Abstract
Objectives
To evaluate the ability of the Ad28.gfap.atoh1 to promote hair cell regeneration and hearing recovery in cochlea injured with kanamycin and furosemide.
Study Design
In vivo model of hair cell ablation and subsequent treatment with Atoh1
Methods
The hair cells of C57BL/6 mice were ablated with systemic administration of kanamycin and furosemide. The left ears were treated with Ad28.gfap.atoh1. Right ears were not treated. Pre-ablation audiograms and DPOAEs were compared to one or two month post-ablation studies. Harvested cochleae were examined for histologic evidence of hair cell regeneration and spiral ganglion cell survival.
Results
Delivery of Ad28.gfap.atoh1 results in development of auditory hair cells. There was no recovery of DPOAEs at one or two months post-treatment. Two months after delivery of Ad28.gfap.atoh1, the left ear exhibited a moderate recovery of hearing at 4 and 8 kHz (p<0.01). There was no significant difference at 16 or 32 kHz. One month after treatment, Myosin VII positive immunohistochemical staining can be seen in both the inner and outer hair cells of the treated ear. In the untreated ear, minimal myosin VII positive debris is seen, with no indication of normal hair cells. Two months after ablation, there is evidence of hair cell recovery on the treated side, while the untreated cochlea demonstrates a flattened epithelium. Untreated ears showed decreased spiral ganglion cell density at the basal turn compared to treated ears.
Conclusions
Ad28.gfap.atoh1 promotes hair cell regeneration in cochlea ablated with kanamycin and furosemide resulting in moderate hearing recovery.
Keywords: Hair cell regeneration, atoh1, adenovector, hearing
Introduction
Hearing loss is a widespread problem that not only impedes effective communication, but also impairs the patient’s ability to interact with society as a whole. 16.1% of US citizens between the ages of 20 and 69 suffer from some degree of hearing loss. Not surprisingly, the elderly are disproportionately afflicted by hearing loss, with up to 60 to 83% demonstrating loss. This can presumably be attributed to the fact that this population has had more time and opportunity to accumulate acoustic injuries.1 A recent study, however, has also demonstrated that the prevalence of hearing-related disorders appears to be increasing among patients 20 to 29 years old.2 Sensorineural hearing loss (SNHL) may be congenital, noise induced, infection related or due to exposure to ototoxic agents. This form of hearing loss is typically considered to be permanent due to the irreversible loss of sensory hair cells. Progenitors to mammalian hair cells exit the cell cycle early in development and are unable to undergo further division in response to damage.3,4,5 Currently, treatment for SNHL is limited to amplification and cochlear implantation.
Over the last decade, gene therapy has emerged as a promising alternative for treating disorders of the inner ear, to include SNHL. Over-expression of the basic helix-loop-helix transcription factor Atoh1 (also known as Math 1) is now known to be sufficient to promote supporting cell trans-differentiation and restoration of hair cells.6,7 Several different vector systems have been examined for delivery of the Atoh1 gene. Due to the ease of production, high transduction efficiency and well-studied pharmacodynamics, adenovirus is frequently used as a vehicle to deliver genes to the inner ear.8,9 Using an adenoviral vector, investigators have not only demonstrated morphologic recovery of the sensory epithelium, as well as improvement in auditory brainstem response (ABR) thresholds after treatment.10
Over 51 serotypes of the adenovirus have been identified to date. These have been further classified into subgroups A-F. Most of these initial studies using adenovirus as a delivery vehicle were performed using the common Ad5 serotype. This particular serotype is known to be immunogenic in humans, with approximately 65 to 75% of the population demonstrating neutralizing antibodies to the virus.11,12 The presence of these antibodies potentially lowers the safety and efficacy profiles of these vectors for translational studies in humans. Our lab recently investigated alternate adenovector serotypes. The Ad28 serotype not only displayed superior transfection kinetics compared to Ad5, but it also demonstrated preferential distribution into supporting cells (Schlecker C, Praetorius M, Brough DE, Presler RG, Hsu C, Plinkert PK and Staecker H. Selective atonal gene delivery improves balance function in a mouse model of vestibular disease. Gene Therapy, 2011, In Press). This, in addition to the relatively low prevalence of neutralizing antibodies in human population studies, makes Ad28 a strong candidate for gene delivery for disorders of the inner ear.
In this study, we ablated cochlear hair cells in C57Bl/6 mice using a combination of subcutaneous kanamycin and intraperitoneal furosemide as described by Oesterle et al.13 We then treated the left ear of each animal by injecting Ad28.gfap.atoh1 vector into the perilymph via a posterior canal injection, leaving the right ear untreated to serve as an internal control. The mice were then examined for functional recovery of hearing by comparing pre- and post-treatment auditory brainstem responses (ABR) and distortion product otoacoustic emissions (DPOAE). Histological evidence of hair cell recovery was obtained via immunohistochemistry and histology at one and two months post vector delivery.
Methods
Animals and Anesthesia
All studies used 3 month-old C57BL/6 mice and were approved by the University of Kansas guidelines for animal care and housing. Prior to audiologic testing or surgical procedures, mice were anesthetized with a mixture of Ketamine (100mg/kg), Xylazine (5mg/kg) and Acepromazine (2mg/kg) administered intraperitoneally.
Auditory brainstem response and distortion product otoacoustic emissions
Auditory brainstem responses (ABR) were measured bilaterally in each animal using the Smart EP Program (Intelligent Hearing Systems Corp, Miami, Florida). Pure tone stimulation of 500 μs duration was administered at 4kHz, 8kHz, 16kHz and 32kHz using a high frequency transducer. Thresholds were obtained forty-eight hours prior to ablation and thirty days after treatment with the vector. For each animal, pre- and postoperative thresholds at each frequency and were determined and examined for statistically significant differences between the treated (left) and non-treated (right) ears by ANOVA for repeated measures (Sigma Stat 3.5). Hearing outcomes were determined prior to vector aminoglycoside treatment, at 10 days post aminoglycoside treatment and at 30 and 60 days post vector delivery (40 and 70 days post aminoglycoside treatment).
While under anesthesia for ABR testing, distortion product otoacoustic emissions (DPOAE) were also obtained. A range of pure tones from 2 to 32 kHz (16 sweeps) was used to obtain the DPOAE for each ear. The pure tone ratio F2/F1 was set at 1.22 and the pure tone amplitudes were fixed at 65dB for L1 and 55dB for L2 (Intelligent Hearing System, SmartOAE Program, Intelligent Hearing Systems Corp, Miami, Florida). Similarly, pre- and post-treatment DPOAEs were evaluated for statistically significant differences in treated and non-treated ears by ANOVA for repeated measures (Sigma Stat 3.5). Amplitude of DPOAEs is reported in dB for 2211, 3130, 4422, 6244, 8844, 12503, 17672, 24990 and 35344 Hz.
Hair cell ablation
Hair cell ablation was carried out via a single subcutaneous injection of kanamycin dissolved in phosphate buffered saline (1 mg/g body weight, Sigma-Aldrich Co., St. Louis, Mo) followed 30 minutes later by an intraperitoneal injection of furosemide (0.4 mg/g body weight, Sigma-Aldrich Co., St. Louis, Mo). For verification of efficacy of the ototoxin treatment, mice were treated as described above and evaluated for presence or absence of DPOAEs at 10 days post ototoxin treatment (n=5). Subsequently, temporal bones were harvested and evaluated for hair cell integrity by immunofluorescent staining for myosin VIIa. All subsequent studies used a 10-day period after ototoxin exposure prior to administration of vector.
Vector production and application
The construction of Ad28-based adenovectors was conducted as described in Gall et. al.14 The Ad28 vectors for these studies are deleted in both adenovirus E1A and E1B (E1), and are constructed and produced using 293-ORF6 cells. The transgene expression cassette is contained in the E1 region.15
For the construction of Ad28 vectors, a full length Ad28 virus genome was cloned into a plasmid based system. An Ad28 specific left end shuttle plasmid was built in the same manner as described for Ad5. The regions encoding the Ad28 E1A and E1B region were replaced with the transgene expression cassette. Full length E1 deleted Ad28 vector genomes were then constructed and produced using 293-ORF6 cells. The 293-ORF6 cell line allows for growth of E1 deleted adenoviruses from multiple serotypes.16 The expression cassettes used in these studies include a human CMV i.e. promoter driven cassette and a human glial fibrillary acidic protein (GFAP) promoter driven cassette. Use of the glial fibrillary acidic protein (GFAP) promoter has been shown to induce expression of the delivered transgene to supporting cells.17 The atonal expressing Ad28 adenovector used in these studies expresses the mouse atonal gene (Ad28.gfap.atoh1) driven by the human glial fibrillary acidic protein (GFAP) promoter with an SV40 poly-adenylation site and transcriptional stop 3’ of the open reading frame. Total particles are analyzed by a spectrophotometric assay that has been standardized and qualified to reliably and robustly quantitate the total particles (PU) within a lot of adenovirus. All dosing was done by total pu. After purification vector lots are aliquoted and stored at - 80°C.
The vectors were administered into the perilymph of the left posterior semicircular canal. The canal was approached through a post-auricular incision. Once adequately exposed, the canal was entered with a drill. One μL of vector was injected into the posterior canal with a Hamilton micro-syringe. The bony defect was closed with bone wax (Ethicon Inc. USA) and covered with muscle. The right ear was not treated and served as an internal control. All vectors were delivered 10 days after ototoxin administration and verification of hearing loss by DPOAEs. At one month (n=7) and two months (n=9) post vector administration left and right hearing was retested by ABR and DPOAEs and subsequently temporal bones were harvested.
Immunohistochemistry
Cochleae were harvested after completion of audiologic testing in order to assess for histological evidence of hair cell regeneration. The mice were anesthetized with Beuthanasia-D (10ml/kg, Schering-Plough Animal Health) and perfused with 4% paraformaldehyde. Both temporal bones were removed and fixed in 4% paraformaldehyde overnight. Temporal bones were decalcified and processed for either frozen sections, paraffin embedding or plastic sectioning. For evaluation of hair cell integrity 10 days after ototoxin cochleae were frozen in OCT and 10 micron sections were cut and mounted on silane coated slides. At one month post vector delivery animals were processed for paraffin embedding and immunofluorecent or confocal microscopy. After decalcification in RDO rapid decalcification solution (Apex Engineering Products Corporation, Aurora, IL) for 30 minutes, the tissue was embedded in paraffin. Ten-micron sections were cut and placed on slides. After deparaffinization and antigen retrieval (Dako Target Retrieval Solution, Dako, Denmark), representative sections were stained with anti-Myosin VIIa (rabbit, Invitrogen, USA, 1:50) as a primary antibody. A fluorescent labeling was performed with a secondary anti-body (Alexa Fluor 488, goat anti-rabbit IgG, Invitrogen, USA, 1:100). Sections were cover-slipped with ProLong Gold anti-fade medium (Invitrogen, USA) and examined by confocal microscopy (Nikon Confocal Microscope C1, Nikon corp. Japan). The untreated right ears were used as controls and processed and examined in the same manner.
Plastic Sections
The mice were anesthetized with Beuthanasia-D (10ml/kg, Schering-Plough Animal Health) and perfused with 4% paraformaldehyde. The cochleae were retrieved from the temporal bone, and the round window membrane was opened. After rinsing the cochlea in ddH2O, the specimens were placed in a 15 mL conical tube filled with Osmium Tetroxide for 1 hour. The cochlea were then rinsed again in ddH2O, before being decalcified for 30 minutes in RDO Rapid Decalcifier Specimens were then subjected to serial rinses in PBS, ddH2O and increasing concentrations of EtOH. Subsequently, the cochlea were bathed for 30 minutes on Propylene Oxide (PO) before being placed in a 1:1 mixture of Araldite (Electron Microscopy Sciences, Hatfield, PA) and PO for an additional hour. Specimens were then transferred to a 2:1 mixture of Araldite and PO and place on an orbital shaker overnight. Specimens were transferred to a capsule filled with Araldite mix and allowed to de-gas in a vacuum chamber for two hours. The cochleae were then placed in a 60 degree centigrade oven for 48 hours to allow the Araldite plastic to harden. Forty micrometer sections were made of the specimens, which were then mounted on Colorfrost Plus Slides (Fisher Scientific, Pittsburgh, PA).
Results
Model Development
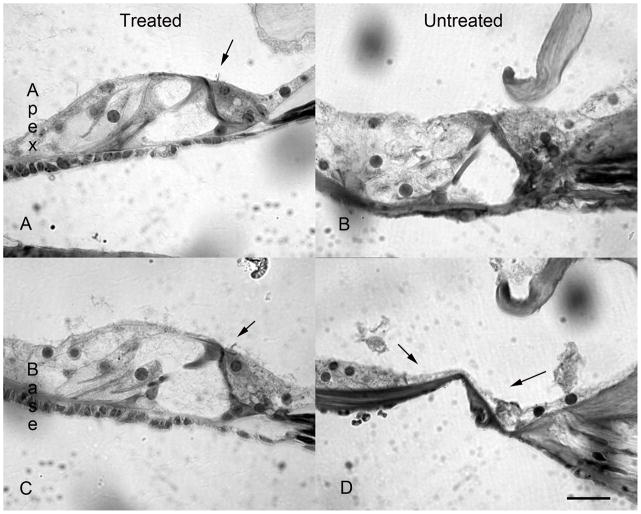
To ensure that aminoglycoside and diuretic treatment adequately ablated all hair cells a subset of animals was tested for hair cell survival at ten days post kanamycin and furosemide treatment. At 10 days post ototoxin delivery, mice (n=5) were anesthetized and evaluated for presence or absence of DPOAEs in order to evaluate outer hair cell function. As seen in Fig 2A, prior to kanamycin and furosemide treatement, mice demonstrated robust DPOAEs in both the right and left ears at 8844 to 17672 Hz. Higher frequency emissions were within the noise floor. By 10 days after ototoxin ablation, DPOAEs were eliminated on both the right and the left (Fig 2B). Evaluation of inner hair cell and functional hearing by ABR (Fig 3) demonstrated that 3 month-old C57Bl/6 mice had thresholds in the 35–40 dB range across all frequencies tested prior to ototoxin exposure. By 10 days post ototoxin administration, their hearing dropped to the 100 dB range in the low frequencies and was immeasurable at the high frequencies (Fig 3) in both left and right ears. Histologic sections of these temporal bones were obtained to confirm structural ablation of hair cells in addition to functional ablation. A merged phase contrast and DAPI view of a mid turn organ of Corti is seen in Fig 1A. There is collapse of the tunnel but as seen by nuclear staining, there are remaining cells in the region of Corti's organ. Immunofluorescent staining for myosin VII did not show any cellular labeling in the region of the organ of Corti (arrow, Fig 1B). Similarly, there was no survival of hair cells at the apical or basal turns in either ear (data not shown).
Figure 2.
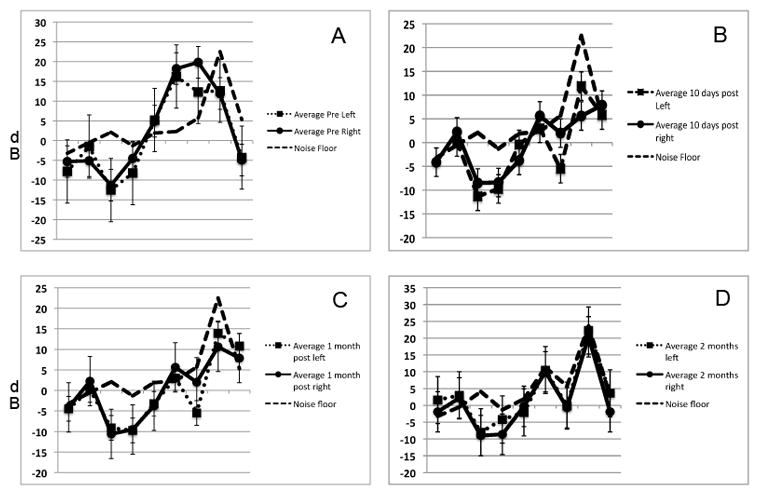
Effect of kanamycin and furosemide treatment on DPOAE generation. Mice were tested for the presence of DPOAEs pre kanamycin treatment (A), ten days after otototoxin (B), and one (C) and two (D) months after delivery of Ad28.gfap.atoh1. At ten days post ototoxin there was complete loss of DPOAEs in both the right and the left ear. No recovery of OAEs was seen at either one or two months after atoh1 delivery.
Figure 3.
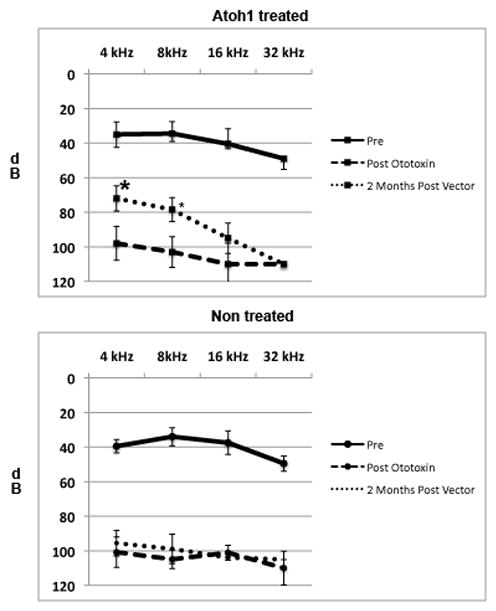
Effect of ototoxin treatment and delivery of Ad28.gfap.atoh1 on hearing. Adult mice were tested for hearing loss prior to administration of aminoglycosides and diuretic. Treatment with kanamycin and furosemide resulted in a reduction of hearing thresholds to and average of 100 dB in all frequencies tested in both the left and right ears. Two months after delivery of Ad28.gfap.atoh1 to the left ear there was a moderate recovery of hearing at 4 and 8 kHz. There was no recovery in the right, non vector treated ear. Difference in hearing at 4 and 8 kHz was statistically significant (p<0.01) when comparing the left and right sides. There was no significant difference in hearing at 16 or 32 kHz when comparing the treated to the untreated side.
Figure 1.
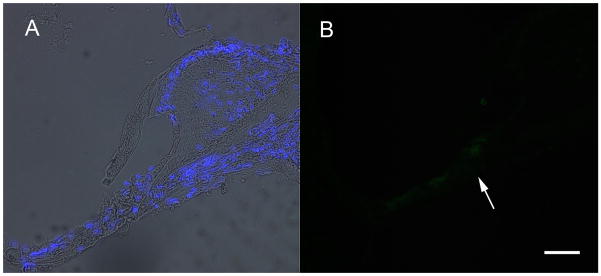
To ensure that aminoglycoside and diuretic treatment adequately ablated all hair cells, a subset of animals was tested for hair cell survival at ten days post kanamycin and furosemide treatment. A merged phase contrast and DAPI view of a mid turn organ of Corti is seen in A. There is collapse of the tunnel but, as seen by nuclear staining, there are remaining cells in the region of Corti's organ. Immunofluorescent staining for myosin VII did not show any cellular labeling in the region of the organ of Corti (arrow, B). Bar =10 um
Effect of Ad28.gfap.atoh1 delivery at one month
A subset of animals was treated with aminoglycosides/diuretic followed by Ad28.gfap.atoh1 and evaluated at one-month post vector delivery to the inner ear. Evaluation of DPOAEs demonstrated that there was no recovery of outer hair cell function at this point in time (Fig 2C). Temporal bones were evaluated for the presence of hair cells one month after delivery of atoh1 by immunofluorescent staining for myosin VII and analysis by confocal microscopy. In the apical turn of treated (left) ears myosin positive staining can be seen in both the inner and outer hair cell regions (Fig 4A). The arrow indicates a myosin VII positive cell in Corti’s tunnel below a normal appearing inner hair cell. Two myosin VII positive cells can be seen lateral to the normal outer hair cell region. In the mid-turn of the treated side (Fig 4B), multiple myosin VII positive cells are seen in the inner hair cell region (arrow) indicating the presence of supernumerary hair cells. Three myosin VII positive cells that do not extend to the basement membrane can be seen in the outer hair cell region. In the untreated ear only minimal myosin VII positive debris can be seen in the apical turn (Fig 4C), with no indication of myosin-expressing hair cells in any of the turns.
Figure 4.
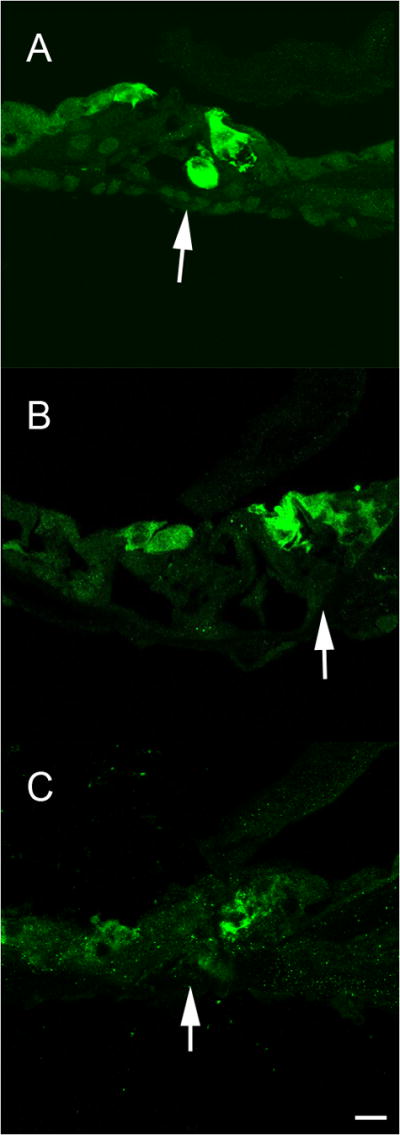
Delivery of Ad28.gfap.atoh1 results in development of auditory hair cells. Temporal bones were evaluated for the presence of hair cells one month after delivery of atoh1 by immunofluorescent staining for myosin VII. In the apical turn of treated (left) ears, myosin positive staining can be seen in both the inner and outer hair cell regions (A). The arrow indicates a myosin VII positive cell in Corti’s tunnel below a normal appearing inner hair cell. Two myosin VII positive cells can be seen lateral to the normal outer hair cell region. In the midturn of the treated side (B), multiple myosin VII positive cells are seen in the inner hair cell region (arrow) indicating the presence of supernumerary hair cells. Three myosin VII positive cell that do not extend to the basement membrane can be seen in the outer hair cell region of the midturn organ of Corti. In the untreated ear only minimal myosin VII positive debris can be seen in the apical turn (C), with no indication of normal hair cells in any of the turns.
Evaluation of hearing and hair cell regeneration 2 months after atoh1 delivery
Two months after vector delivery ears that had been treated with atoh1 demonstrated improved hearing compared to the contralateral untreated ear (Fig 3). Treatment with kanamycin and furosemide resulted in a reduction of hearing thresholds to and average of 100 dB in all frequencies tested in both the left and right ears. Two months after delivery of Ad28.gfap.atoh1 to the left ear there was a moderate recovery of hearing at 4 and 8 kHz. There was no recovery in the right, non-vector treated ear. Difference in hearing at 4 and 8 kHz was statistically significant (p<0.01) when comparing the left and right side, with thresholds in the atoh 1 treated ear reaching 75–80dB. There was no significant difference in hearing at 16 or 32 kHz when comparing the treated to the untreated side. DPOAE function did not recover in either the treated or the untreated ear (Fig 2D).
Histological evaluation of the two-month cohort was carried out by evaluation of osmium stained 5 micron cut plastic sections. Two months after delivery of Ad28.gfap.atoh1 to the damaged ear, significant differences can be seen between the atoh1 treated and untreated sides. In the apical turn of the atoh1 treated ear there is clear evidence of the presence of inner hair cells, but not outer hair cells. The arrow in Fig 5A points to the apical turn stereocilia of the inner hair cell. The apical turn of the untreated side (Fig 5B) shows no evidence of inner or outer hair cells. In the basal turn of the treated side (Fig 5C), inner hair cells (arrow) can still be identified. The basal turn of the untreated side shows a flattened epithelium without any of the features of Corti’s organ (Fig 5D). At two months post atoh1 treatment, differences could also be seen in spiral ganglion density. The apical turn of atoh1 treated ears (Fig 6A) and non-treated ears (Fig 6B) had a similar spiral ganglion density. The basal turn of the treated (Fig 6C) and untreated (Fig 6D) ears showed differences in spiral ganglion density with the spiral ganglion area that corresponded to the region of missing organ of Corti (Fig 5D).
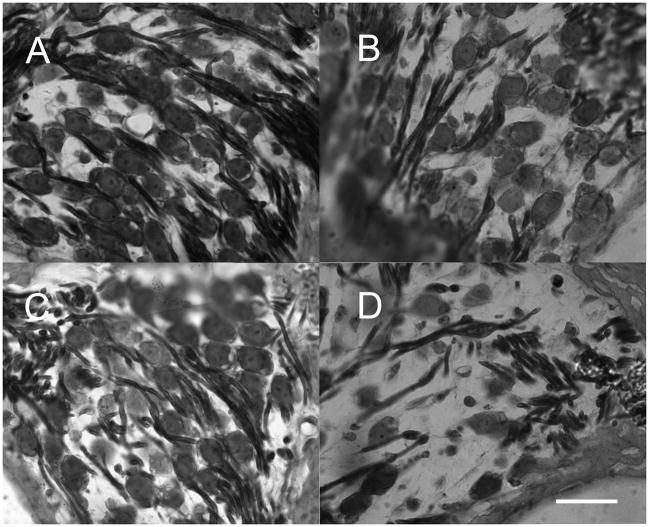
Figure 5.
Two months after delivery of Ad28.gfap.atoh1 to the damaged ear, significant differences can be seen between the atoh1 treated and untreated sides. Anti-Myosin VIIa staining of plastic sections. In the apical turn of the atoh1 treated ear there is clear evidence of the presence of inner hair cells. The arrow in (A) points to the apical turn stereocilia of the inner hair cell. The apical turn of the untreated side (B) shows no evidence of inner or outer hair cells. In the basal turn of the treated side (C), inner hair cells (arrow) can still be identified. The basal turn of the untreated side shows a flattened epithelium without any of the features of Corti’s organ (D).
Figure 6.
Survival of spiral ganglion cells in atoh1 treated and non-treated ears. Osmium staining of plastic sections. At two months post atoh1 treatment differences could be seen in spiral ganglion density in the basal turns. The apical turn of atoh1 treated ears (A) and non-treated ears (B) had a similar spiral ganglion density. The basal turn of the treated (C) and untreated (D) ears showed differences in spiral ganglion density with the spiral ganglion area that corresponded to the region of missing organ of Corti (5 D) showing reduced spiral ganglion density.
Discussion
Utilization of a single dose aminoglycoside and diuretic treatment has enabled the reliable ablation of hearing and cochlear hair cells in the mouse (Figs 1–3). Delivery of atoh 1 by an Ad28 capsid vector and driven by the supporting cell specific gfap promoter resulted in restoration of inner hair cells that could be seen by one month post atoh1 delivery. Inner hair cells were maintained through two months post treatment (Fig 5) at which point recovery of low frequency hearing could be documented (Fig 3). DPOAE function was ablated shortly after aminoglycodside treatment and did not recover in with the atoh1 treated or contralateral untreated control ear (Fig 2).
In this particular study, we elected to ablate the hair cells using kanamycin and furosemide as described by Osterle et al.13 These ototoxic agents are administered systemically, thus ablating both cochleae simultaneously. The untreated cochleae can then be used as an internal control for comparison to the treated ear, eliminating some of the variability that is sometimes seen with aminoglycoside treatment in mice. As described by Oesterle et. al., use of a single treatment of aminoglycoside and diuretic should preferentially ablate cochlear hair cells. It appears from our immunohistochemical sections that in our hands the combination of kanamycin and furosemide may be more destructive than originally anticipated. The untreated ear sections clearly demonstrate disruption of the entire sensory epithelium with only cellular debris and no intact hair cells on immunohistochemical staining and imaging (Figs 1,4,5). This may be due to strain differences since different mouse strains are known to have differential susceptibility to aminoglycosides.18 Similarly testing by DPOAEs or ABR demonstrated a profound hearing loss in the animals treated with this combination of ototoxins (Figs 2,3).
Histological sections of Ad28.gfap.atoh1 treated ears demonstrated hair cell regeneration in the organ of Corti compared to the untreated side (Figs 4,5). There were, however, several structural irregularities. Supernumerary inner hair cells could be observed at one-month post atoh1 delivery (Fig 4). Additionally the cells labeling in the outer hair cell region appeared to be in the region of the Hensen cells but not in the associated Dieter’s cells, which raises the question as to which supporting cells are actually undergoing trans-differentiation or producing new hair cells. At two months post atoh1 delivery, inner hair cells could be demonstrated in the treated ear but not in on the untreated side (Fig 5). Myosin-expressing cells were no longer seen in either group. Collapse of Corti’s organ continued in the basal turn of the untreated side leaving a “flat epilthelium” that has previously been described in severe aminoglycoside injury in guinea pigs.19 As noted in the aforementioned study, the overly damaged epithelium does not appear to respond to atoh1, possibly explaining some of the limits of hair cell recovery that are seen in this series of experiments.
Post-ablation audiometric testing showed a significant increase in ABR thresholds as well as decreased DPOAEs. We failed to demonstrate any improvement in hearing in the treated ear ABRs or DPOAEs compared to the untreated ear at one-month post atoh1 delivery, but at two months post delivery there was a statistically significant recovery of ABR thresholds ear in the low frequencies (Fig 3). Izumikawa similarly noticed in guinea pig studies that statistically significant improvements in hearing were not demonstrable until 8 weeks after treatment with the gene product.10 Future studies will need to address the sequence of events involved in remodeling and reinnervation of the regenerated sensory epithelium and the time frame required for optimal recovery. Although there was evidence of ABR recovery at two months, DPOAEs never recovered after ablation. This is consistent with the histologic data, in which the myosin-expressing cells in the outer tunnel seen at one month were not maintained in the two month specimens. It may be that additional factors may be required for the differentiation and maintenance of outer hair cells in addition to the over-expression of atoh 1 in this model.
Despite the presence of inner hair cells in the basal half of the atoh1 treated ear, recovery of hearing was not seen in the high frequency range. There did appear to be some possible secondary benefits to hair cell regeneration in this region compared to the contralateral ear. In the non-treated basal turn, a distinct difference in spiral ganglion density could be seen when comparing treated to untreated ears. Potentially the basal turn is overall is too damaged by this ototoxin treatment to recover hearing but the presence of a regenerated hair cell may allow neurotrophin production and tropic support of the spiral ganglion in this region (Figs 5,6). This has significant implications with regards to the potential for combined modality treatment, specifically the use of cochlear implantation in the severely damaged ear.
In the last decade, several experiments have demonstrated the ability to regenerate hair cells via Atoh 1 delivery to the inner ear.14,15 Izumikawa and colleagues subsequently over-expressed Atoh 1 in deafened guinea pigs and not only regenerated hair cells, but were also able to show improved hearing function.10 This experiment differs from that original work in two important ways. Most prior atoh1 delivery studies were performed using the common Ad5 serotype, to which a large portion of the human population possesses neutralizing antibodies. Antibodies to the vector not only have the potential to lower the efficacy of the therapy, but are known to be immunogenic in humans, thus lowering the safety profile of the therapy. Recently, we investigated several alternate adenoviral serotypes in order to identify other candidate vectors. The Ad28 serotype was found to demonstrate superior kinetics to Ad5 while supporting cell specific tropism (Schlecker, et al). Ultimately the differences in vector capsid effects and delivery space will need to be addressed in a quantitative fashion, but the potential for improved specificity, efficacy and safety has significant implications when considering translational research in humans.
The Izumikawa experiment was designed so that the atonal expressing vector was delivered into the scala media via a cochleostomy through the stria vascularis. In this study we delivered our novel vector into the perilymph of the vestibular system via a canalostomy. While previous studies have demonstrated endolymph delivery to be more effective,20 this approach has been successful in regenerating hair cells in the vestibular system.21 . The benefit of the perilymphatic delivery approach is that there are several well-established methods to access the perilymphatic space in animal and human models. The scala vestibuli can be access via stapedotomy or cochleostomy and the scala tympani via the round window. The larger volume of the perilymphatic space potentially would allow for the delivery of more viral particles.22 From a target cell perspective, all but the apical surface of the targeted supporting cell is in perilymph, suggesting that there may be differences in vector binding along the apical vs. basal portions of the cell. Ultimately, the efficacy of perilymph versus endolymph vector delivery may vary due to differences in vector binding between species. Additionally, the differences in antigen recognition between vector serotypes may dictate which delivery method is most effective for each model or application. This will have to be investigated further as researches move toward translational studies.
Conclusions
Combined aminoglycoside and diuretic treatment in C56Bl/6 mice is a severe ototoxic trauma model that results in ablation of inner and outer hair cells in and is associated with profound hearing loss. Perilymphatic delivery of Ad28.gfap.atoh1 in this model induced regeneration of inner hair cells with recovery low frequency hearing as demonstrated by ABR. Further examination of vector kinetics in the cochlea and recovery of sensory hair cells after transdifferentiation will improve our understanding of this process.
Acknowledgments
This work was supported by NIDCD R01DC008424 to HS and NICHD HD02528 Core Grant as well as DOD PR081241 to HS
Footnotes
The authors have no financial conflict of interest
Level of Evidence: N/A
References
- 1.Helzner EP, Cauley JA, et al. Race and sex differences in age-related hearing loss: the Health, Aging and Body Composition Study". J Am Geriatr Soc. 2005;53(12):2119–2127. doi: 10.1111/j.1532-5415.2005.00525.x. [DOI] [PubMed] [Google Scholar]
- 2.Moscicki EK, Elkins EF, et al. Hearing loss in the elderly: an epidemiologic study of the Framingham Heart Study Cohort. Ear Hear. 1985;6(4):184–190. [PubMed] [Google Scholar]
- 3.Ruben RJ, Sidman RL. Serial section radioautography of the inner ear. Histological technique. Arch Otolaryngol. 1967;86(1):32–37. doi: 10.1001/archotol.1967.00760050034007. [DOI] [PubMed] [Google Scholar]
- 4.Lee YS, Liu F, et al. A morphogenetic wave of p27Kip1 transcription directs cell cycle exit during organ of Corti development. Development. 2006;133(15):2817–2826. doi: 10.1242/dev.02453. [DOI] [PubMed] [Google Scholar]
- 5.Roberson DW, Rubel EW. Cell division in the gerbil cochlea after acoustic trauma. Am J Otol. 1994;15(1):28–34. [PubMed] [Google Scholar]
- 6.Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3(6):580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- 7.Kelley MW. Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci. 2006;7(11):837–849. doi: 10.1038/nrn1987. [DOI] [PubMed] [Google Scholar]
- 8.Staecker H, Brough DE, et al. Drug delivery to the inner ear using gene therapy. Otolaryngol Clin North Am. 2004;37(5):1091–1108. doi: 10.1016/j.otc.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Praetorius M, Baker K, et al. Pharmacodynamics of adenovector distribution within the inner ear tissues of the mouse. Hear Res. 2007;227(1–2):53–58. doi: 10.1016/j.heares.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Izumikawa M, Minoda R, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11(3):271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- 11.Vogels R, Zuijdgeest D, et al. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J Virol. 2003;77(15):8263–8271. doi: 10.1128/JVI.77.15.8263-8271.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kidd AH, Banatvala JE, et al. Antibodies to fastidious faecal adenoviruses (species 40 and 41) in sera from children. J Med Virol. 1983;11(4):333–341. doi: 10.1002/jmv.1890110409. [DOI] [PubMed] [Google Scholar]
- 13.Oesterle EC, Campbell S, et al. Sox2 and JAGGED1 expression in normal and drug-damaged adult mouse inner ear. J Assoc Res Otolaryngol. 2008;9(1):65–89. doi: 10.1007/s10162-007-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gall JG, Lizonova A, EttyReddy D, McVey D, Zuber M, Kovesdi I, Aughtman B, King CR, Brough DE. Rescue and production of vaccine and therapeutic adenovirus vectors expressing inhibitory transgenes. Mol Biotechnol. 2007;35:263–73. doi: 10.1007/BF02686012. [DOI] [PubMed] [Google Scholar]
- 15.Brough DE, Lizonova A, Hsu C, Kulesa VA, Kovesdi I. A gene transfer vector-cell line system for complete functional complementation of adenovirus early regions E1 and E4. J Virol. 1996;70:6497–501. doi: 10.1128/jvi.70.9.6497-6501.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abrahamsen K, Kong HL, Mastrangeli A, Brough D, Lizonova A, Crystal RG, Falck-Pedersen E. Construction of an adenovirus type 7a E1A- vector. J Virol. 1997;71:8946–51. doi: 10.1128/jvi.71.11.8946-8951.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Praetorius M, Hsu C, Baker K, Brough DE, Plinkert P, Staecker H. Adenovector-mediated hair cell regeneration is affected by promoter type. Acta Otolaryngol. 2009;129:1–8. doi: 10.3109/00016480903019251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu WJ, Sha SH, McLaren JD, Kawamoto K, Raphael Y, Schacht J. Aminoglycoside ototoxicity in adult CBA, C57BL and BALB mice and the Sprague-Dawley rat. Hear Res. 2001;158:165–78. doi: 10.1016/s0378-5955(01)00303-3. [DOI] [PubMed] [Google Scholar]
- 19.Izumikawa M, Batts SA, Miyazawa T, Swiderski DL, Raphael Y. Response of the flat cochlear epithelium to forced expression of Atoh1. Hear Res. 2008;240:52–6. doi: 10.1016/j.heares.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Y, Chi F, Han Z, Yang J, Gao W, Li Y. New ectopic vestibular hair cell-like cells induced by Math 1 gene transfer in post-natal rats. Brain Res. 2009;1276:31–38. doi: 10.1016/j.brainres.2009.04.036. [DOI] [PubMed] [Google Scholar]
- 21.Baker K, Brough DE, Staecker H. Repair of the vestibular system via adenovector delivery of Atoh1: a potential treatment for balance disorders. Adv Otorhinolaryngol. 2009;66:52–63. doi: 10.1159/000218207. [DOI] [PubMed] [Google Scholar]
- 22.Staecker H, Brough DE, Praetorius M, Baker K. Drug delivery to the inner ear using gene therapy. Otolaryngol Clin North Am. 2004;37:1091–1108. doi: 10.1016/j.otc.2004.05.001. [DOI] [PubMed] [Google Scholar]