geyer, 1995; but see Gogos et al, 2005, for contrasting results), which leads to a negative modulation on the firing rate of 5-HTergic neurons and 5-HT release in forebrain areas (Sharp and Hjorth, 1990; Blier et al, 1998). Thus, the observed functional desensitization of 5-HT1A receptors with respect to gating regulation may reflect the reduced availability of 5-HT, which may limit the ability of 5-HT1A receptors to regulate its release from presynaptic terminals. In line with this interpretation, Rigdon and Weatherspoon (1992) found that the PPI-disrupting properties of 8-OH-DPAT were ablated in rats treated with reserpine and tetrabenazine, two potent inhibitors of 5-HT release. While the interpretation of these findings was limited by the possibility of “floor effects” caused by the intrinsic gating deficits induced by these substances, the rats fed with TR− diet in our study and previous reports showed only a very mild, not significant reduction of average baseline PPI levels (Bortolato et al., 2008).
In line with our previous studies (Bortolato et al, 2008), TRP chronic depletion did not produce a significant reduction in PPI values. Previous studies have shown that pharmacological conditions that induce a near-complete inhibition of 5-HT synthesis or release can significantly attenuate PPI (Rigdon and Weatherspoon, 1992; Fletcher et al., 2001; Prinssen et al, 2002). The failure of our diet to induce robust PPI changes is likely dependent on the insufficient level of 5-HT depletion. In previous studies, our group reported that the 2-week TRP-poor diet used in this study resulted in 35–45% reduction of 5-HT brain levels (Fadda et al, 2000). In contrast, Prinssen and colleagues (2002) documented that a mild, yet significant PPI deficit, corresponded to the reduction in 5-HT content of 88.8%. In fact, the authors of that study suggested that only very marked decreases in 5-HT levels (>95%) were able to abolish PPI and that the magnitude of PPI disruption depended on the magnitude of depletion of 5-HT (Prinssen et al, 2002).
Alternatively, the observed results may depend on the desensitization (or down-regulation) of specific populations of 5-HT1A presynaptic receptors as a homeostatic adaptive changes in response to chronic TRP deprivation. Indeed, previous studies documented that long-term TRP depletion resulted in a reduction of 5-HT1A receptor binding in the frontal cortex (Kawai et al, 1992; but see also Cahir et al, 2007).
The reduction of startle in TR− rats is consistent with our previous report (Bortolato et al, 2008), which showed a reduction of startle amplitude following 14 days of TRP-deprivation. This phenomenon may have been influenced by concomitant reductions in body weight. Irrespective of this issue, the full dissociation between changes in startle amplitude and PPI in these experiments fully supports the contention that the latter effect is completely independent from startle variations.
The dietary restriction of TRP did not affect the sensitivity of 5-HT2 receptors, indicating a lack of compensatory adaptations of the latter targets to the effects of 5-HT depletion. DOI has been shown to disrupt PPI through the activation of postsynaptic 5-HT2A receptors without affecting startle parameters. It has been shown that 5HT2A receptors in the ventral pallidum regulate sensorimotor gating pathways (Sipes and Geyer 1997). It has been suggested that the activation of 5-HT2A receptors on GABAergic presynaptic terminals within the ventral pallidum may affect PPI by reduce GABA neurotransmission (Swerdlow et al., 2003). Although DOI stimulates both 5-HT2A and 5HT2C receptors, the PPI-disrupting properties of this drug have been ascribed to the 5HT2A receptors, since this effect is reversed by the selective 5-HT2A–receptor antagonist M100907, but not by either a selective 5-HT2C–receptor antagonist or a non-selective 5-HT1–receptor antagonist (Sipes and Geyer, 1997). Thus, our results further confirm previous evidence on the role of 5HT2A in mediating PPI disruption and appear to suggest that TRP-deprivation does not elicit functional alterations of 5-HT2A receptors on gating regulation, despite previous reports of an increased cortical 5-HT2A binding following three weeks of TRP-deficient diet (Cahir et al, 2007).
Gating paradigms are a powerful tool for the operative analysis of perceptual and pre-attentional disturbances in psychotic disorders (Geyer and Markou, 1995; Koch, 1999). In particular, the bulk of evidence has shown that PPI deficits in schizophrenia likely reflect the inability to adequately filter and process information. In turn, this pre-attentional impairment may lead to a number of cognitive disturbances in psychosis, such as sensory distortion, hallucinations, depersonalization, derealization and formal thought disorders (Braff et al., 2001). Indeed, distinct mechanisms can plausibly contribute to the alteration of gating functions, which are mediated by different neurotransmitters. In our previous studies, we identified that TRP deprivation sensitizes to the PPI-disrupting properties of amphetamine through a dopaminergic mechanism (Bortolato et al., 2008). Our present results show that the same treatment reduced the impact of 5HT1A receptor activation and left the outcomes of 5HT2 receptor agonists unaffected. Taken together, these results further emphasize that the 5-HTergic mechanisms underlying gating deficits are plausibly partially unrelated to the dopaminergic processes responsible for the same phenomenon.
Several limitations should be acknowledged in the present study. First, the behavioral analyses were not paralleled by biochemical evaluations of 5-HT1A or 5-HT2A expression induced by the dietary restriction of TRP; second, we did not evaluate whether other behavioral responses to 5-HT1A may be affected by prolonged TRP dietary restriction. Finally, our study did not encompass the analysis of the impact of TRP-deprivation in female rats; indeed, previous studies have shown that the PPI-disrupting effects of 8-OH-DPAT are reduced by estrogens (Gogos and van den Buuse, 2004; Gogos et al., 2006; Gogos et al., 2010).
While future studies are warranted to further explore these issues, the present study highlights novel neurobiological mechanisms underpinning the environmental adaptation of 5-HTergic system to the regulation of gating, and the neurochemical substrates of schizophrenia and psychotic disorders. Furthermore, in view of the emerging role of 5-HT1A receptors in the modulation of the PPI-disrupting properties of entactogen and hallucinogen agents (Krebs-Thompson et al, 2006; van den Buuse et al, 2011), the present findings may help elucidate the neurochemical underpinnings of these psychotomimetic drugs.
Acknowledgments
Role of funding source
This work was supported by grants from National Institute of Health (R21 HD070611, to M.B.), Tourette Syndrome Association (to M.B), USC Zumberge Research Grant (to M.B.), Sardinia Regional “Master and Back” fellowships (to R.F., V.B). The collaboration between the Universities of Southern California and Cagliari was also supported by COST Action CM1103.
The authors gratefully acknowledge the assistance of Sean Godar and Simone Tambaro in manuscript preparation.
Footnotes
Contributors
RS took part in the design of the study and contributed to the first draft of the manuscript. RF contributed to the first draft of the manuscript, performed behavioral tests and managed literature search. Maria C and VB performed behavioral tests and contributed to the data analysis. FF and Manolo C discussed and revised the paper. MB supervised the experimental design and execution, monitored data collection, performed statistical analyses, wrote and revised the manuscript. All authors read and approved the final manuscript.
Conflicts of interest
The Authors certify that there is no actual or potential conflict of interest in relation to this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bard JA, Zgombick J, Adham N, Vaysse P, Branchek TA, Weinshank RL. Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J Biol Chem. 1993;268:23422–6. [PubMed] [Google Scholar]
- Baumgarten HG, Grozdanovic Z. Role of serotonin in obsessive-compulsive disorder. Br J Psychiatry. 1998;35 (Suppl):13–20. [PubMed] [Google Scholar]
- Blier P, Piñeyro G, El Mansari M, Bergeron R, De Montigny C. Role of Somatodendritic 5-HT Autoreceptors in Modulating 5-HT Neurotransmission. Annals of the New York Academy of Sciences. 1998;861:204–216. doi: 10.1111/j.1749-6632.1998.tb10192.x. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Frau R, Orrù M, Collu M, Mereu G, Carta M, Fadda F, Stancampiano R. Effects of tryptophan deficiency on prepulse inhibition of the acoustic startle in rats. Psychopharmacology. 2008;2:191–200. doi: 10.1007/s00213-008-1116-9. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Human and animal model studies. Arch Gen Psychiatry. 1990;47:181–8. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156:234–58. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Cahir M, Ardis T, Reynolds GP, Cooper SJ. Acute and chronic tryptophan depletion differentially regulate central 5-HT1A and 5-HT 2A receptor binding in the rat. Psychopharmacology. 2007;4:497–506. doi: 10.1007/s00213-006-0635-5. [DOI] [PubMed] [Google Scholar]
- D’Souza DN, Zhang Y, Damjanoska KJ, Carrasco GA, Sullivan NR, Garcia F, Battaglia G, Doncarlos LL, Muma NA, Van de Kar LD. Estrogen reduces serotonin-1A receptor-mediated oxytocin release and Galpha(i/o/z) proteins in the hypothalamus of ovariectomized rats. Neuroendocrinology. 2007;80:31–41. doi: 10.1159/000080795. [DOI] [PubMed] [Google Scholar]
- Fadda F, Cocco S, Stancampiano R. A physiological method to selectively decrease brain serotonin release. Brain Res Brain Res Protoc. 2000;5:219–222. doi: 10.1016/s1385-299x(00)00016-7. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Selhi ZF, Azampanah A, Sills TL. Reduced brain serotonin activity disrupts prepulse inhibition of the acoustic startle reflex. Effects of 5,7-dihydroxytryptamine and p-chlorophenylalanine. Neuropsychopharmacology. 2001;24:399–409. doi: 10.1016/S0893-133X(00)00215-3. [DOI] [PubMed] [Google Scholar]
- Frau R, Orrù M, Fà M, Casti A, Manunta M, Fais N, Mereu G, Gessa GL, Bortolato M. Effects of topiramate on the prepulse inhibition of the acoustic startle in rats. Neuropsychopharmacology. 2007;32:320–31. doi: 10.1038/sj.npp.1301115. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Markou A. Animal models of psychiatric disorders. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology. The Fourth Generation of Progress. Raven Press; New York: 1995. pp. 787–798. [Google Scholar]
- Gogos A, Kwek P, Chavez C, van den Buuse M. Estrogen treatment blocks 8-hydroxy-2-dipropylaminotetralin- and apomorphine-induced disruptions of prepulse inhibition: involvement of dopamine D1 or D2 or serotonin 5-HT1A, 5-HT2A, or 5-HT7 receptors. J Pharmacol Exp Ther. 2010;333:218–27. doi: 10.1124/jpet.109.162123. [DOI] [PubMed] [Google Scholar]
- Gogos A, Nathan PJ, Guille V, Croft RJ, van den Buuse M. Estrogen prevents 5-HT1A receptor-induced disruptions of prepulse inhibition in healthy women. Neuropsychopharmacology. 2006;4:885–9. doi: 10.1038/sj.npp.1300933. [DOI] [PubMed] [Google Scholar]
- Gogos A, Kusljic S, van den Buuse M. 8-OH-DPAT-induced effects on prepulse inhibition: pre- vs. post-synaptic 5-HT1A receptor activation. Pharmacol Biochem Behav. 2005;81:664–72. doi: 10.1016/j.pbb.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Gogos A, Van den Buuse M. Estrogen and progesterone prevent disruption of prepulse inhibition by the serotonin-1A receptor agonist 8-hydroxy-2-dipropylaminotetralin. J Pharmacol Exp Ther. 2004;309:267–74. doi: 10.1124/jpet.103.061432. [DOI] [PubMed] [Google Scholar]
- Harmer CJ. Serotonin and emotional processing: does it help explain antidepressant drug action? Neuropharmacology. 2008;55:1023–8. doi: 10.1016/j.neuropharm.2008.06.036. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Ison JR. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychol Rev. 1980;87:175–189. [PubMed] [Google Scholar]
- Kawai K, Yokota N, Sera H, Tanra AJ, Yamawaki S, Seo A. Effect of long-term feeding with tryptophan-free diet on the circadian rhythm in rats. Yakubutsu Seishin Kodo. 1992;12:75–84. [PubMed] [Google Scholar]
- Kehne JH, Padich RA, McCloskey TC, Taylor VL, Schmidt CJ. 5-HT modulation of auditory and visual sensorimotor gating: I. Effects of 5-HT releasers on sound and light prepulse inhibition in Wistar rats. Psychopharmacology. 1996;124:95–106. doi: 10.1007/BF02245609. [DOI] [PubMed] [Google Scholar]
- Koch M. The neurobiology of startle. Prog Neurobiol. 1999;2:2107–28. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Ruiz EM, Masten V, Buell M, Geyer MA. The roles of 5-HT1A and 5-HT2 receptors in the effects of 5-MeO-DMT on locomotor activity and prepulse inhibition in rats. Psychopharmacology. 2006;189:319–29. doi: 10.1007/s00213-006-0566-1. [DOI] [PubMed] [Google Scholar]
- Martinez DL, Geyer MA. Characterization of the disruptions of prepulse inhibition and habituation of startle induced by alpha-ethyltryptamine. Neuropsychopharmacology. 1997;16:246–255. doi: 10.1016/S0893-133X(96)00240-0. [DOI] [PubMed] [Google Scholar]
- Merens W, Willem Van der Does AJ, Spinhoven P. The effects of serotonin manipulations on emotional information processing and mood. J Affect Disord. 2007;103:43–62. doi: 10.1016/j.jad.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Padich RA, McCloskey TC, Kehne JH. 5-HT modulation of auditory and visual sensorimotor gating: II. Effects of the 5-HT2A antagonist MDL 100,907 on disruption of sound and light prepulse inhibition produced by 5-HT agonists in Wistar rats. Psychopharmacology. 1996;124:107–116. doi: 10.1007/BF02245610. [DOI] [PubMed] [Google Scholar]
- Prinssen EP, Assié MB, Koek W, Kleven MS. Depletion of 5-HT disrupts prepulse inhibition in rats: dependence on the magnitude of depletion, and reversal by a 5-HT precursor. Neuropsychopharmacology. 2002;26:340–347. doi: 10.1016/S0893-133X(01)00348-7. [DOI] [PubMed] [Google Scholar]
- Rigdon GC, Weatherspoon JK. 5-Hydroxytryptamine 1a receptor agonists block prepulse inhibition of acoustic startle reflex. J Pharmacol Exp Ther. 1992;263:486–93. [PubMed] [Google Scholar]
- Roth BL, Meltzer HY. The role of serotonin in schizophrenia. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology. The Fourth Generation of Progress. Raven Press; New York: 1995. pp. 1215–1228. [Google Scholar]
- Sharp T, Hjorth S. Application of brain microdialysis to study the pharmacology of the 5-HT1A autoreceptor. J Neurosci Methods. 1990;34:83–90. doi: 10.1016/0165-0270(90)90045-h. [DOI] [PubMed] [Google Scholar]
- Sipes TE, Geyer MA. DOI disrupts prepulse inhibition of startle in rats via 5-HT2A receptors in the ventral pallidum. Brain Res. 1997;761:97–104. doi: 10.1016/s0006-8993(97)00316-8. [DOI] [PubMed] [Google Scholar]
- Sipes TA, Geyer MA. 8-OH-DPAT disruption of prepulse inhibition in rats: reversal with (+)WAY 100,135 and localization of site of action. Psychopharmacology. 1995;117:41–8. doi: 10.1007/BF02245096. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Shoemaker JM, Platten A, Pitcher L, Goins J, Crain S. Heritable differences in the effects of amphetamine but not DOI on startle gating in albino and hooded outbred rat strains. Pharmacol Biochem Behav. 2003;75:191–7. doi: 10.1016/s0091-3057(03)00078-9. [DOI] [PubMed] [Google Scholar]
- van den Buuse M, Becker T, Kwek P, Martin S, Ruimschotel E, Risbrough V. Disruption of prepulse inhibition by 3,4-methylenedioxymethamphetamine (MDMA): comparison between male and female wild-type and 5-HT(1A) receptor knockout mice. Int J Neuropsychopharmacol. 2011;14:856–61. doi: 10.1017/S1461145711000101. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gray TS, D’Souza DN, Carrasco GA, Damjanoska KJ, Dudas B, Garcia F, Zainelli GM, Sullivan Hanley NR, Battaglia G, Muma NA, Van de Kar LD. Desensitization of 5-HT1A receptors by 5-HT2A receptors in neuroendocrine neurons in vivo. J Pharmacol Exp Ther. 2004;1:59–66. doi: 10.1124/jpet.103.062224. [DOI] [PubMed] [Google Scholar]


